Combustion, Thermal Decomposition and Application of Metal Hydride in Energetic Materials: an Extensive Literature Survey
ZHANG Yang, ZHAO Feng-qi, XU Si-yu, GUO Jing, LI Meng, YANG Yan-jing
(Science and Technology on Combustion and Explosion Laboratory, Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The research status and prospect of using metal hydride as additives in energetic materials (EMs) are summarized in this study through three aspects: thermodynamics, energy and combustion performance of metal hydride in EMs. The results show that the combustion heat, energy level and some other performance indices of system are improved effectively, while opportune percentages of metal hydride are added in EMs. Despite huge potentials exist for the addition of metal hydride in EMs, some practical difficulties hinder the general application of them. Among them, the appropriate thermal stability of metal hydride should be developed and payed attention. Doping and nanocrystallization are two useful ways to improve the thermodynamics performance of metal hydride.
Keywords:applied chemistry; metal hydride; energetic materials; energy and combustion performance; thermodynamics performance; propellant; explosive
Introduction
Hydrogen is a green energy carrier with large gravimetric energy density, abundant reserves and cleanness. The calorific value of hydrogen is as high as -121061kJ/kg, higher than that of methane, gasoline, ethanol and methanol. In recent years, with the development of hydrogen storage technology, numerous hydrogen-storage materials have been developed. At the same time, the application of hydrogen-storage materials in energetic materials, has attracted extensive attention in the academic world. Based on the current research at home and abroad, the energetic materials in which hydrogen storage materials could be applied, mainly involve solid propellants and explosives. Considering the specific impulse generated by propellant consist of liquid oxygen and liquid hydrogen reaches 3822N·s/kg(390s)[1], visible hydrogen is very suitable for use in energetic materials. The specific impulse (Isp), which is the most important parameter for propellant, with introducing H2into solid propellant combustion process, the average molecular weight of gas can be reduced significantly. In addition, the combustion of metal and H2emit a large amount of energy, increasing the temperature of combustor, then theIspwas improved. The propellants mainly consist of adhesive, oxidant (high energy explosive), plasticizer, high energy combustion agent, combustion catalyst. The metal hydride could be added into propellants as high energy combustion agent. As a result, the energy level of propellant can be effectively improved by storing H2in propellant components, releasing it for participating in combustion of propellant[2]. The addition of hydrogen storage materials in explosives can increase the total energy of explosion[3], and can also significantly improve the explosive properties of emulsion explosives[4-5].
There are mainly three types of hydrogen storage technologies: high pressure gaseous hydrogen storage, cryogenic liquid hydrogen storage and solid hydrogen storage[6]. Among them, only solid hydrogen storage can meet the requirements of the application in energetic materials. Solid state hydrogen storage refers to storing hydrogen by physical or chemical interactions between hydrogen and solid materials. According to the interacting mechanisms, the solid materials can be divided into two categories: physical adsorption hydrogen storage materials and chemical adsorption hydrogen storage materials. For the former, such as carbon nanotubes, activated carbon, metal organic frameworks (MOFs), self-microporous polymer (PIMS) and zeolite compounds, the hydrogen storage is possible only at low temperature or at room temperature under high pressure[7]. Therefore, the working condition of such materials limits their application in energetic materials. On the other hand, the latter type of materials function when chemical reaction or interaction between hydrogen and materials occur[8]. Wide categories of chemical adsorption hydrogen-storage materials exist, including metal hydrides, metal alloys hydrides, coordination hydrides, and so on. The physical/chemical properties of the hydrogen storage materials are mainly dependent on substances/absorption of thermodynamic/kinetic characteristics. This kind of hydrogen storage materials own the advantages of large hydrogen storage capacity and variable properties. Hence, they have good prospects in the field of energetic materials. Although the application of hydrogen storage materials in energetic materials has various advantages, but there are still some problems to resolve before successfully applying hydrogen storage materials in energetic formulations.
