碳纳米管纱作为填料应用于硝基芳烃污水处理
Sushil R. Kanel, Heath Misak, Dhriti Nepal, Shankar Mall, Seth W. Brittle,Ioana Sizemore, David M. Kempisty, Mark N. Goltz
(1. Department of Systems Engineering and Management, Air Force Institute of Technology, 2950 Hobson Way,Wright-Patterson AFB, OH45433-7765, USA;2. Department of Aeronautics and Astronautics, Air Force Institute of Technology, 2950 Hobson Way, Wright-Patterson AFB, OH45433-7765, USA;3. Materials and Manufacturing Directorate, Air Force Research Laboratory, Wright-Patterson AFB, Ohio45433-7702, USA;4. Department of Chemistry, Wright State University, 3640 Colonel Glen Highway, Dayton, OH45435, USA)
碳纳米管纱作为填料应用于硝基芳烃污水处理
Sushil R. Kanel1,Heath Misak2,Dhriti Nepal3,Shankar Mall2,Seth W. Brittle4,Ioana Sizemore4,David M. Kempisty1,Mark N. Goltz1
(1.DepartmentofSystemsEngineeringandManagement,AirForceInstituteofTechnology, 2950HobsonWay,Wright-PattersonAFB,OH45433-7765,USA;2.DepartmentofAeronauticsandAstronautics,AirForceInstituteofTechnology, 2950HobsonWay,Wright-PattersonAFB,OH45433-7765,USA;3.MaterialsandManufacturingDirectorate,AirForceResearchLaboratory,Wright-PattersonAFB,Ohio45433-7702,USA;4.DepartmentofChemistry,WrightStateUniversity, 3640ColonelGlenHighway,Dayton,OH45435,USA)
碳纳米管纱(CNTY)因具有优异的力学强度、化学稳定性、热稳定性和高比表面积而成为去除废水中有机污染物的潜在材料。本文将CNTY用于含2,4-二硝基甲苯(DNT)的污水处理。CNTY对DNT的吸附能力与文献报道值作对比研究,同时探讨吸附动力学。采用SEM-EDX、HRTEM、Raman与XPS表征CNTY吸附DNT前后的尺寸、表面形貌及表面化学。结果表明,经CNTY对DNT污水处理后的水质达到实验室无离子水级纯度。CNTY对DNT吸附符合Freundlich吸附等温线,Freundlich参数由KF为55.0 mg/g (L/mg)1/n,指数1/n为 0.737得到,表明其比活性炭吸附性弱,但更易再生。CNTY比活性炭的吸附性速率更快,遵循拟二级动力学模型。CNTY吸附DNT引起D、G常偏移,归因于CNTs与DNT间电子受体和供体效应。
碳纳米管纱; 2,4-二硝基甲苯; 吸附; Freundlich吸附等温线; 新兴技术
1 Introduction
Activated carbon (AC) is a common adsorbent that is used to remove contaminants from water[1]. Though AC is an excellent adsorbent, it is not ideally suited for removal of polar molecules. In addition, the application of AC in water treatment has several challenges, due to its slow adsorption kinetics and resource requirements for regeneration and reactivation[2]. Modified carbon nanotubes (CNTs) have the ability to remove polar molecules[3-6],adjustable structures and surface chemistries, and unique properties such as chemical stability, mechanical and thermal stability as well as high surface area. Given these characteristics, CNTs have great potential application as a third generation carbonaceous adsorbent[2]. In addition to water treatment, CNTs have found a number of other uses in hydrogen storage, protein purification, and biomedical applications[7-11].
In addition to their use as sorbents[2,5,12], CNT-based nanotechnologies have been applied as catalysts[13,14], membrane filters[2,7], and electrodes[10,15]as well as in water treatment. As sorbents, multi-wall CNTs (MWCNTs) and single-wall CNTs (SWCNTs) have been used to remove organic and inorganic pollutants from aqueous solutions as well as gaseous mixtures[12,16]. Although CNTs have several advantages over conventional sorbents like ACs (e.g., faster kinetics and the ability to add functional groups to enhance adsorption and/or degradation of target contaminants[12,16]), their use in practical applications presents challenges[2]. Due to their small size, CNTs cannot be filtered or settled, making recovery problematic[2,9]. Therefore, recent research into water treatment applications of CNTs as sorbents has focused on using CNT incorporated into sponge-like structures[7,12,17]. It has been demonstrated that such three-dimensional structures can adsorb about 3.5 times more mass of a dissolved organic compound (e.g., o-dichlorobenzene) than CNTs used alone[18]. In addition, because of their sponge-like structure, the CNTs can be easily collected, regenerated, and reused[9,19]. A new spinning method has recently been developed to prepare CNT yarn (CNTY)[11]. CNTY consist of assemblies of individual CNT bonded together by van der Waals forces and mechanical interlocking[20]. The yarns were found to be mechanically robust with exceptionally high specific surface area. Although CNTY have been applied in a variety of ways (e.g., medical scaffolding, intelligent clothing, electrical sensors)[12,14], they have not been used in environmental applications to our knowledge. Based on the advantages that have been observed when CNTs are made into sponge-like structure[17,19,21], investigation of CNTY as a sorbent to remove organic contaminants from water is warranted.
