点缺陷引起中子辐照MgO(110)单晶的铁磁性
曹梦雄,王兴宇,马亚茹,马春林,周卫平,*,王晓雄,王海欧,谭伟石,3,*
1南京理工大学理学院应用物理系,软化学与功能材料教育部重点实验室,南京 210094
2杭州电子科技大学材料物理研究所,杭州 310018 3湖南城市学院信息与电子工程学院,湖南 益阳 413002
1 Introduction
In recent years, the ferromagnetism (FM) has been reported in undoped oxides, such as HfO2, TiO2, In2O3, CeO2, Al2O3, SnO2and ZnO1-7. This observed ferromagnetism in materials, containing no intrinsic magnetic elements, is termed d0ferromagnetism8. The d0ferromagnetism can be explained by invoking the formation of point defects but the nature of defects is still debatable. MgO is one of the most attractive materials for investigating d0ferromagnetism because of its wide bandgap, which yields a spin-polarized p band9. Hu et al.10reported that MgO nanocrystalline powders, prepared by sol-gel method, exhibited room temperature ferromagnetism (RTFM). The vacuum annealing of MgO powders reduced FM. They attributed the RTFM in MgO to Mg vacancies at/near the surfaces of nanograins. The X-ray photoelectron spectroscopy (XPS) showed that MgO powders exhibited Mg-deficiency, indicating that the RTFM in MgO powders was related to Mg vacancies11. Li et al.12suggested that the observed RTFM in MgO thin films, which were prepared under various oxygen pressures, depended strongly on Mg vacancies concentration. On the basis of X-ray absorption near-edge fine structure spectroscopy (XANES), it could be contemplated that excitation to the localized bound state at surface state might be responsible for the FM in MgO, which was due to Mg vacancies at surface states13. These results were in accordance with some theoretical predictions14-16. Using ab initio calculations based on density functional theory, Kuang et al.14demonstrated that both neutral and singly charged Mg vacancy could introduce magnetic moment of MgO, and the magnetic moment mainly originated from the spin polarization of partially occupied 2p orbitals of the nearest O atoms surrounding Mg vacancies. By the full potential linearized augmented plane wave L/APW+lomethod, Merabet et al.16predicted that the cation vacancies caused the FM in MgO, with a spin magnetic moment of 0.21 μB·atom-1.
However, some theoretical calculations indicated that oxygen vacancies played a key role in FM spin-order in MgO17,18. Using ab initio calculations based on density functional theory, Zhang et al.18showed that local magnetic moment in MgO could be induced by the composite defects around the oxygen vacancies, when the exchange split of the oxygen vacancies was enhanced. Experimentally, Kumar et al.19reported the correlation between defects and ferromagnetism in synthesized MgO nanocrystallite. Their study suggested that the oxygen vacancies, namely singly ionized anionic vacancies and dimers, induced the room temperature ferromagnetic spin-order. Maoz et al.20noticed that air annealing reduced magnetic spin-order in MgO nanosheets and suggested that the ferromagnetic spin-order was due to the unpaired electrons trapped at oxygen vacancies.Mishra et al.21reported that Al-doped MgO nanoparticles exhibited weak RTFM. Using XPS, they highlighted the dominant role of oxygen vacancies in the development of RTFM in MgO. The RTFM in Fe-doped MgO could be explained by oxygen vacancies interaction based on the bound magnetic polaron (BMP) model22.
Although there are many theoretical and experimental studies regarding FM in MgO, the origin of FM in MgO is still controversial. The point defects can account for the FM in MgO, but it is still uncertain whether the defects should be anion defects or cation defects. Thus, a detailed insight into the role of point defects on ferromagnetism in MgO(110) single crystals is obtained in this paper. We study the point defects configuration in neutron irradiated MgO(110) single crystals by the X-ray diffuse scattering and UV-Vis absorption spectra. The magnetic properties in MgO are characterized by a superconducting quantum interference device (SQUID) magnetometer. The relevance between defects and ferromagnetism in MgO single crystals is discussed.
