利用生理生化指标评价花生种质耐低温性
Vincent Malizukiswe Vacu,张 君,关淑艳,Thobela Louis Tyasi, 王丕武,王秀贞,唐月异*,王传堂,*
(1. 吉林农业大学农学院,吉林 长春 130118; 2. 山东省花生研究所,山东 青岛 266100)
Introduction
Groundnut or peanut plays an important role in nutrition of many developing nations around the world. It is grown mostly in tropical and subtropical parts around the world. China is the world's largest peanut producer[1](Liao, 2014). The Northern parts of China experience low temperatures at sowing period which results from long winter season. For peanut growers this is challenging as it affect the time taken from sowing to germination and consequently the seedling stand. In addition to that, low-temperature stress has a negative impact on product quality because it reduces synthesis, accumulation, storage of proteins and polysaccharides, and consequently affects the nutritional and taste profiles of the product[2](Sannita di Toppi, and Pawlick-Skowronska, 2003). This affects the market product reception. Seed quality is an important attribute of long shelf life. The changes in seed biochemical composition has been suggested to alter seed germination, seed and seedling vigour, seedling survival in cases where planting is done during stressful conditions[3](Sun et al.,2014). Apart from growth and production, chill temperatures also affect the geographic distribution of several agricultural crops[4](Liu et al., 2013).The mean optimum temperature for vegetative growth and reproduction of groundnut ranges between 24℃ and 33℃[5-6](Upadhyaya et al., 2008; Kulkarni et al., 2015). Several molecular studies had been conducted on seeds and seedlings to understand groundnut genetic behaviour when exposed to chill stress[7-10](Chen et al., 2014; Zhang et al., 2016;Tang et al., 2011; Tang et al., 2012).Amongst others, the late embryogenesis abundant (LEA) genes have been found to enhance tolerance to chill stress at both germination and seedling stages. Chill stress facilitates changes in biological processes such as changes in protein, soluble metabolites, signal transduction and transcript regulations[7](Chen et al., 2014). Each of the biological processes is controlled by the up-regulation and the down-regulation of genes by plant response to low temperature stress. The present study was undertaken to evaluate the physiological and biochemical response of selected groundnuts to low temperatures (2℃ and 5℃) and 2 growth stages (germination and seedling). We further wanted to describe the physical variation in groundnut genotypes under chill stress. The 69 genotypes were randomly selected from Shandong and Jilin provinces of China and their performance at chill stress was unknown. Because the adaptation to low temperature involves several physiological and biochemical properties, we explored seed biochemical parameters such as sucrose, total soluble sugars (TSS), vitamin E, erucic acid, protein, palmitic acid, oil, linoleic acid and oleic acid. The response of 2 weeks old groundnut seedlings to chilling stress was investigated by measuring the change in peroxidase (POD), chlorophyll content, proline and MDA concentration. We measured these parameters of seedling leaves at both low temperature and room temperature (≥25℃) and compared them. We further compared all the parameters measured in this study using Pearson's correlation coefficients to determine any significant correlation that might exist. The current study provides the research knowledge about differential response of groundnut crop to chill stress and also provide knowledge of LT response of groundnut plant materials used in Jilin and Shandong provinces of China.
1 Material and Methods
1.1 Material
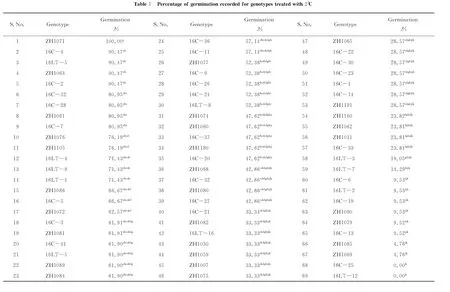
69 genotypes as listed in Table 1 were used in germination test. Only 18 of them (Table 2), representing highly sensitive (0%~49%), sensitive (50%~69%), tolerant (70%~89%), highly tolerant (90%~100%) at germination stage, were randomly selected for measuring biochemical indices at seedling stage.