The main goal of this study is to investigate the application of CNTY to remove organic contaminants from water. A nitroaromatic compound such as 2,4-dinitrotoluene (DNT), frequently detected in surface and ground water[22], will be used as a model organic contaminant. The main objectives of this study are (i) to characterize CNTY using microscopy and spectroscopy, (ii) to determine the rate and extent of DNT adsorption onto CNTY, and (ⅲ) to compare these results with the rates and extent of adsorption of DNT onto alternative sorbents (e.g., AC and CNT powder) reported in literatures.
2 Experimental
2.1Materials and chemicals
Sodium hydroxide (NaOH), hydrochloric acid (HCL), nitric acid (HNO3) and DNT were purchased from Sigma-Aldrich (St. Louis, MO). All reagents were used as received. All experiments were conducted in ultra-pure water (18 MΩ-cm) produced by a Millipore water purification system (Millipore, Billerica, MA).
The CNTY (60-yarn) were procured from Nanocomp Technologies, Inc., which were produced by a direct spinning method. The process is proprietary, but in general CNTY are made by injecting grain alcohols and iron-based catalysts into a horizontal reactor furnace with hydrogen as the carrier gas. Free CNT and CNT bundles are produced and exit the reactor as an aerogel-like material. In a post processing operation the CNTY is drawn through an acetone bath and twisted prior to collection on a spool[23].
2.2Methods
Batch kinetic and equilibrium adsorption experiments were conducted in triplicate with CNTY. The adsorption experiments were performed in 100 mL amber serum bottles having aluminum-coated septum caps. The pH values for all the samples before and after the experiments were measured (Mettler Toledo Instruments). The ionic strength of the solution was maintained at 1 mM using NaCl, the pH was maintained at 6.5, and the experiments were conducted at room temperature.
For the kinetic experiments, 50 mL of 10 mg·L-1of DNT solution was added to 100 mL amber serum bottles containing ~1 mg CNTY. Ionic strength was maintained at 1mM with NaCl. The pH of the solution was adjusted to pH 6.5 using NaOH. Bottles were prepared in triplicate and mixed using a magnetic stir bar. Aliquot samples of 0.2 mL were removed from the bottles at different time intervals (0-72 h) to determine the rate of adsorption. Samples were diluted with 0.8 mL of deionized water and filtered using a 0.2 μm filter and analyzed by a UV-Vis absorption spectrophotometer (UV-Cary 60, Agilent Technologies). Control samples (triplicate) were run in parallel to confirm that significant DNT losses were not occurring without the adsorbent.
For the adsorption isotherm studies, a known mass of CNTY (~1 mg) was transferred to 100 mL bottles that contained 50 mL of solution at various DNT concentrations (0, 1, 2, 4, 8, 16, and 24 mg·L-1). The isotherms were conducted using a similar method as reported by Randtke[24]. The contents of the bottles were mixed with a magnetic stirrer. After 72 h the bottles were centrifuged and a 4 mL aliquot sample was withdrawn to determine the dissolved DNT concentration at equilibrium using gas chromatography/mass spectrometry (GC/MS) (GC 7890A; Agilent Technologies). The solid phase concentration of DNT was determined by mass balance. Preliminary work showed no further reduction in DNT concentration after 24 h and therefore 72 h was conservatively chosen as the experimental equilibrium time.
Details about the surface morphology of CNTY were obtained using a high resolution scanning electron microscope (Quanta SEM 450 (2 kV using EDAZ (20 kV), FEI). For SEM, CNTY was mounted as received onto an aluminum stub using carbon tape. Images were taken at multiple magnifications. Additional internal morphology of CNTY was determined using high resolution transmission electron microscopy (HR-TEM) at 200 kV using a Philips TEM (CM200 LaB6, Philips Corporation).