2 Experimental
Commercial MgO(110) single crystals with purity > 99.99% were purchased from Hefei Kejing Materials Technology Co., Ltd. (China) and then irradiated by slow neutron with different doses in the range from 1.0 × 1016to 1.0 × 1020cm-2. The neutron energy is continuous but lower than 20 eV. Based on Huang′s scattering theory, the isointensity profiles of cubic and double-force point defects induced X-ray diffuse scattering were calculated using MATLAB software. The measurement of X-ray diffuse scattering intensity distribution curves near the 220 reciprocal-lattice point of MgO single crystals was carried out at Beijing Synchrotron Radiation Facility (BSRF) diffuse scattering station at room temperature. The wave length was 0.15406 nm. The ω-2θ scans and rocking curves close to the symmetric (220) reflection were measured with a step width of 0.005°. The reciprocal space mappings (RSMs) around 220 reciprocal-lattice point were conducted with a step width of 0.01° at room temperature. The UV-Vis absorption spectra were obtained at room temperature using TU-1901 double beam spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., China). The data were recorded in a wavelength range from 200 to 800 nm. The magnetization as a function of an applied magnetic field at different temperature and as a function of temperature (zero field cooled and field cooled) under an applied field of 500 Oe in the temperature range of 2-300 K were measured by a Quantum Design MPMS XL-5 SQUID magnetometer. All samples were cleaned with alcohol and acetone in an ultrasonic bath before measurements.
3 Results and discussion
As the Fourier transform of a defect-induced displacement field is given, the isointensity profile of X-ray diffuse scattering near the reciprocal-lattice point can be calculated23. The purpose of such a calculation is to compare calculated profiles with experimental results and, afterwards, to confirm the point defect configurations. To investigate the irradiation induced point defect configurations in MgO, the isointensity profiles of X-ray diffuse scattering caused by the cubic and double-force point defects are calculated based on Huang′s scattering formula23using MATLAB software.
It is worth noting that the cubic defect along <100> direction induced diffuse scattering isointensity profiles are identical for the cubic crystal23. Therefore, the scattering isointensity profiles near 200, 020 and 002 are uniform for MgO. Thus, we just calculated the Huang′s scattering isointensity profiles near 200 and 220 reciprocal-lattice points for diffuse X-ray scattering induced by the cubic defect in MgO, as shown in Fig.1. Three independent elastic constants for MgO used for calculation are given as follows: C11 = 297.8 GPa, C12 = 95.8 GPa, C44 = 154.7 GPa. The profiles near 200 reciprocal-lattice point are almost of the lemniscate type, as seen in Fig.1a.
In the same way, the isointensity profiles near 200, 020, 002 and 220 reciprocal-lattice points for a double-force defect in MgO are calculated, shown in Fig.2. The isointensity profiles near different reciprocal-lattice points for a double-force defect show various distribution types. Our results are similar with Flocken et al.′s results in cubic metals23.
Obviously, the isointensity profiles near different reciprocal- lattice points for the double-force defect are different. Whatever, near the same reciprocal-lattice point, the isointensity profiles for cubic defects and double-force defects are diverse and distinguishable distinctly. Thus, we can confirm the point defects configuration by comparing the experimental RSMs with calculated diffuse scattering intensity curves.
Fig.3 shows the X-ray diffraction patterns of irradiated MgO(110), which are recorded with 2θ in the range from 30° to 80°. It is worth noting that the patterns cannot reveal the diffuse scattering accurately. Evidently, only diffraction peak of (220) reflection at about 62.2° can be observed in Fig.3. It indicates that the samples are (110)-oriented MgO single crystals without any impurity phase. It is noteworthy that we cannot study the defects in MgO basing the X-ray diffraction patterns in Fig.3. It is necessary to measure the ω-2θ curves and rocking curves for studying the defects in MgO(110) single crystals.