At present, AlH3has been successfully used in propellants. Compared with conventional propellants, the energy performance andIspof AlH3propellants are greatly improved. For example, the measured specific impulse for high energy propellant AlH3/ADN developed by Russia exceeds 2940N·s/kg, which is currently the highest level for solid propellant in the world[9]to the knowledge of the authors. In order to successfully apply metal hydride in energetic formulations, numerous properties of metal hydrides have to be obtained by theoretical or experimental means. The hydrogen desorption properties of hydrogen storage materials are characterized by two aspects: thermodynamic and desorption kinetics. In order to make a full use of the stored hydrogen, the thermal stability of hydrogen storage materials should be matched with the combustion temperature, propellant composition and processing technology, which request appropriate thermodynamic properties of the materials. In addition, the combustion of solid propellant is a rapid integrating oxidation and reduction processes, which require the metal hydrogen storage materials emit H2at a fast rate, namely excellent desorption kinetics. Therefore, this paper mainly focuses on the thermodynamics, kinetics of metal hydrogen storage materials, and the combustion/energy performance when applying them in solid propellants.
1 Energy Performance of Metal Hydride in Propellants
The propellant is contained in the combustion chamber of rocket engine. During launching process, the propellant is ignited by the ignition device for regular combustion, and a large amount of heat and gas are released at the same time. This high temperature, high pressure gas is ejected at a high speed through the nozzle at the tail of the engine, which generates a strong reaction force and enables the rocket to fly upwards. It can be seen that propellant is the energy source for launching rockets, and its working process is a process of energy conversion. Energy performance and combustion performance are crucial for propellant. Whether certain metal hydrogen-storage materials can be used in energetic materials, greatly depends on the combustion performance of the metal hydrogen-storage materials and the combustion characteristics in energetic materials. The physical-chemical properties of the main reported metal hydrides were showed in Table 1.

Table 1 The physical-chemical properties of the main reported metal hydrides [10-16]
The acceleration of thermal decomposition of AP by MgH2is obviously stronger than that by Mg according to Liu et al.[17]. The low and high decomposition heats are decreased while the apparent decomposition heats are increased by MgH2. In the AP/Al/HTPB composite propellant with MgH2, the temperature of thermal decomposition is decreased by catalyzing the decomposition of AP and the heat of reaction has increased, which showed enhancement effect in the burning rate by 13.9% for 1.3% addition of MgH2[18].
The application of MgH2in emulsion explosive was studied by researchers[4-5], the results show that addition of MgH2and TiH2can significantly improve the emulsifying and detonating properties of emulsion explosives, further evidencing the great potential of metal hydride containing compounds. The effects of MgH2on the explosive properties of some explosives such as TNT, Tetryl and C-4 were studied by Hradel et al.[19]. The results show that MgH2can improve the organic explosive explosives. In addition, the effects of various metal hydrides on the energy performance of the propellants were investigated by theoretical calculations. Li et al.[20]used the specific impulse of propellants containing Al as reference to evaluate the impacts of adding metal hydrides in composite propellants, and chemical equilibrium calculation was conducted through the principle of minimum free energy. The single metal hydrides under discussion include AlH3, MgH2, TiH2, CaH2, ZrH2, SrH2, BaH2and CsH. The results prove that the contribution of AlH3and MgH2to standard theoreticalIspare greater than Al, while the contribution of TiH2, CaH2, ZrH2, SrH2, BaH2and CsH are less than Al. The effects of AlH3, MgH2, TiH2, ZrH2, SrH2, BaH2and CsH on the energy characteristics of 3,3- two azideoxetane (BAMO) and 3-3- methoxy methyl azideoxetane (AMMO) block copolymer p (BAMO-AMMO) based propellants, were studied by Pei et al.[21]. Among the above hydrides, the contribution of AlH3to the increase in propellant energy is the most obvious, followed by MgH2, while the rest will decrease the energy of propellants.