Raman spectroscopy is an extensively used analytical tool for the characterization of structure and transformations of materials including CNTs. Thus, to analyze the possible interactions between CNTY and DNT, Raman spectra were measured on the CNTY samples and controls using a LabRamHR 800 (Horiba Scientific Inc) equipped with a high resolution BX41 confocal microscope. The excitation source was a He-Ne laser (632.8 nm) set to an output of 17 mW. The laser beam (~1 μm in diameter) was directed onto the solid samples placed on microscope glass slides through an Olympus 100x objective. Acquisition parameters were holographic grating of 600 grooves mm-1, confocal hole of 300 μm, and accumulation time of 5 s. Under these conditions, the spectral resolution was about 1 cm-1. Each micro-Raman spectrum was averaged over 5 cycles, and was collected with the help of a thermo-electric cooled charge coupled device (CCD) detector (1 024 × 526 pixels). Spectral data was processed and plotted in Origin 8 software.
Using molecular orbital theory and the binding energy of electrons, X-ray photoelectron spectroscopy (XPS) provides information about the chemical structure of materials. In this work, XPS was used to determine differences in the CNTY pre- and post-adsorption. An XPS (M-Probe, Surface Sciences Instruments) was operated at a base pressure of 3×10-7Pa using an operating voltage of 10 kV and a spot size of 800 μm2. The samples were attached with indium foil. Binding energies were calibrated relative to the C1s peak at 284.6 eV. The raw XPS spectra were deconvoluted by curve fitting peak components using the software CASAXPS with no preliminary smoothing. Symmetric Gaussian-Lorentzian product functions were used to approximate the line shapes of the fitting components after a Shirley-type background subtraction. Atomic ratios were calculated from experimental intensity ratios and normalized by atomic sensitivity factors.
3 Results and discussion
3.1SEM-EDX and HR-TEM
Adsorbent characterization was first performed using a standard digital camera, SEM-EDX, and HR-TEM. Images can be seen in Fig. 1. The two images (Fig. 1a) were taken with a standard digital camera to demonstrate the physical size of the CNTYs, an operational advantage compared to conventional unwound nanomaterials such as CNTs. The Fig. 1a inset shows that the CNTY did not unravel after its submersion in DNT aqueous solution. Fig. 1b illustrates an individual CNTY segment consisting of different cylinder-shaped structures wound together. The fine details provided by HR-TEM analysis showed that CNTYs incorporated fine particles (black specs in Fig. 1c). Previous characterization work has revealed the presence of iron in CNTs[25]; thus the small specs are believed to be of iron nature. The CNTY structure did not appear to change after adsorption of DNT. HR-TEM images taken post-adsorption of DNT are indistinguishable from pre-adsorption (Fig. 2). Using SEM-EDX, the elemental composition of the sample was determined. Fig. 1d shows that CNTY is comprised of 94.3% carbon, 5.6% oxygen, and 0.1% of iron.

Fig. 1 (a) Digital picture of CNTY. The inset shows a vial containing CNTY in a DNT aqueous solution;

Fig. 2 HR-TEM of CNTY post-adsorption to DNT.
3.2Adsorption kinetics
Results for the kinetic experiments of DNT adsorption onto CNTY are shown in Fig. 3a. DNT concentrations decrease dramatically initially then equilibrate after 24 h. Since no further adsorption of DNT was observed after 24 h, 72 h was selected as a conservatively sufficient time to achieve equilibrium for the isotherm study. Shen et al (2009) studied the adsorption of nitroaromatic compounds onto multi-walled CNTs with 4 different kinetic models[26]. Apseudo second-order kinetic model was the only model that consistently fit results accurately and was therefore used in this effort. The pseudo-second order model considers both the external mass transfer and the intraparticle diffusion mechanisms responsible for adsorption and uses the difference between the equilibrium solid phase concentration and the solid phase concentration at any time as shown in equation 1[27]:
(1)


Fig. 3 (a) Adsorption kinetics of DNT on CNTY;
3.3Adsorption isotherm
Results from the DNT isotherm experiments are presented in Fig. 3c whereqe(mg·g-1) andCe(mg·L-1) are the equilibrium solid-phase DNT concentration on the CNTY and the equilibrium DNT concentration in the aqueous phase, respectively. The adsorption data were fitted with a Freundlich isotherm[24,28,29].
(2)
WhereKF(mg/g*(L/mg)1/n) is the Freundlich affinity coefficient andqerepresents the equilibrium adsorption capacity and 1/n (unitless) is the Freundlich exponent representing the heterogeneity of adsorption site energies. For the DNT sorption isotherm in this study, eq. 2 appears to hold true, with a linear relation between logqeand logCe(Fig. 3b). Determination of the Freundlich parameters from the isotherm data provided aKF= 55.0 mg/g *(L/mg)1/nand a 1/n value of 0.737. Adsorption is believed to be the sole mechanism responsible for the removal of DNT from solution. Although previous research has shown metal impurities in CNTs, which react with contaminants[30], and the CNTY in this work contained iron, this phenomenon was not observed since GC/MS results showed no DNT degradation products. Table 1 compares these Freundlich isotherm values with the parameter values obtained for DNT adsorption onto various other sorbents found in the literatures. Not surprisingly, the value of the Freundlich affinity coefficient,KF, for CNTY was found to be most similar to the value ofKFobtained for single-wall CNTs (SWCNTs).KFvalues for adsorption onto powder activated carbon (PAC) and granular activated carbon (GAC) were higher, indicating that forCe= 1 mg/L, adsorption of DNT onto GAC and PAC is greater than adsorption onto CNTs and CNTY.