The radial scattering intensity distribution along [220] reciprocal vectors are measured at room temperature. Fig.4(a, b) represents the ω-2θ curves and rocking curves of (220) reflection for pristine and irradiated MgO(110) single crystals. As shown in Fig.4a, in comparison with the case for pristine MgO, the diffraction intensity of (220) reflection decreases dramatically for irradiated samples. The diffraction intensity is almost inversely proportional to the irradiation dose. Meanwhile, the diffraction peak moves obviously toward to the low angle for the irradiated MgO with higher dose (1.0 × 1019and 1.0 × 1020cm-2), implying the presence of lattice imperfection due to the neutron irradiation. Using Bragg equation, the lattice parameter a in bulk MgO has been calculated. The lattice parameter a in pristine MgO is 0.4211 nm, while the values of lattice parameter a in irradiated MgO with the doses of 1.0 × 1019and 1.0 × 1020cm-2are 0.4217 and 0.4222 nm, respectively. As shown in Fig.4b, the intensity of rocking curve decreases with increasing irradiation dose. It should be mentioned that the split of peak in rocking curve is distinct for the irradiated MgO with higher dose. The results obviously indicate that a number of point defects are introduced and hence result in lattice distortions in MgO(110) single crystals via neutron irradiation. The point defects-induced diffuse scattering was also observed in Au+ion irradiated MgO single crystals24. Using X-ray diffraction methods, Pillukat et al.25demonstrated that the electron irradiation could generate point defects and result in the increase of diffuse scattering intensity close to different reflections in GaAs. Karsten et al.26measured X-ray diffuse scattering close to different Bragg reflections in irradiated InP. They confirmed that electron irradiation generated Frenkel defect pairs in irradiated InP, leading to the diffuse scattering.
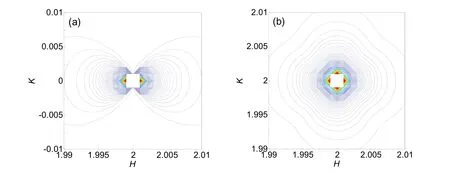
Fig.1 Calculated isointensity profiles of X-ray diffuse scattering near (a) 200 and (b) 220 reciprocal-lattice points for the cubic point defect in MgO.

Fig.2 Calculated isointensity profiles of X-ray diffuse scattering near reciprocal-lattice points (a) 200, (b) 020, (c) 002, and (d) 220 for the double-force point defect in MgO.
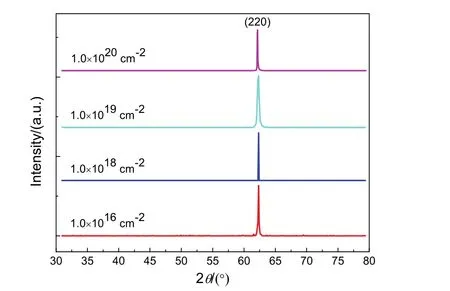
Fig.3 X-ray diffraction patterns of neutron irradiated MgO(110) single crystals with different doses.
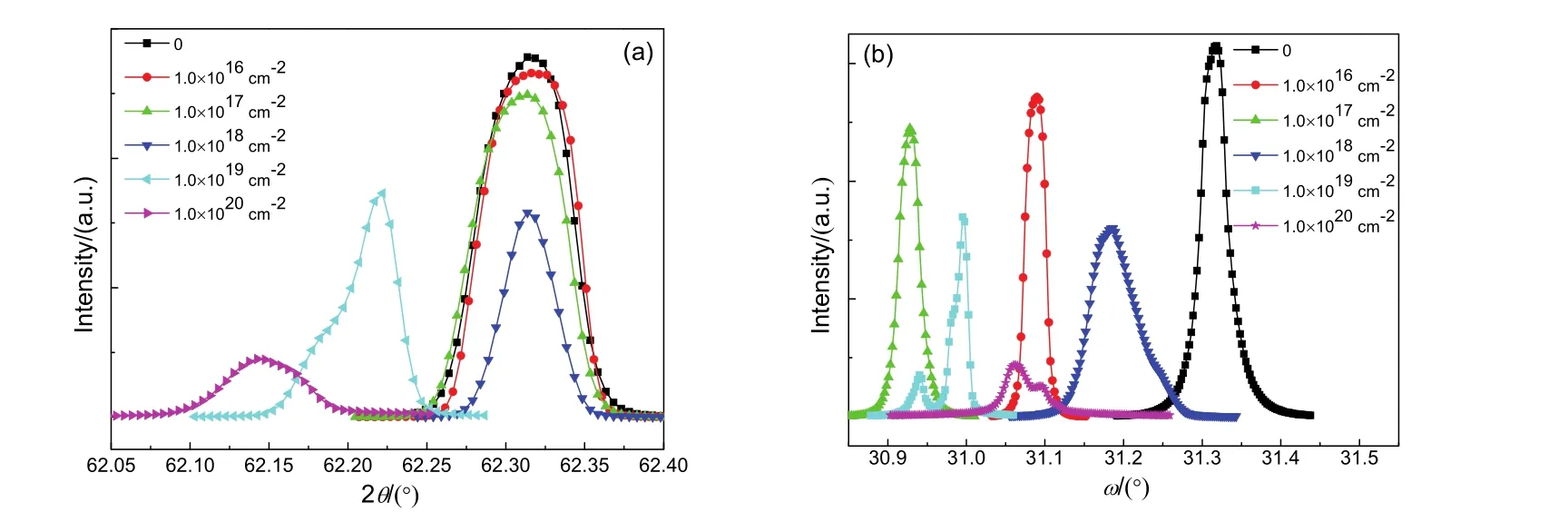
Fig.4 ω-2θ curves (a) and rocking curves (b) in the vicinity of (220) reflection for MgO(110) single crystals. To adjust position of ω and 2θ axes accurately in our experiment, we fix one of these two axes and scan the other one repeatedly until the maximum of diffraction intensity appears. We do not change the zero point of ω and 2θ axes of goniometer during measurement and therefore, the ratio of 2θ/ω is not strictly twice in the measured results. The reason for this case is due to the angle of inclination between the surface and diffracting plane of MgO single crystals.