Feng et al.[3]used Mg-Al-B(hydrogen storage capacity is 1.1%) and Mg-B based hydrides (hydrogen storage capacity is 4.3%) to replace pure Al as additive into RDX based explosives, and 30% mixing of the abovementioned hydrides was adopted. The specific energy and specific bubble energy of the explosive were measured by underwater blasting experiment, and the sum of them is taken as the total energy of underwater explosion. The results show that compared with Al-containing explosive, the specific bubble energies of the two hydride-containing explosives are increased by 9.3% and 5.1%, respectively, and the total energies are increased by 7% and 3% respectively. Many aspects may account for such enhancement effects of total energy, e.g. combustion, heat releasing, water vapor and the oxidation of aluminum and boron metal.
The application of metal aluminum hydride compounds in propellants has not been reported yet, but their possible effects on the energy performance of propellants have been investigated by chemical equilibrium calculations. The effects of LiAlH4and Mg(AlH4)2on the energy performance of HTPB-based composite propellants with three or four components, were researched by Li et al[9]. The results show that the contribution of these compounds to the standard theoretical specific impulse is greater than Al, and an optimal value of the energy characteristic parameter exists. The positive effect of LiAlH4is more significant than Mg(AlH4)2. The energy performance effects of the following two aluminum hydride top(BAMO-AMMO) based propellants. The obtained laws are similar to HTPB-based composite propellants, and the energy level of propellant can be improved through the substitution of Al by the two compounds. However, similar to metal boron hydrogen compounds, metal aluminum hydrogen compounds also have strong reducibility. Therefore, it is also necessary to regulate the interaction between metal aluminum hydride and other propellant components.
2 Combustion Performance of Metal Hy-dride in EMs
The physical/chemical properties and ballistic performance of propellant containing AlH3were studied by Deluca et al.[22], who found that using AlH3instead of Al will cause the combustion temperature to decrease and the combustion products of CO2, [OH] and H2O to reduce, thereby weakening the throat erosion.

Fig.1 Combustion flames of the DB propellants with Al and ZrH2[23]
The combustion of ZrH2in double-base propellant was studied experimentally by Yang et al.[23]. As shown in Fig.1 (a) and (b), the typical “dark zones” are present for the combustion of double-base propellant with Al at both 2 and 4MPa, and the thickness of the dark zone decreases with the increase in pressure due to the enhanced combustion reactions. The occurrance of the “dark zones” could be attributed to the relatively low temperature in certain region of combustion. As for the propellant with ZrH2, a completely different combustion behavior from that of Al-containing propellant is observed, see Fig.1 (c) and (d). The combustion of ZrH2occurs on the burning surfaces of propellants and no “dark zone” is observed. The improved combustion characteristics for ZrH2-containing propellant, can be explained as follows. Decomposition of ZrH2forms Zr and H2which both participate in combustion. Zr melts and then combusts on the burning surface, favoring the heat feedback to the propellant.
The effects of MgH2on the combustion characteristics of nitrocellulose (NC) were studied by Jing et al.[24]through oxygen bomb calorimetry and DSC. They found that the addition of MgH2can increase the combustion heat of NC system, and the effect is proportional to the amount of MgH2added. With 5% mass fraction of MgH2, the heat of combustion system is increased by 6.5%. However, with the increase of the amount of MgH2, the combustion efficiency of the system first increases and then decreases, which peaks at a mass fraction of 2%.
Effects of hydrogen storage alloys hydride on combustion of AP/HTPB propellant were researched by Dou et al.[25-26]. The results show that hydrogen storage alloys hydride can increase the propellant combustion efficiency in the sidewall of the unit. At the same time, the decomposition of hydrogen combustion, ignition characteristics, combustion velocity and combustion efficiency are all significantly improved for the AP/HTPB propellant.
The Al cladding Mg-Ni-B based hydrogen storage alloy was prepared through ball-milling by Dou et al.[27-28], whose hydrogen storage capacity is 1.0% and density is 2.371g/cm3. The test results of oxygen bomb analyzer show that practical combustion heat is -31525kJ/kg and the combustion efficiency is 94.32%, which are higher than Al. Further studies show the addition of the alloy has two-fold functions of adsorption and promotion for the heat decomposition of AP. The ignition delay time of propellant is shortened, explosion heat, combustion velocity, combustion area temperature are all increased by a large margin when the Mg-Ni-B based hydrogen storage alloy is added into the composite propellant using HTPB, GAP and PET as adhesive.