3.4Raman analysis
Raman spectroscopy measurements were performed in triplicate on the CNTY samples (~0.02 g·L-1) exposed for 3 days to various concentrations of DNT (0, 1, 2, 4, and 8 mg·L-1) to confirm the interaction between DNT and CNTY and to examine the concentration dependence of the DNT adsorption behavior (Fig. 4). The identity and purity of the CNTY was confirmed by the presence of characteristic Raman bands at 196 (radial breathing mode), 1 328 (D-disordered mode), 1 588 (G-tangential mode), and 2 640 cm-1(D' or second orderG'), which closely matched the literature values for CNTs[31-34](Fig. 4). The bands at 213 and 257 cm-1(Fig. 4a inset) are probably the result of two contributions: 1) the presence of iron oxide particles as also revealed by SEM-EDX and HR-TEM[35,36], and 2) the radial breathing modes of CNTs[31,37]. The appearance of the CNT Raman bands post-adsorption to DNT indicates that the identity of CNTY remained intact, which is in agreement with the HR-TEM observations. Post-adsorption, no vibrational modes characteristic to DNT were detected in the Raman spectra (Fig. 4b) probably due to the limited sensitivity of the micro-Raman technique at these DNT concentrations. However, CNTY exhibited changes in their Raman spectral profile (Fig. 4b), which became more significant with increasing DNT concentration (Fig. 4c-e). For example, theDandGbands experienced Raman shifts to lower wavenumbers after adsorption of DNT. The largest shifts were observed post-adsorption to 8 mg·L-1of DNT, namely 8 and 15 cm-1for theD- andG-band, respectively. Additionally, theD-band decreased more than 20% in full width at half maximum (FWHM) and increased more than 100% in theID-band/IG-bandratio with the increase in DNT concentration from 0 to 8 mg·L-1. Molecules containing electron-acceptor groups like DNT are known to modify the electronic structure of electron-donor CNTs, leading to important changes in their Raman spectra, in particular for the disorder-inducedD-band[38,39]. In a recent study, Hung et al. have showed that theelectrons on the sidewall of SWCNTs may interact with nitroaromatic compounds like DNT viaπ-πstacking[38]. Thus, the spectral changes observed for the Raman characteristic peaks of CNTY are indicative of non-covalent interaction with DNT in a dose dependent manner and the possible formation of an electron donor-acceptor complex between CNTY and DNT.

Table 1 Freundlich model coefficients (KF and 1/n) obtained from DNT adsorption onto various adsorbents.
Note: 2,4 DNT: 2,4 dinitrotoluene, G/PAC( - ): granular/powdered activated carbon (type), NA: Not available, SWCNT: single wall carbon nanotube, CNTY- carbon nanotube yarn.