Fig.5 RSMs around the (220) reflection for MgO(110) irradiated with different doses of (a) 1.0 × 1016 cm-2, (b) 1.0 × 1017 cm-2,(c) 1.0 × 1019 cm-2, and (d) 1.0 × 1020 cm-2.
To further understand point defect configurations, the RSMs around 220 reciprocal-lattice point are measured at room temperature and shown in Fig.5. The RSMs in irradiated MgO are like rhombic and diffuse scattering can be observed clearly. It reveals that a certain number of point defects are introduced in all neutron irradiated MgO(110) single crystals. The RSMs in irradiated MgO with higher dose are more complex, indicating that the defects concentration is higher in irradiated MgO with higher dose. The result is in agreement with that of ω-2θ scans and rocking curves. The RSMs around 220 reciprocal-lattice point of irradiated MgO can be well compared with the calculated diffuse scattering intensity profile near 220 reciprocal-lattice point for double-force point defects, demonstrating that interstitial atoms or divacancies are introduced in irradiated MgO. It is well believed that the interstitial atom is more stable in face-center cubic crystal. It makes clear that lots of interstitial atoms rather than divacancies are introduced in irradiated MgO(110) single crystals. To keep neutral, equal amount of vacancies will be generated if interstitials are presented in materials without impurity. Thus, we conclude that the Frenkel defects are introduced in MgO(110) single crystals by means of neutron irradiation.
It is still unknown that the introduced defects are cation or anion Frenkel defects. So, the UV-Vis absorption spectra of pristine and irradiated MgO(110) single crystals are recorded and shown in Fig.6. It can be seen that the absorption spectrum of pristine bulk MgO is a smooth curve without any absorption peak, implying that defects and impurities are absent in pristine MgO(110). However, the broad peak, centered at about 250 nm, can be observed for all irradiated samples. But the intensity is much lower for some samples. This absorption peak at about 250 nm is associated with single anion vacancies (F or F+centers)27,28. It indicates that O vacancies are presented in irradiated MgO(110) single crystals. Depending on the charge of anion vacancy, the F-type color centers in MgO are classified as F-centers (O vacancies with two electrons trapped), F+-centers (O vacancies with one electron trapped) or F2+-centers (O vacancies without electrons trapped). Notably, the absorption peak around 570 nm is obviously observed for irradiated samples with higher dose (1.0 × 1019and 1.0 × 1020cm-2). The absorption around 570 nm is due to the aggregation of F centers27. Once samples are irradiated with higher dose, the introduced single vacancies can acquire enough energy, resulting in migrating easily. As a consequence, the single vacancies will be aggregated. Thus, the single vacancies can be aggregated when the irradiation dose exceeds 1.0 × 1019cm-2.