1.2 Methods
1.2.1Measurementofgerminationpercentage
The rolled paper towel procedure was used for laboratory germination of groundnut genotypes as described by Upadhyaya et al (2001)[11]. 9 seeds of each genotype were wrapped with paper towel and plated in a rectangular metallic tray. Each sample replicated 3 times. Immediately a 0.05% ethrel (w/v), 0.315mL/L tebuconazole-carbendazim fungicide mixture was applied once to provide enough moisture for the seeds. This ethrel fungicide mixture was applied to overcome possible seed dormancy and to escape fungal pathogen damage to the seeds prior germination. The seeds were incubated at 2℃ for 4 days. After 4 days the seeds were incubated at 28℃ day and night for 7 days. Distilled water was used to provide constant moisture throughout. After 7 days the germination percentage was recorded. Seeds with approximately 2mm and above were regarded as germinated. A calliper was used for measurement. Germination percentage was calculated. Seeds with 71%~100% germination were considered to be tolerant to low temperature[5](Upadhyaya et al. 2008).
1.2.2Planting
Data from germination percentage was used as a basis for selection of genotypes from the 69 genotypes tested. Seeds of 18 genotypes consist of 6 tolerant[5](Upadhyaya et al., 2008), 6 in a range of 50%~70% germination and 6 in a range of 0%~49% germination were planted. The genotypes planted are indicated in table 2. A 2:1 soil and vermiculite mixture was used. Prior the total coverage of seeds with soil mixture, a full coverage of 0.05% ethrel (w/v), 0.625 mL/L tebuconazole-carbendazim fungicide mixture spray was applied. 2 weeks old seedlings were taken to a growing chamber set at 5℃ for 120 hours at 12h photoperiod. Control plants were constantly grown at room temperature. After 48 hours the leaves of both treated and control plants were sampled, frozen in liquid nitrogen and stored at -80℃ until required.
1.2.3Determinationofbiochemicalparametersfromthekernelsof18genotypes
Sucrose, total soluble sugars (TSS), vitamin E, erucic acid, protein, palmitic acid, oil, linoleic acid and oleic acid were measured using the near infrared reflectance spectroscopy (NIRS) following different models. Briefly, freshly harvested pods were carefully shelled to obtain 50 seeds for each genotype. The seeds of each genotype were placed intact into the clean sample cup with a diameter of 5 cm and were scanned using MatrixTM-E spectrometer (Bruker Optics, Germany). The wavelengths used were 1000 nm and 2500 nm. For protein, oil, oleic acid and linoleic acid we followed models as described by Zhang et al. (2009)[12]. For determination of sucrose we followed a developed model by Tang et al. (2018) and for the total soluble sugars (TSS)[13], vitamin E, erucic acid and palmitic acid we followed a model as described by Wang and Zhang (2013)[14]. All the parameters were measured three times.
1.2.4Determinationofchlorophyll
For chlorophyll determination, a method by Zhang and Huang (2013) was followed[15]. With the use of mortar and pestle, approximately 0.2 g of leaf sample was ground into powder using liquid nitrogen and homogenized with 2mL 100% NN-Dimethylformamide. The supernatant was collected by spinning at 10000r/min for 10 min. The optical density of the supernatant was measured at 664 nm and 647 nm. Each genotype replicated 3 times. The total chlorophyll in mg/L was calculated using the formula by Inskeep and Bloom (1985), [chlorophyll a+chlorophyll b]= 17.90×A647+8.08×A664[16].
1.2.5Determinationofproline
For the estimation of proline, the sulphosalicylic acid ninhydrin method by Zhang and Huang (2013) was followed[15]. Briefly, approximately 0.5g of leaf sample was ground into powder using liquid nitrogen and homogenized with 2mL sulphosalicylic acid in a test tube. The homogenized mixture was centrifuged at 5000r/min for 5min. 1mL of the supernatant was transferred to a new tube, 1mL of acetic acid and 1mL of ninhydrin reagent was added. The reaction was catalysed by boiling the mixture at 100℃ for 45min. The reaction was immediately stopped in ice for 30min and 3mL of toluene was added and mixed vigorously and centrifuge at 1000r/min for 5 min. The absorbance was measured at 520 nm using the Shimadzu UVmini-1240 spectrophotometer. The samples were replicated 3 times. The absorbance of serial standards was measured to make the curve from which the concentration (μmol/L) of the samples was calculated. Using excel spreadsheet the equationy=0.0079xwithR2value of 0.9384 was obtained from the curve. The proline concentration in μmol/g was calculated following equation by Bates et al. (1973)[17].