Fig. 4 Raman spectra of CNTY (a) pre-adsorption (inset shows the 150-350 cm-1 spectral range) and
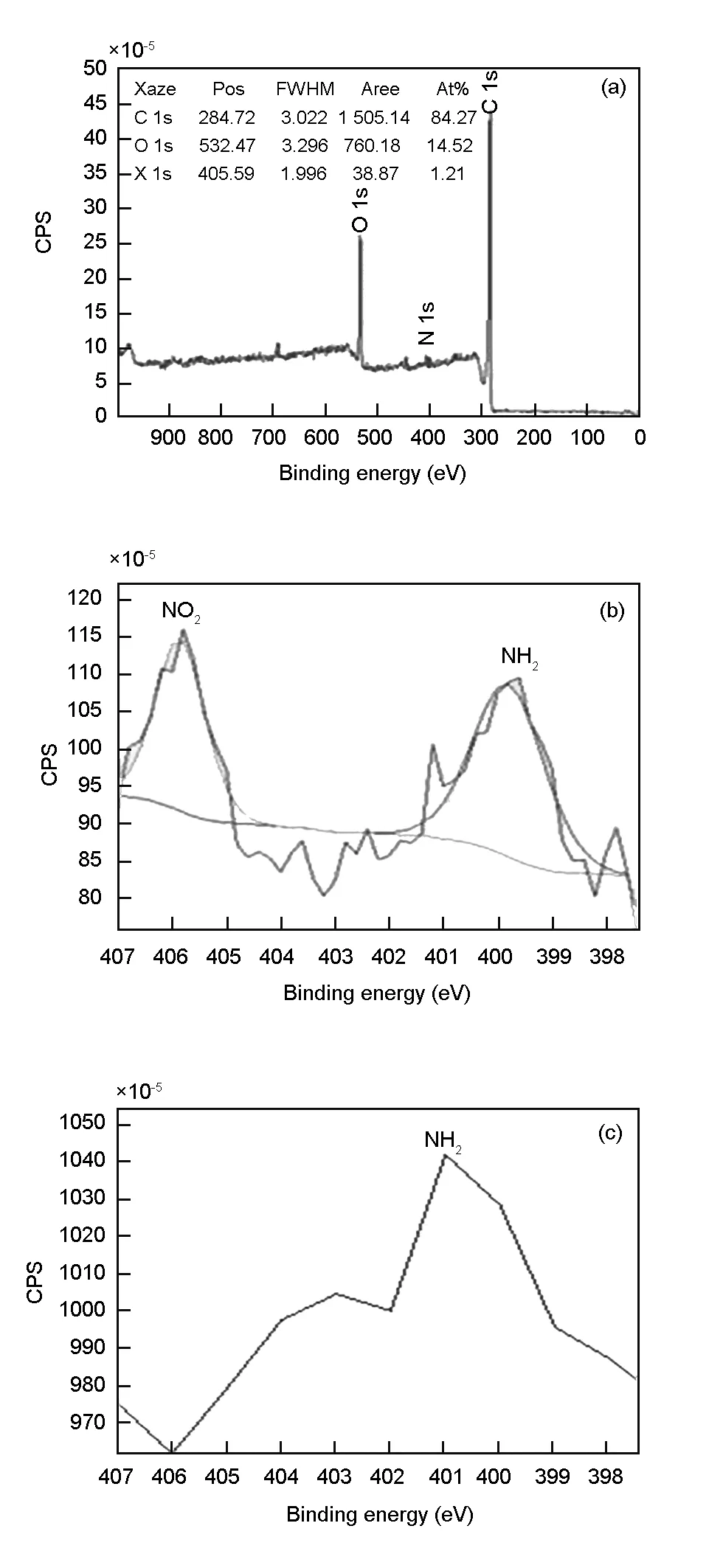
Fig. 5 XPS study of CNTY pre and post-adsorption to DNT:
3.5XPS analysis
XPS study of CNTY post adsorption with DNT revealed a N 1s peak (405.47 eV) with 1.17% of its atomic percentage confirming molecular adsorption of DNT. In addition to the N 1s peak, the survey scan (Fig. 5a) showed C 1s (284.6 eV), O 1s (532.35 eV) and Fe 2p (706.78 eV) peaks. As expected C 1s is the most predominant with atomic percentage 84.27% confirming it as the major backbone of the CNTY. Oxygen species present on the CNTY are also confirmed with the observation of defects by D-band shift from Raman analysis. Trace amount (0.07%) of Fe 2p also corroborates with SEM-EDAX results discussed earlier. N 1s spectrum shows two distinct peaks. The first peak at the highest binding energy (406 eV) can be attributed to NO2(Fig. 5b). This peak is absent on all the pre-adsorption CNTY surface spectra. This confirms that CNTY substrates reacted with DNT having the NO2functional group on their surfaces. The second peak (Fig. 5b and 5d) around 400 eV can be assigned, either, to the atmospheric nitrogen (this peak is always evidenced when the sample is prepared apart from the preparation chamber of the XPS spectrometer) and/or, as reduced nitrogen (amines)[43]. The same peak (400 eV), although weaker, is also observed in pre-adsorption CNTY (Fig. 5c; atomic percentage of N 1s is < 0.06%) whereas the NO2peak (406 eV) is completely absent.
4 Conclusions
Advantages of using CNTY to treat contaminated waters include its ease of recovery, the ability to add functional groups to it to allow both degradation and adsorption of specific contaminants, and potentially, due to its structure, higher hydraulic conductivities than competing sorbents like PAC and CNTs. In this study, we have shown that CNTY has an adsorption capacity toward a model organic compound that is comparable with, if not slightly better than, that of CNTs. In essence, CNTY has many of the advantages of CNTs as a sorbent (e.g., rapid kinetics, ability to functionalize), while not having the disadvantages (e.g., post-treatment separation problems, low conductivity, release to the environment). Potentially, CNTY can be used in both batch and flow-through modes as an adsorbent with high capacity and rapid kinetics. It may also be possible to apply CNTY in situ to treat contaminated ground water, perhaps as an adsorbent in a permeable reactive barrier. To explore these potential applications, future research is needed to investigate CNTY functionalization, CNTY hydraulic conductivity, rate and extent of adsorption of other organic (polar and non-polar) and inorganic compounds onto CNTY, and adsorption behavior in water with a background matrix containing natural organic matter.