The effects of NaBH4on the Ba(NO3)2-RP-Mg combustion pyrotechnic composition system were studied by Gui et al.[29]. They found that the calorific value is increased by 14.3% after adding 20% of NaBH4. In addition, NaBH4can reduce the sensitivity and improve the comprehensive performance of combustion agents. An extensive review about the use of boron hydrides in high burning rate propellant was presented by Wang et al.[30]. They pointed out that ionic borohydride salt and carborane, which are simple and inexpensive to synthesize, can regulate the burning rate of solid propellant in large range.
From the above research, the metal hydrides are helpful to improve explosion performances of different kinds of energetic materials, there is great application potential for metal hydrides in propellants, explosives and pyrotechnic compound.
3 Thermodynamics Performance of Met-al Hydride in EMs
Some key thermodynamics parameters like hydrogen desorption characteristic temperature (Tt), enthalpy change (ΔH) and entropy change (ΔS), can be used to judge whether the metal hydrogen-storage materials are suitable or not for the practical application conditions, say, as additive in solid rocket propellants.
3.1 Hydrogen desorption characteristic temperature
Besides the content of hydrogen, the thermal stability of hydrides is also important for application in energetic compounds. If the thermal stability of EMs are too low, decomposition may occur in the production process, causing security risks. The thermal stability of metal hydrogen-storage materials can be represented by hydrogen desorption characteristic temperature (Tt). Light metal hydrides include lithium hydride (LiH), sodium hydride (NaH), magnesium hydride (MgH2), calcium hydride (CaH2) and aluminum hydride (AlH3), etc.[20]. Their hydrogen storage capacities are more than 4.2%, and the hydrogen storage capacity of LiH reaches 12.7%.
Excellent performance was shown when AlH3was used in double base propellant by Flynn[31], the theoretical and measured specific impulses were increased up to 2874.34N·s/kg and 2675.4N·s/kg, respectively. Nevertheless, the thermal stability of AlH3is low largely due to the composition of two reductive elements. Even forα-AlH3, the most stable structure among all variants, slow hydrogen desorption and decomposition will occur. From this aspect, it cannot fully satisfy the requirements for propellant development[32]. MgH2is also widely investigated as a promising additive by researchers. The hydrogen storage capacity of MgH2is 7.6%, and its thermal stability is higher than AlH3.Generally MgH2decomposes at more than 300℃to release H2[33].
The hydrogen storage alloys are mainly composed of transition metal elements, and hydrogen reacts with them to form interstitial hydrides. Among them, the rare earth hydrogen storage alloys represented by LaNi5have good thermodynamic and kinetic properties, but their hydrogen storage capacity is generally lower than 2%. On the other hand, the hydrogen storage capacity of the magnesium alloys represented by Mg2Ni is relatively high(up to 3.6%), and the hydrogen releasing temperature is about 250℃, indicating good thermal stability. At the same time, the combustion heat value of the Mg, a common metal fuel for solid propellant, is as high as -24773kJ/kg. The composition of hydrides of Mg-based hydrogen storage alloys can be expressed as Mg2HxLy, where L represents one or more active metals other than Mg (e.g. Al, Ni, Cu, Mn, La, Co, Li, Zn, Fe). The stability, density, oxygen consumption and combustion heat of the alloy hydrides containing Mg, Al, Ni, B, H and other elements were investigated by Dou et al.[34]. It is found that the alloy hydrides have good storage stability in dry air, density and oxygen consumption comparable to Al, and combustion heat value higher than Al. They can be used as high energy combustion agent for propellants.