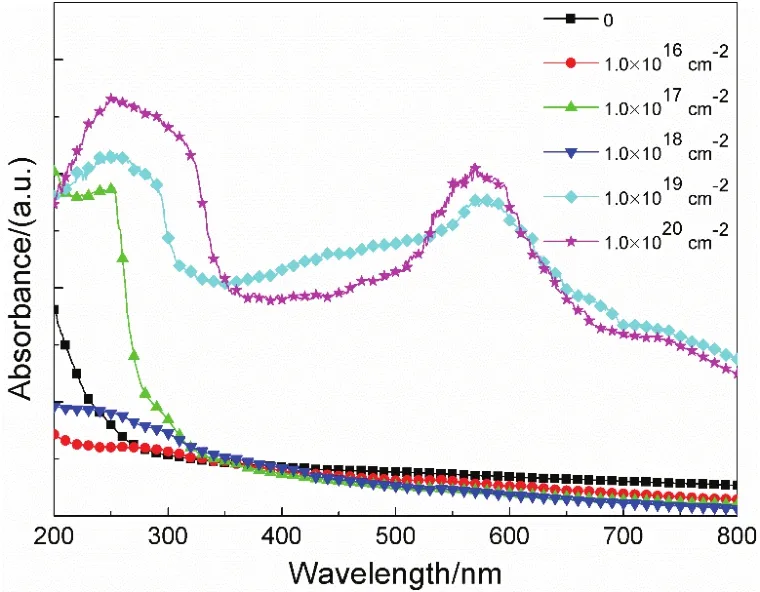
Fig.6 UV-Vis absorption spectra of neutron irradiated MgO(110) single crystals with different doses.
The peak intensity is related to the defects concentration. It seems that the defects concentration is dependent on the neutron irradiation dose but not in directly proportion to it. The defects concentration in irradiated MgO with the dose of 1.0 × 1020cm-2is highest. The results of RSMs and UV-Vis spectra indicate clearly that neutron irradiation can introduce O Frenkel defects in MgO(110) single crystals and aggregation is formed in samples with higher dose. It is important to point out that the absorption peak around 350 nm does not appear, which is related to O divacancies28. It is confirmed that the O divacancies are not presented in irradiated bulk MgO, which is in agreement with the result of RSMs.
Fig.7(a-d) displays the mass magnetization (M) versus magnetic field (H) curves of the neutron irradiated MgO(110) with doses of 1.0 × 1016, 1.0 × 1017, 1.0 × 1019and 1.0 × 1020cm-2. The curves are typical diamagnetic above 20 K, revealing the neutron irradiated MgO is still diamagnetism above 20 K. However, the curves show obvious ferromagnetic signal as the temperature is below 10 K, indicating the irradiated bulk MgO is ferromagnetism as the temperature is below 10 K. The low temperature ferromagnetism in all irradiated MgO(110) single crystals can be observed. Notably, the saturation field of all samples is about 2.5 T at 2 K. The saturation magnetization is different for irradiated MgO, which may be due to the defects concentration. The maximum saturation magnetization is about 0.058observed in irradiated MgO with the dose of 1.0 ×. And the defects concentration in this sample is highest. The low temperature ferromagnetism in irradiated MgO single crystals can be attributed to the neutron irradiation induced oxygen vacancies. The neutron irradiated MgO(110) single crystals exhibit rich population of oxygen defects, subsequently resulting in the low temperature ferromagnetism. The ferromagnetism in undoped MgO nanocrystallites and nanosheets could be also due to O vacancies19,20. Using the X-ray photoelectron spectroscopy (XPS), Mishra et al.21highlighted the dominant role of O vacancies in the development of RTFM in Al-doped MgO nanoparticles. Based on the BMP model, O vacancies could account for the RTFM in Fe-doped MgO22. Glinchuk et al.′s calculations showed that long-range ferromagnetic ordering in MgO films was induced by magnetic O vacancies17. The ab initio calculations also showed that O vacancies might induce local magnetic state, as long as exchange split of O vacancies was enhanced enough18.