Acknowledgments
This research was supported by the Department of Defense’s Environmental Restoration Account. The authors acknowledge Dr. Daniel Felker for his help in GC/MS analysis and gratefully acknowledge the technical assistance efforts of research assistant Tala Ebrahimian. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the United States Air Force, the Department of Defense, or the U.S. Government.
[1]Pal S, Joardar J, Song JM. Removal of E. coli from water using surface-modified activated carbon filter media and its performance over an extended use[J]. Environmental Science & Technology, 2006, 40(19): 6091-6097.
[2]Liu XT, Wang MS, Zhang SJ, et al. Application potential of carbon nanotubes in water treatment: A review[J]. J Environ Sci, 2013, 25(7): 1263-1280.
[3]Nepal D, Geckeler KE. pH-sensitive dispersion and debundling of single-walled carbon nanotubes: Lysozyme as a tool[J]. Small, 2006, 2(3): 406-412.
[4]Nepal D, Geckeler KE. Proteins and carbon nanotubes: Close encounter in water[J]. Small, 2007, 3(7): 1259-1265.
[5]Kim DS, Nepal D, Geckeler KE. Individualization of single-walled carbon nanotubes: Is the solvent important[J]. Small, 2005, 1(11): 1117-1124.
[6]Wang HT, Lin KY, Jing BX, Krylova G, et al. Removal of oil droplets from contaminated water using magnetic carbon nanotubes[J]. Water Res, 2013, 47(12): 4198-4205.
[7]Basu-Dutt S, Minus ML, Jain R, et al. Chemistry of carbon nanotubes for everyone[J]. Journal of Chemical Education, 2012, 89(2): 221-229.
[8]Chun K-Y, Kim SH, Shin MK, et al. Hybrid carbon nanotube yarn artificial muscle inspired by spider dragline silk[J]. Nature Communications, 2014, 5(2): 163-180.
[9]Mubarak NM, Sahu JN, Abdullah EC, et al. Removal of heavy metals from wastewater using carbon nanotubes[J]. Sep Purif Rev, 2014, 43(4): 311-338.
[10]Nepal D, Geckeler K. Functional Nanomaterials. In: Geckeler K, Rogenburg E, editors. Functionalization of Carbon Nanotubes[M]. Valencia: American Scientific Publishers, 2006: 57-79.
[11]Wei G, Yu H, Quan X, et al. Constructing all carbon nanotube hollow fiber membranes with improved performance in separation and antifouling for water treatment[J]. Environmental Science & Technology, 2014, 48(14): 8062-8068.
[12]Wang S, Zhai Y-Y, Gao Q, et al. Highly Efficient removal of acid red 18 from aqueous solution by magnetically retrievable chitosan/carbon nanotube: Batch Study, Isotherms, Kinetics, and Thermodynamics[J]. Journal of Chemical & Engineering Data, 2013, 59(1): 39-51.
[14]Wirth CT, Bayer BC, Gamalski AD, et al. The phase of iron catalyst nanoparticles during carbon nanotube growth[J]. Chemistry of Materials, 2012, 24(24): 4633-4640.
[15]Nepal D. Dispersion, individualization and novel supramolecular adducts of carbon nanotubes[D]. Gwangju, South Korea: Gwangju Institute of Science and Technology, Ph.D. Thesis, 2006.
[16]Sheng GD, Shao DD, Ren XM, et al. Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes[J]. Journal of Hazardous Materials, 2010, 178(1-3): 505-516.
[17]Gui X, Wei J, Wang K, et al. Carbon nanotube sponges[J]. Advanced Materials, 2010, 22(5): 617-621.
[18]Camilli L, Pisani C, Gautron E, et al. A three-dimensional carbon nanotube network for water treatment[J]. Nanotechnology, 2014, 25(6): 65701-65707.
[19]Hashim DP, Narayanan NT, Romo-Herrera JM, et al. Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions[J]. Scientific Rep, 2012, 2(363), doi:10.1038/srep00363.
[20]Ma W, Liu L, Yang R, et al. Monitoring a micromechanical process in macroscale carbon nanotube films and fibers[J]. Advanced Materials, 2009, 21(5): 603-608.