Metal borohydride compounds (M(BH4)n) and metal aluminum hydride compounds (M(AlH4)n), two typical coordination hydrides compounds with high hydrogen content and chemical activity[35], are hotspots in the field of hydrogen storage[36-40]. Metal boron hydride compounds include alkali metal, boron hydrogen compounds, alkaline earth metal boron hydrogen compounds, transition metals, boron hydrogen compounds, lanthanide metal boron hydrogen compounds, actinide metals, boron hydrogen compounds, etc[41]. Among them, lithium borohydride (LiBH4, the hydrogen storage capacity of 18.1%), boron magnesium hydride (Mg(BH4)2, the hydrogen storage capacity 14.9%) are called metal boron hydrogen compounds. In these light metal borohydride compounds, Al(BH4)3is liquid at room temperature and has a low thermal stability. It easily decomposes and releases B2H6, so it is not suitable for use in propellants. Except for Al(BH4)3, the other light metal borohydride compounds are solid at room temperature and have good thermal stability. The major hydrogen desorption temperatures of LiBH4and NaBH4are above 400 and 450℃[42-43]. The hydrogen release of Mg(BH4)2and Ca(BH4)2begin at 250 and 320℃[44-45]. According to the large hydrogen content and high thermal stability of the light metal boron hydride compounds, they have great potential for use in propellants. On the other hand, aluminum hydrides compounds can release hydrogen at lower temperature, for example, LiAlH4decomposes into Li3AlH6and LiH and releases H2at about 110℃in an exothermic process. The product can continue to decompose and release hydrogen at higher temperature of about 400℃, completing the hydrogen desorption process[46]. The thermal stability of KAlH4is obviously higher than LiAlH4and NaAlH4, for which the temperature of decomposition and desorption of hydrogen is more than 300℃[47]. The initial desorption temperature of alkaline earth metal hydride Mg(AlH4)2is about 115℃, and the decomposition reaction is a weak exothermic reaction[48]. The initial hydrogen desorption temperature of Ca(AlH4)2is about 170℃, and the thermal stability is higher than Mg(AlH4)2[49]. In addition, the hydrogen desorption behavior of the above two alkaline earth metal hydrogen compounds is obviously different from that of alkali metal aluminum hydride. The enthalpy of dehydrogenation reaction for Mg(BH4)2is about 37kJ/mol H2[50-51], much lower than Mg-based alloys hydride and MgH2.
In order to tune the hydrogen desorption characteristics of metal borohydride compounds, many methods have been attempted by researchers. The influence of Fe, Co, Ni, Cu and Ti dopants on both stability and hydrogen dissociation of LiBH4were investigated by Huang et al[52]. The calculation results indicate that all dopants considered tend to occupy interstitial sites instead of the Li atom site, and that the hydrogen removal energies for the H atoms of TM (transition metal)-doped LiBH4are smaller than those of pure LiBH4(H1-H4). The occupation energy for TM-doped LiBH4was calculated to be much higher, suggesting a larger energy input during preparation of such materials, say, by the high-energy ball milling. The analyses of dioctylsebacate(DOS), Bader atomic charge and bond length between the boron and hydrogen atoms reveal that the modification of LiBH4with transition metals may decrease its stability by weakening the B—H bonding interactions, which is beneficial for the dehydrogenation of LiBH4. Considering the occupation energy and hydrogen removal energy, Ti-doping performs the best among the TM addition systems for improving the dehydrogenation properties of LiBH4.
3.2 Enthalpy change and entropy change
The entropy of formation indicates the trend of hydride reaction. The higher the value is, the lower the equilibrium decomposition temperature will be, and the more stable the hydride will be. When the temperature is constant, the equilibrium pressure of hydrogenation dehydrogenation reaction depends on the enthalpy change. The smaller the enthalpy change, the lower the equilibrium pressure of the corresponding hydrogen will be, which means that the hydride is more likely to give off hydrogen. Therefore, it is possible to evaluate and predict the dehydrogenation performance of hydrides by the enthalpy change[53-57].