The d0ferromagnetism is closely related to the defects concentration. For the case of long-range ferromagnetic ordering in CaO or MgO, there exists a minimum concentration of vacancies to establish the percolation29,30. The vacancies tend to be presented at/near the surfaces of samples, but our irradiated bulk MgO(110) single crystals do not have high specific surface area. Thus, we conclude that the insufficient defects concentration should account for the unchanged diamagnetism in irradiated MgO(110) single crystals above 20 K. The defects concentration may be less than the threshold value for achieving long-range ferromagnetism at room temperature.

Fig.7 M-H curves at different temperatures for irradiated MgO(110) of (a) 1.0 × 1016 cm-2, (b) 1.0 × 1017 cm-2, (c) 1.0 × 1019 cm-2, and (d) 1.0 × 1020 cm-2.

Fig.8 Temperature dependence of ZFC and FC magnetization of irradiated MgO of 1.0 × 1020 cm-2.
Fig.8 shows the field-cooled (FC) and zero-field-cooled (ZFC) magnetization curves in the temperature range of 2 to 300 K at a magnetic field of 0.05 T for the irradiated MgO with the dose of 1.0 × 1020cm-2. The ZFC and FC processes are consistent and reversible for the irradiated sample, which is similar with the case of previously report on the magnetic properties of pristine MgO31. It indicates that neutron irradiated MgO(110) single crystals are not ferromagnetic at room temperature, which is consistent with the results of M-H curves. It also can be seen that there is no blocking temperature in the temperature range, indicating that the ferromagnetic contamination can be ruled out in irradiated samples5,32,33. The result confirms that low temperature d0ferromagnetism in irradiated MgO single crystals is surely intrinsic rather than from magnetic impurities.
To account for the connection of O vacancies to the observed ferromagnetism in bulk MgO single crystals, we consider the F-center exchange mechanism. The F-center exchange mechanism, a subcategory of the BMP model, is very useful to explain O vacancies induced ferromagnetism in insulators34,35. The correlation between the origin of ferromagnetism and O vacancies in Al2O3, SnO2, TiO2and CeO2has been described using F-center exchange mechanism5,35-37.
Previous report indicated that electrons in these singly charged oxygen vacancies (F+) are strongly localized34,37. The electron associated with a particular defect will be confined in a orbital of radius

where ε is the high-frequency dielectric constant, m is the electron mass, m*is the effective mass of the donor electron and a0is the Bohr radius.
The F+centers, basically the electrons in the singly occupied oxygen vacancies lying deep in the bandgap, will be able to favor a ferromagnetic state34. As the concentration of F+centers reaches the threshold value for magnetic percolation, the F+centers can overlap each other, leading to the long-range ferromagnetic ordering.
The neutron irradiation can introduce anion defects in MgO(110) single crystals, as determined by the UV-Vis spectroscopic results. The electrons can be trapped by these vacancies, forming significant density of F+centers. To keep neutral, the trapped electrons can be bound with Mg in irradiated MgO, accordingly leading to the bound magnetic polarons.
As the temperature is decreased, the lattice thermal vibration effect will be decreased, resulting in increasing the range of interaction of the polarons. The neighbouring polarons will overlap each other easily. Thus, the magnitude of ferromagnetism is larger at low temperature. The magnitude of ferromagnetism should be directly related with the concentration of O vacancies in irradiated bulk MgO. On the contrary, the magnitude of ferromagnetism is decreased or vanished even with increasing temperature. Thus, the neutron irradiated MgO(110) single crystals is ferromagnetic at low temperature, but diamagnetic at room temperature.
4 Conclusions
In summary, the defects and magnetic properties in neutron irradiated MgO(110) single crystals have been investigated. The ω-2θ curves and rocking curves demonstrated that neutron irradiation led to a lattice distortion in irradiated MgO. The experimental RSMs showed the significant scattering diffuse in irradiated samples. Both the RSMs and UV-Vis absorption spectra revealed that the O Frenkel defects were introduced in irradiated samples. The defects concentration was higher in irradiated MgO(110) single crystals with higher dose. The magnetic measurements showed that irradiated MgO(110) single crystals exhibited the d0ferromagnetism at low temperature. But, the saturation magnetization was different for irradiated MgO with different dose. The maximum saturation magnetization was about 0.058 emu·g-1, observed in irradiated MgO with the dose of 1.0 × 1020cm-2. The defects concentration determines the saturation magnetization. The neutron irradiated MgO(110) single crystals can obtain the low temperature d0ferromagnetism by introducing the oxygen vacancies through neutron irradiation. The ferromagnetism in oxygen-deficient MgO has been analyzed in terms of F+center exchange mechanism. Our results revealed a close correlation between d0ferromagnetism and oxygen vacancies in neutron irradiated MgO(110) single crystals.