1.2.6Determinationofmalondialdehyde(MDA)
The MDA content was measured using the protocol by Zhang and Huang (2013) with slight modifications[15]. Briefly, about 100mg leaf sample of similar age was ground into powder with liquid nitrogen and added in eppendorf tube containing 1 mL 0.1% (w/v) trichloroacetic acid (TCA) and homogenized by inverting several times. The homogenized sample was centrifuged at 10000 r/min for 10 min and 0.8 mL of the supernatant was transferred to a new tube. 4mL of 20% TCA containing 0.5% thiobarbituric acid (TBA) and the mixture was boiled at 100℃ for 15 min. The reaction was stopped in ice and the mixture was centrifuged at 10000r/min for 5 min. The absorbance reading was taken both at 532 nm and 600 nm. Formula by Heath and Packer (1968) was used to calculate the MDA content in nmol/mL, thus MDA (nmol/mL) = [(A532-A600)/155000]×106[18]. The samples were replicated 3 times.
分离胶浓度为12%,浓缩胶浓度为5%,进样量为10 μL,电泳槽中加入电泳缓冲液(Tris 3.04 g、甘氨酸14.42 g、SDS1 g、用蒸馏水溶解、调pH 8.3后定容至1000 mL),开始电压为80 V,进入分离胶后电压加大至170 V,需1~2 h。
1.2.7Determinationofperoxidaseenzymeactivity(POD)
About 0.5 g of peanut leaves was ground thoroughly with 100 mmol/L phosphate buffer (pH6) using an ice cold mortar and pestle. The homogenate was centrifuged at 4500 r/min for 13 min at 4℃. The supernatant was transferred to a new test tube and the sediment was re-homogenized with equal volume of 100 mmol/L phosphate buffer and centrifuged in the same conditions as mentioned. 1 mL of the supernatant was mixed with 3 mL of reaction buffer contained 50mL phosphate buffer, 28 μL guaiacol and 19 μL H2O2. POD was measured per minute at 470 nm based on the change in absorbance of the resulting brown mixture.
The test tubes were always placed on ice. The activity of POD in each sample was calculated using the following equation POD (U·g-1·min-1)=ΔA470×VT/W×VS×0.01×t, where ΔA470is the POD activity at 470nm,VTis the total volume of the solution,VSis the volume of the sample taken for POD measurement,Wis the weight of plant sample used andtis the time taken to record the change in POD.
1.2.8Identificationofphysicalresponsetolowtemperaturestress
Using physical observation we were able to differentiate between highly tolerant, sensitive and highly sensitive genotypes. The genotypes that recovered within 2 days after exposure to chill stress, with no lost leaves were regarded as highly tolerant, the genotypes that lost basal leaves after 2 to 5 days as sensitive and genotypes that lost both basal and cauline leaves as highly sensitive.
1.2.9Statisticalanalysis
Analysis of variance(ANOVA) was used to determine the variation amongst the samples. In cases of significant variation among individual means student's t-test was used. Statistical Analysis Software(SAS)version 9.0 was used for analysis. Statistical Package for the Social Sciences (SPSS) version 16 was used for correlation analysis. The difference (Δ) between the values obtained from the seedlings under treatment conditions and control conditions was used for the correlation.