For metal hydrides the thermodynamics of dehydrogenation relate to their structures. The structural stability and dehydrogenation thermodynamics of MgH2surface were studied by Zhang et al.[58], the average desorption enthalpy and single H atom dissociation enthalpy with regard to MgH2(0 0 1) and MgH2(1 1 0) surfaces were calculated, respectively. The computational formula of average desorption enthalpy and single H atom dissociation enthalpy are showed as Eq. (2) and (3)[59]:
(1)
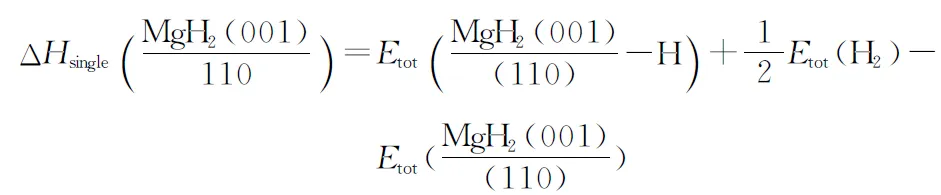
(2)
The calculation results were showed in Table 2.

Table 2 Average desorption enthalpy (ΔHaverage) and single H atom dissociation enthalpy (ΔHsingle) of MgH2(0 0 1), MgH2(1 1 0) and bulk MgH2[58]
The average desorption enthalpy of MgH2(0 0 1) and MgH2(1 1 0) were determinedas 32.4325kJ/mol and 51.0249kJ/mol,which are reduced obviously comparing to the average desorption enthalpy of bulk MgH2(62.2971kJ/mol)[59]. It showed that MgH2surface own better thermodynamic properties of dehydrogenation than bulk. The geometries, energetic and electronic structures of MgH2(0 0 1) and (1 1 0) surfaces were calculated to investigate the surface stabilities, dehydrogenation thermodynamics and their intrinsic relations with the microatomic and microelectronic structures. The results show that no apparent reconstruction occurs either for MgH2(0 0 1) surface or for MgH2(1 1 0), suggesting that both are stable cleavage planes of MgH2crystal. Comparatively, MgH2(1 1 0) surface exhibits a higher structural stability. The calculations of average desorption enthalpy and single H atom dissociation enthalpy show that MgH2(0 0 1) surface presents better dehydrogenation thermodynamics. Besides, the adsorption characteristics of water molecules on (0 0 1) and (1 1 0) surfaces of magnesium hydride were investigated by Dai et al.[60]through first principle calculations. The adsorption mechanisms of water molecule on the two surfaces were clarified from electronic structures (Fig.2 Projections of charge difference distributions on different surface), and the (1 1 0) surface shows a higher reactivity with H2O molecule owing to the larger adsorption energy than the (0 0 1) surface.

Fig.2 Projections of charge difference distributions on (001) plane for surface adsorption[60]
At present, the research on the application of light metal hydrides to propellants are mainly focused on AlH3and MgH2, and a series of achievements have been made. The phase transition of pure and Li-doped AlH3was investigated by DFT[61]. It is necessary to point out that AlH3is a kinetically stable hydride, the decomposition and desorption of hydrogen is a weak exothermic reaction[62], which means that slow decomposition will occur during storage at room temperature. This change will be detrimental to the long storage stability of the propellant and needs more attention.
Doping and nanocrystallization are two useful ways to improve the thermodynamics performance of metal hydrogen-storage materials. First-principle calculations were carried out to provide insights into the improved dehydrogenation performance and hydrogen storage properties of elements doping by Wu et al.[63-64]. It was found that both kinetic and thermodynamic properties are improved by interstitially doping metalloid B into Mg-based alloys. The relative formation enthalpies of Mg-B-based alloys and their hydrides could reach 0.508 and 0.303 eV/mol, respectively, on account of the mutual interactions between B and Mg atoms. The dehydrogenation energy and desorption temperature of Mg-B-based hydrides could be reduced respectively by 32.2% and 166℃, due to the weakening of the bonding effects between Ni and H atoms caused by the hybridization of Bs, Bp and Hs orbitals. The study results show that interstitially doping metalloid B not only helps to lower the dehydrogenation energy and desorption temperature, but also benefits the reduction of the thermal stability for Mg-based hydrogen storage materials. Numerous studies[65-68]demonstrate that nanostructuring allows facile transition metal doping and provides a route toward improved hydrogen storage thermodynamics. The predicted relationship between radius of MgH2particle and dehydrogenation enthalpy is showed in Fig.3. Apart from the smallest clusters, the enthalpy is larger for the particle than for the bulk. Using accurate calculations of the dehydrogenation enthalpy, the authors show that considering the new low-energy structures strongly favored in the nanostructuring process, nanostructuring at these sizes do not necessarily improve dehydrogenation thermodynamics. Indeed, nanostructuring of MgH2causes a slight worsening of dehydrogenation thermodynamics. The study attributed this to the fact that the (MgH2)nclusters reconstruct from the bulk into new structures with reduced surface energy. This surface energy effects can be generalized to other hydrogen storage materials, although whether the effects will enhance or reduce stability also depends on the stability of the dehydrided material.