(1) Venkatesan, M.; Fitzgerald, C. B.; Coey, J. M. D. Nature 2004, 430, 630. doi: 10.1038/430630a
(2) Wang, S.; Pan, L.; Song, J.; Mi, W.; Zou, J.; Wang, L.; Zhang, X. J. Am. Chem. Soc. 2015, 137, 2975. doi: 10.1021/ja512047k
(3) Patel, S. K. S.; Dewangan, K.; Srivastav, S. K.; Gajbhiye, N. S. Curr.Appl. Phys. 2014, 14, 905. doi: 10.1016/j.cap.2014.04.007
(4) Phokha, S.; Swatsitang, E.; Maensiri, S. Electron. Mater. Lett. 2015, 11, 1012. doi: 10.1007/s13391-015-4164-4
(5) Yang, G.; Gao, D.; Zhang, J.; Zhang, J.; Shi, Z.; Xue, D. J. Phys.Chem. C 2011, 115, 16814. doi: 10.1021/jp2039338
(6) Bhaumik, S.; Sinha, A. K.; Ray, S. K.; Das, A. K. IEEE Trans. Magn. 2014, 50, 2400206. doi: 10.1109/TMAG.2013.2292575
(7) Das, A. K.; Srinivasan, A. J. Magn. Magn. Mater. 2016, 404, 190. doi: 10.1016/j.jmmm.2015.12.032
(8) Coey, J. M. D. Solid State Sci. 2005, 7, 660. doi: 10.1016/j.solidstatesciences.2004.11.012
(9) Seike, M.; Sato, K.; Katayama-Yoshida, H. Jpn. J. Appl. Phys. 2011, 50, 090204. doi: 10.1143/jjap.50.090204
(10) Hu, J.; Zhang, Z.; Zhao, M.; Qin, H.; Jiang, M. Appl. Phys. Lett. 2008, 93, 192503. doi: 10.1063/1.3021085
(11) Khamkongkaeo, A.; Mothaneeyachart, N.; Sriwattana, P.; Boonchuduang, T.; Phetrattanarangsi, T.; Thongchai, C.; Sakkomolsri, B.; Pimsawat, A.; Daengsakul, S.; Phumying, S.; Chanlek, N.; Kidkhunthod, P.; Lohwongwatana, B. J. Alloy. Compd. 2017, 705, 668. doi: 10.1016/j.jallcom.2017.02.170
(12) Li, J.; Jiang, Y.; Bai, G.; Ma, T.; Yang, D.; Du, Y.; Yan, M. Appl.Phys. A 2014, 115, 997. doi: 10.1007/s00339-013-7922-x
(13) Singh, J. P.; Chen, C. L.; Dong, C. L.; Prakash, J.; Kabiraj, D.; Kanjilal, D.; Pong, W. F.; Asokan, K.; Superlattices Microstruct. 2015, 77, 313. doi: 10.1016/j.spmi.2014.10.035
(14) Kuang, F.; Kang, S.; Kuang, X.; Chen, Q.; RSC Adv. 2014, 4, 51366. doi: 10.1039/C4RA06340F
(15) Uchino, T.; Yoko, T. Phys. Rev. B 2013, 87, 144414. doi: 10.1103/PhysRevB.87.144414
(16) Merabet, B.; Kacimi, S.; Mir, A.; Azzouz, M.; Zaoui, A. Mod. Phys.Lett. B 2015, 29, 1550147. doi: 10.1142/S021798491550147X
(17) Glinchuk, M. D.; Eliseev, E. A.; Khist, V. V.; Morozovska, A. N. Thin Solid Films 2013, 534, 685. doi: 10.1016/j.tsf.2013.02.135
(18) Zhang, Y.; Feng, M.; Shao, B.; Lu, Y.; Liu, H.; Zuo, X. J. Appl. Phys. 2014, 115, 17A926. doi: 10.1063/1.4867228
(19) Kumar, A.; Kumar, J.; Priya, S. Appl. Phys. Lett. 2012, 100, 192404. doi: 10.1063/1.4712058