2 Results and discussion
2.1 Germination response to cold treatment
2.2 Phenotypic response to cold treatment
From the 3 groupings mentioned earlier, genotypes in each group were randomly sampled and planted. After 2 weeks, healthy seedlings of the same age from each genotype were incubated at 5℃ for 120 hours. During the incubation period, the seedlings showed wilting and chlorophyll leaching at the edges of the basal leaves. After 2 days at room temperature the following genotypes, 16C-4, 16C-26, 16LT-5, 16LT-8, ZH1071, ZH1084, ZH1061, ZH1081 and ZH1086 recovered well and regarded as highly tolerant. On the other hand, the other genotypes were still showing signs of damage until the 5thday when genotypes ZH1063, ZH1085, 16LT-12, 16C-37, ZH1072, ZH1069, ZH1105 their leaves were yellowish in colour and lost some basal leaves, and these were regarded as sensitive. Genotypes ZH1059 and ZH1160 were highly sensitive to low temperature (LT). There was obvious difference amongst the genotypes. Studying groundnut response at low temperature, Upadhyaya et al., (2008)[5]and Upadhyaya et al., (2001)[11]used higher temperature than 5℃. However this is the first time groundnut seedlings incubated at 5℃ for 120 hours.
2.3 Estimation of malondialdehyde (MDA) changes in seedlings under cold stress
Membrane integrity is compromised by exposure to cold stress.The most susceptible sites for low temperature stress in plants are thylakoids and plasma membrane[19](Campos et al., 2003). When plant is exposed to low temperatures, lipid composition of the membrane may change from fluid, liquid-crystalline state to solid or gelled state[20](Pike et al., 1979). These changes from liquid to solid phase may lead to cell rupture. To quantify the cell membrane injuries and deterioration in cellular metabolism, we have measured the MDA (nmol/mL) in leaves of the young seedlings exposed to chilling stress and normal temperature as a control. There was a significant difference (P<0.05) between control and cold treated seedlings of each genotype (see table 2). We have recorded slightly high MDA values for low temperature treated seedlings compared to control. MDA has been used as a lipid peroxidation marker in mammals[21-22](Torun et al., 2009; Ray et al., 2014). This is true with plants. The lipid peroxidation of plants under cold stress has been studied measuring the MDA levels accumulated. Amongst all the genotypes investigated, ZH1160, ZH1105, ZH1084, ZH1085, 16LT-12, and 16C-37, had the highest increase in MDA content (see table 2). These results are consistent with our phenotypic observation of the genotypes except for ZH1084 (belongs to highly tolerant group). Previous studies have also shown an increase in MDA content of stress sensitive genotypes[4,19](Campos et al., 2002; Liu et al., 2013). The high MDA content is detrimental to plant survival. In our study the genotypes with lowest MDA content increase at LT had a shortest recovery period. These results indicate that the cell membrane was little damaged on genotypes with less MDA accumulation in response to LT and severely damaged for the genotypes that couldn't recover in 2 days after exposure to LT. Furthermore, the ability of ZH1084 to tolerate LT even though it experienced high lipid peroxidation (detected by an increase in MDA content) confirms a complex mechanism of response to LT by different genotypes of the same species.
2.4 Chlorophyll content estimation of seedlings under cold stress
Stress factors that affect chlorophyll consequently affect the photosynthesis process. Cold stress causes severe structural changes which negatively affect the chlorophyll; these include swelling of chloroplast, deformation and swelling of thylakoids, and deteriorating of starch granules[23](Kalisz et al., 2016). This affects the ability of plant to absorb light and transform it into chemical energy (Liu et al., 2013). In our experiment we have measured the chlorophyll content from the seedling leaves at 5℃ for 120 hours and from the control plants. As reported in previous studies[4,23](Liu et al., 2013; Kalisz et al., 2016), the chlorophyll content had slightly decreased in cold treated seedlings compared to control. Among the 18 genotypes examined, there was statistical significant difference (P<0.05) in-betweenLT incubated seedlings and between LT incubated seedlings and control (table 2). As shown in table 2, the range of total chlorophyll (TC) measured in leaves of peanut seedlings at room temperature between all genotypes tested was 63.2644~72.1278 mg/L. Under LT the chlorophyll ranged between 59.2234~64.9593 mg/L (see table 2). 61.1% of the total number of genotypes tested had a very slight decrease in TC when exposed to LT. Of this percentage, the highly tolerant genotypes, ZH1084, 16C-4, 16C-26, 16LT-5, 16LT-8, ZH1061, ZH1081 and ZH1086 were included except ZH1071. Chlorophyll content has been reported to continuously decline over time of expose to cold stress[4,24](Liu et al., 2013; Gupta et al., 2015). We recorded a high TC decrease on genotypes ZH1071, ZH1105 and ZH1160. These results suggest that about 61.1% of groundnut genotypes investigated have some degree of LT tolerance. The high tolerance of ZH1071 might be accounted for by some other biochemical or physiological parameters.