[21]Upadhyayula VKK, Deng S, Mitchell MC, et al. Application of carbon nanotube technology for removal of contaminants in drinking water: A review[J]. Science of The Total Environment, 2009, 408(1): 1-13.
[22]O'Mahony AM, Wang J. Nanomaterial-based electrochemical detection of explosives: a review of recent developments[J]. Anal Methods, 2013, 5(17): 4296-4309.
[23]Wu AS, Chou T-W, Gillespie JW, et al. Electromechanical response and failure behaviour of aerogel-spun carbon nanotube fibres under tensile loading[J]. Journal of Materials Chemistry, 2012, 22(14): 6792-6798.
[24]Randtke SJ, Snoeyink VL. Evaluating GAC adsorptive capacity[J]. J Am Water Work Assoc, 1983, 75(8): 406-413.
[25]Kashiwagi T, Grulke E, Hilding J. Thermal degradation and flammability properties of poly(propylene)/carbon nanotube composites[J]. Macromol Rapid Commun, 2002, 23(13): 761-765.
[26]Shen X-E, Shan X-Q, Dong D-M, et al. Kinetics and thermodynamics of sorption of nitroaromatic compounds to as-grown and oxidized multiwalled carbon nanotubes[J]. Journal of Colloid and Interface Science, 2009, 330(1): 1-8.
[27]Azizian S, Yahyaei B. Adsorption of 18-crown-6 from aqueous solution on granular activated carbon: A kinetic modeling study[J]. Journal of Colloid and Interface Science, 2006, 299(1): 112-115.
[28]Kanel SR, Manning B, Charlet L, et al. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron[J]. Environmental Science & Technology, 2005, 39(5): 1291-1298.
[29]Kanel SR, Choi H, Kim J-Y, et al. Removal of arsenic(III) from groundwater using low-cost industrial by-products-Blast furnace slag[J]. Water Quality Research Journal of Canada, 2006, 41(2): 130-139.
[30]Tian X, Zhou S, Zhang Z, et al. Metal impurities dominate the sorption of a commercially available carbon nanotube for Pb(II) from water[J]. Environmental Science & Technology, 2010, 44(21): 8144-8149.
[31]Graupner R. Raman spectroscopy of covalently functionalized single-wall carbon nanotubes[J]. J Raman Spectrosc, 2007, 38(6): 673-683.
[32]Osswald S, Havel M, Gogotsi Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy[J]. J Raman Spectrosc, 2007, 38(6): 728-736.
[33]Sato-Berrú RY, Basiuk EV, Saniger JM. Application of principal component analysis to discriminate the Raman spectra of functionalized multiwalled carbon nanotubes[J]. J Raman Spectrosc, 2006, 37(11): 1302-1306.
[34]Valcárcel M, Cárdenas S, Simonet BM. Role of carbon nanotubes in analytical science[J]. Analytical Chemistry, 2007, 79(13): 4788-4797.
[35]Bersani D, Lottici PP, Montenero A. Micro-Raman investigation of iron oxide films and powders produced by sol-gel syntheses[J]. J Raman Spectrosc, 1999, 30(5): 355-360.
[37]Choi JH, Nguyen FT, Barone PW, et al. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes[J]. Nano Letters, 2007, 7(4): 861-867.
[38]Hung W-C, Elias G, Wai CM. Interaction of aromatic derivatives with single-walled carbon nanotubes[J]. ChemPhysChem, 2010, 11(16): 3439-3446.
[39]Menanteau T, Levillain E, Breton T. Spontaneous grafting of nitrophenyl groups on carbon: Effect of radical scavenger on organic layer formation[J]. Langmuir, 2014, 30(26): 7913-7918.
Instructions to Authors
NewCarbonMaterialsis a bimonthly journal published with the permission of the Ministry of Science and Technology and of the State News and Publication Agency. The journal is sponsored by the Institute of Coal Chemistry, Chinese Academy of Sciences, and is published by Science Press.Aims and Scope
NewCarbonMaterialspublishes research devoted to the physics, chemistry and technology of those organic substances that are precursors for producing aromatically or tetrahedrally bonded carbonaceous solids, and of the materials that may be produced from those organic precursors. These materials range from diamond and graphite through chars, semicokes, mesophase substances, carbons, carbon fibers, carbynes, fullerenes and carbon nanotubes, etc. Papers on the secondary production of new carbon and composites materials (for instance, carbon-carbon composites) from the above mentioned various carbons are also within the scope of the journal. Papers on organic substances will be considered if research has some relation to the resulting carbon materials.
Manuscript Requirements
1.NewCarbonMaterialsaccepts Research Paper, Short Communication and Review. The number of words in each Research Paper should be less than 6000 words. Short Communication < 3500 words. There is no maxium of words for Review.