(20) Maoz, B. M.; Tirosh, E.; Sadan, M. B.; Markovich, G. Phys. Rev. B 2011, 83, 161201. doi: 10.1103/PhysRevB.83.161201
(21) Mishra, D.; Mandal, B. P.; Mukherjee, R.; Naik, R.; Lawes, G.; Nadgorny, B. Appl. Phys. Lett. 2013, 103, 182204. doi: 10.1063/1.4804425
(22) Phokha, S.; Klinkaewnarong, J.; Hunpratub, S.; Boonserm, K.; Swatsitang, E.; Maensiri, S. J. Mater. Sci.: Mater. Electron. 2016, 27, 33. doi: 10.1007/s10854-015-3713-9
(23) Flocken, J. W.; Hardy, J. R. Phys. Rev. B 1970, 1, 2472. doi: 10.1103/PhysRevB.1.2472
(24) Bachiller-Pere, D.; Debelle, A.; Thomé, L.; Crocombette, J. J. Mater.Sci. 2016, 51, 1456. doi: 10.1007/s10853-015-9465-3
(25) Pillukat, A.; Karsten, K.; Ehrhart, P. Phys. Rev. B 1996, 53, 7823. doi: 10.1103/PhysRevB.53.7823
(26) Karsten, K.; Ehrhart, P. Phys. Rev. B 1995, 51, 508. doi: 10.1103/PhysRevB.51.10508
(27) Ruan, Y.; Ma, P.; Liu, J.; Li, W.; Liu, C. J. Rare Earths 2006, 24, 56.
(28) Monge, M. A.; Popov, A. I.; Ballesteros, C.; González, R.; Chen, Y.; Kotomin, E. A. Phys. Rev. B 2000, 62, 9299. doi: 10.1103/PhysRevB.62.9299
(29) Gao, F.; Hu, J.; Yang, C.; Zheng, Y.; Qin, H.; Sun, L.; Kong, X.; Jiang, M. Solid State Commun. 2009, 149, 855. doi: 10.1016/j.ssc.2009.03.010
(30) Osorio-Guillén, J.; Lany, S.; Barabash, S. V.; Zunger, A. Phys. Rev.Lett. 2006, 96, 107203. doi: 10.1103/PhysRevLett.96.107203
(31) Prucnal, A.; Shalimov, A.; Ozerov, M.; Potzger, K.; Skorupa, W. J. Cryst. Growth 2012, 339, 70. doi: 10.1016/j.jcrysgro.2011.11.067
(32) Yang, G.; Gao, D.; Shi, Z.; Zhang, Z.; Zhang, J.; Zhang, J.; Xue, D. J. Phys. Chem. C 2010, 114, 21989. doi: 10.1021/jp106818p
(33) Gao, D.; Li, J. Li, Z.; Zhang, Z.; Zhang, J.; Shi, H.; Xue, D. J. Phys.Chem. C 2010, 114, 11703. doi: 10.1021/jp911957j
(34) Coey, J. M. D.; Venkatesan, M.; Fitzgerald, C. B. Nature Mater. 2005, 4, 173. doi: 10.1038/nmat1310
(35) Singhal, R. K.; Kumari, P.; Samariya, A.; Kumar, S.; Sharma, S. C.; Xing, Y. T.; Saitovitch, E. Appl. Phys. Lett. 2010, 97, 172503. doi: 10.1063/1.3507290
(36) Singhal, R. K.; Kumar, S.; Kumari, P.; Xing, Y. T.; Saitovitch, E. Appl. Phys. Lett. 2011, 98, 092510. doi: 10.1063/1.3562328
(37) Shah, L. R. Ali, B.; Zhu, H.; Wang, W. G.; Song, Y. Q.; Zhang, H. W.; Shah, S. I.; Xiao, J. Q. J. Phys.: Condens. Matter 2009, 21, 486004. doi: 10.1088/0953-8984/21/48/486004