2.5 Proline estimation to determine tolerant or sensitive genotypes
Proline occurs in a wide range of higher plants and reported to increase[4,25-26](Ashraf and Foolad, 2006; Hare and Cress, 1997; Liu et al., 2013) in response to plant stress giving it stress marker properties. It acts as an osmoprotectant by protecting protein structures against denaturation[27](Claussen, 2004). The increase in proline content is said to be associated with many benefits for the survival of plants under stress[28-29](Hayat, 2012; Jain et al., 2001). In response to cold stress, the soluble proline concentration increases in plants[4](Liu et al., 2013). We have investigated the proline concentration of seedling exposed to chilling conditions. The results were compared to those of control plants. There was a noted increase in proline concentration on seedlings under cold stress compared to control as seen in table 2. We noted a significant difference (P<0.05) in proline concentration at both LT and RT between all the genotypes investigated. ZH1072 was significantly different from the rest of genotypes with the maximum proline of ±5.1008 μmole/g at 5℃(see table 2) which accounts for increase of 4.2147 μmol/g proline compared to RT. However this genotype is amongst the genotypes that couldn't recover within 2 days after LT indicating that, the high accumulation of proline did not account for the immediate chilling tolerance in this genotype. Similarly, the proline accumulation in ZH1105 and ZH1160 did not account for their recovery from LT treatment. These results are consistent with previous findings that the proline accumulation is not always positively correlated to plant stress in all higher plants[30-31](Liu and Zhu, 1997; Moftah and Michel, 1986). Genotype 16C-26 had approximately 1.2189 μmol/g proline content at LT and accounts for the smallest proline accumulation of about 1.0072μmol/g (see table 2). The proline osmoprotectant feature helps plant prevent cell burst thereby reducing cell membrane damage[28](Hayat, 2012). This feature might have helped to protect membrane integrity of several genotypes which recovered within 2 days in our study (the highly tolerant group). Similarly a study by Jain et al. (2001)[29], noted an increase in proline accumulation in groundnut genotype JL24 under salt stress, which they assumed it to be associated with protection of membrane integrity. The highly tolerant genotypes, ZH1071 and ZH1084 might have benefitted from the increase in proline content to counter the effect of decrease and increase in chlorophyll and MDA content. Amongst other biochemical parameters measured we may say that, the increase in proline is important for LT tolerance for some genotypes within the same plant species.
2.6 Estimation of changes in antioxidant enzyme under chill stress
Low temperature stress results to the accumulation of free reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide and hydroradicals which causes an oxidative stress[4](Liu et al., 2013). This results to chemical imbalance which leads to lipid oxidation within the plant tissue.The increased ROS directly affects the cellular metabolism, thus leading to cell death[32](Chunthaburee et al., 2015). Plants have evolved defence mechanisms to withstand oxidative stress by increasing the ROS scavenging enzymes such as superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT)[33](Miao et al., 2010). In our study we have measured the change of one antioxidant, thus POD. Like the other enzymes, POD is an important enzyme to protect the cell integrity. Several studies have suggested that changes in POD are relevant to plant stress caused by different stress factors[4,32,34](Furlan et al., 2012; Liu et al., 2013; Chunthaburee et al., 2015). We have noted a general increase in POD estimated in leaves of peanut seedlings under chill stress as compared to RT (see table 2). There was a significant difference (P<0.05) between the genotypes investigated. However between 55.6% of all genotypes in our study there was no significant difference on POD measured at LT. ROS are not only toxic but also play an essential role as stress signalling molecules that regulate several genes including the ones encoding antioxidant enzymes[34-35](Theocharis et al., 2012; Furlan et al., 2012). Amidst the genotypes in our study, ZH1059 and 16LT-8 had the lowest POD activity of 1.125 and 1.095 U.g-1.min-1, accompanied with an increase in MDA and proline concentration together with a moderate decrease in TC. This trend might be due to a delayed response to ROS signalling by these genotypes which resulted in late self defence to LT stress. Interestingly, 16LT-8 recovered within 2 days after LT stress where as ZH1160 with POD increase (5.398U.g-1.min-1) which is higher than that of 16LT-8 was severely damaged. As seen in table 2, in our study we have recorded a POD increment at a range of 1.095~10.942U.g-1.min-1. In this study the change in POD activity was different from one genotype to another. This might indicate the differential response of the investigated genotypes to LT. With the exception of genotype ZH1160 we can say the activity of this enzyme has enhanced tolerance against LT as seen in the recovery of the investigated genotypes.