2. Manuscript including an abstract, graphical abstract, highlight, keywords, reference list, original figures and captions, tables. Manuscripts can be written both in Chinese and English.
3. Manuscript should be accompanied with key words placed after Abstract and a short resume of first author (name, academic degree, professional position) placed in the end of 1st page of text as foot-note. Corresponding author and his (her) E-mail address should also be mentioned.
4. All illustrations, photographs, figures and tables should be on separate sheets, figure captions should be typed separately, not included on the diagram. Authors are requested to submit original photographs, which should have good contrast and intensity.
5. References should be individually numbered in the order in which they are cited in the text, and listed in numerical sequence on separate sheets at the end of the paper, typed in double spacing. Remember that "unpublished works" are not references! In the reference list, periodicals [1], books [2], multi-author books with editors [3], proceedings [4], patents [5], and thesis [6] should be cited in accordance with the following examples:
[1]Mordkovich V Z, Baxendale M, Yoshimura S, et al. Intercalation into nanotubes. Carbon, 1996, 34(10): 1301-1303.
[2]Lovell D R. Carbon and High-Performance Fibers Directory.5th ed., London: Chapman & Hall,1991: 66.
[3]Mochida I, Korai Y. Chemical characterization and preparation of the carbonaceous mesophase. In: Bacha J D, Newman J W, White J L, eds. Petroleum-Derived Carbons. Washington DC: ACS, 1986, 29-31.
[4]Su J, Li G, Hao Z. The research and application of copper impregnated coarse-grain graphite throat.23rd Int'l Biennial Conference on Carbon, Extended Abstract and Program, July 18-23, Pennsylvania 1997, 256-258.
[5]Shigeki T, Jinichi M, Hiroshi H. Manufacture of mesocarbon microbeads. JP 61-222913,1986.
[6]Jones L E. The Effect of Boron on Carbon Fiber Microstructure and Reactivity. Ph.D.Thesis. Penn State University, University Partk, PA 1987.
Note: For the references with more than three authors, please give the first three and mark "et al".
6. Publication of papers in the journal is free of charge. Authors whose paper is published in the journal will receive 10 free offprints and 2 copy of this journal soon after its coming out.
7. Manuscript Submission: Online submission: http://xxtcl.sxicc.ac.cn/EN/volumn/home.shtml
The use of carbon nanotube yarn as a filter medium to treat nitroaromatic-contaminated water
Sushil R. Kanel1,Heath Misak2,Dhriti Nepal3,Shankar Mall2,Seth W. Brittle4,Ioana Sizemore4,David M. Kempisty1,Mark N. Goltz1
(1.DepartmentofSystemsEngineeringandManagement,AirForceInstituteofTechnology, 2950HobsonWay,Wright-PattersonAFB,OH45433-7765,USA;2.DepartmentofAeronauticsandAstronautics,AirForceInstituteofTechnology, 2950HobsonWay,Wright-PattersonAFB,OH45433-7765,USA;3.MaterialsandManufacturingDirectorate,AirForceResearchLaboratory,Wright-PattersonAFB,Ohio45433-7702,USA;4.DepartmentofChemistry,WrightStateUniversity, 3640ColonelGlenHighway,Dayton,OH45435,USA)
Carbon nanotube yarn (CNTY) is a promising material for the removal of organic contaminants from aqueous waste streams owing to its extraordinary mechanical strength, chemical stability, thermal stability and high surface area. CNTY was used to treat water contaminated with a model nitroaromatic compound, 2,4-dinitrotoluene (DNT). The isotherms and kinetics of DNT adsorption onto CNTY were investigated. The adsorption capacities of DNT were compared with the literature values of alternative sorbents. SEM-EDX, HR-TEM, Raman spectroscopy and XPS were used to characterize the size, surface morphology and surface chemistry of the CNTY before and after DNT adsorption. Results indicate that adsorption isotherm of DNT onto CNTY could be fitted by the Freundlich isotherm with a Freundlich constant,KF, of 55.0 mg/g (L/mg)1/nand a Freundlich exponent, 1/n, of 0.737. Adsorption kinetics can be formulated by the pseudo-second order kinetic model. This study demonstrates the ability of CNTY to remove organic contaminants from water.
Carbon nanotube yarn; 2,4-dinitrotoluene; Adsorption; Freundlich isotherm; Emerging technology
date: 2016-05-30Reviseddate: 2016-07-30
David M. Kempisty. E-mail: david.kempisty@afit.edu
1007-8827(2016)04-0415-09
TQ127.1+1
A
David M. Kempisty. E-mail: david.kempisty@afit.edu
10.1016/S1872-5805(16)60021-5
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).