2.7 Pearson correlation, estimation of sucrose, TSS, vitamin E, erucic acid, protein, palmitic acid, oil, linoleic acid and oleic acid
The Pearson correlation coefficients of all parameters measured in our study is seen on table 4. The means of each parameter measured were analyzed by Pearson correlation utilizing SPSS software. The biochemical indices were measured in kernels of 18 peanuts genotypes in order to determine the type of correlation that might exist among them and those measured at seedling stage (cold stress tolerance indices) and to predict their combined role in groundnut tolerance to LT. Several parameters showed significant correlations at bothP<0.05 andP<0.01. In table 4, proline increment had a significant negative correlation with erucic acid(r=-0.573,P<0.05). Several parameters showed a positive significant correlation with sucrose and these were TSS (r=0.593,P<0.01), erucic acid (r=0.693,P<0.01) and palmitic(r=0.474,P<0.05), whereas the oil content had a negative significant correlation (r=-0.779,P<0.01). Vitamin E displayed a negative significant correlation with protein (r=-0.716,P<0.01) and a positive significant correlation with linoleic (r=0.506,P<0.05). As seen in table 4, no significant positive correlation between the parameters investigated on kernels and in the seedling leaves. Our observation is that all the seedling groups (highly tolerant, sensitive and highly sensitive) fall relatively on the same range in all the seed quality parameters measured. At germination, genotype 16LT-12 recorded zero percent germination at 2℃ and it is sensitive to 5℃ at seedling stage, however it falls within the range of other genotypes tested in all the seed quality parameters estimated (see table 4). The genotype ZH1071 is tolerant at both stages of plant growth and it follows the same trend as 16LT-12 with seed quality parameters. Our findings imply that the response to LT by seedlings of genotypes might not highly depend on seed quality.


3 Conclusions
Groundnut is an important food and cash crop for developing nations and is threatened by cold weathers. Chill stress lead to changes in cellular membrane leading to decrease of chlorophyll content accompanied by increase in proline, POD, MDA contents. Among 18 genotypes evaluated for biochemical response, 16C-4, 16C-26, 16LT-5, 16LT-8, ZH1071, ZH1084, ZH1061, ZH1081, ZH1086 might have high levels of tolerance to chilling stress based on their physical, physiological and biochemical response to LT. These genotypes can be useful for further plant breeding programs and for planting in areas where LT is experienced. From the germination percentage grouping results we can say the genotypes that fall at a range of 0%~49% are highly sensitive genotypes at both growth stages as seen by the physical performance of ZH1059 and ZH1160 at LT. However, the use of germination percentage as the only measure to determine tolerant and sensitive groundnut genotypes might have a tendency to underestimate and overestimate some genotypes towards their response to chilling stress. In this study we further showed that not all groundnut genotypes benefit from the increase in both proline and POD. The seed quality parameters effect on seedling response to LT needs to be further studied. In our experiment these parameters had no significant positive correlation with parameters at seedling stage (abiotic stress indicators).
Acknowledgement:
ThisresearchwassupportedbyaGrantfromChinaagriculturalresearchsystem-peanut(CARS-13).TheauthorsaregratefultoMrCorneliusSelloTlotlisoandMrAdamsSeiduofJilinAgriculturalUniversityforthetechnicalsupportprovidedduringdatacollection.