Fig.3 The relationship between radius of MgH2 particle and dehydrogenation enthalpy[65]
As far as the present research is concerned, the application of single metal hydrides in EMs is mainly focused on aluminum hydride and magnesium hydride. The existing research shows that the thermal stability of AlH3cannot satisfy propellants application requirements, hence the most stable crystal (pureα-AlH3) needs to be prepared. In terms of the thermal stability, MgH2meets the requirements of EMs, but it is easy to absorb moisture in the air. On the other hand, the thermal stability of alloy metal hydrides can satisfy the requirements of propellants application, but their hydrogen storage capacity are generally low and cannot give play to the advantage of hydrogen. The coordination hydrides own high hydrogen capacity, but are more active and usually have strong reducibility, which lead to the incompatibility with energetic compounds. Perhaps surface coating by graphene can effectively solve this problem.
Besides the application in propellant, the metal hydrides were also applied in explosives in some preliminary attempts. The explosive power of emulsion explosive can be improved significantly by MgH2according to Cheng et al.[4-5,69-72]. Compared with glass microsphere emulsion explosive, the underwater shock wave total energy of MgH2hydrolysis of emulsion explosives is increased by 32%(up to 3341.2kJ/kg), brisance is increased by 15.5%(up to 18.6mm), the detonation velocity is increased by 8.3%(up to 5023m/s). Through experimental and theoretical studies, the detonation reaction of MgH2sythesized emulsion explosive is higher than that of traditional emulsion explosive. In the detonation reaction of emulsion explosive with MgH2, both hydrogen release from MgH2and the explosion of H2are involved, which is different from conventional emusion explosive.
In order to improve the water resistance of MgH2,Cheng et al.[70-71]used paraffin inclusion process to prepare MgH2composite and synthesized hydrogen storage emulsion explosive with good storage stability. The experimental results showed that after 5 months of storage, the peak value of underwater explosion shock wave of glass microsphere emulsion explosive reduces by 31.3%. However, the peak values of underwater explosion shock wave of MgH2synthesized emulsion explosive and MgH2composite sythesized emulsion explosive are reduced by only 1.20% and 1.09%, respectively.
2%-10%(mass fraction) of MgH2were added into TNT, Tetryl and C-4 by Hradel J R et al.[73]. 50g of the new explosives were detonated by 8 detonator, and pressure relative value were recorded by manograph locating at 1.83m far from the explosion point. The results show that 5% of MgH2can increase the maximum total power of the explosives. The effects of Al powder, Mg powder and their hydrides on the detonation parameters of ammonium nitrate (AN), RDX and HMX were studied by Selezenevet al.[74]. The results show that the detonation velocity of explosives containing metal hydrides powders are higher than the explosives containing metals powder. The NASA Langley research center stated in the "predictions of future wars" that in the future, metal hydrogen storage materials can be sued for the development of fuel air explosive(FAE) and the energy may reach several fold of TNT equivalent weight[75]. MgH2was used in pyrotechnic composition by Ward[76], the ignition rate and combustion time will be increased on condition that fireworks luminance is not decreased.
4 Conclusion
In recent years, the use of hydrogen storage materials to enhance the performance of energetic materials has attracted extensive attention. In the light of the current research on hydrogen storage materials and their applications in energetic materials, the authors believe the future studies will focus on the following aspects:
(1)New hydrogen storage materials with excellent properties, e.g. high hydrogen content, good stability, and compliance with the application environment of energetic materials, should be developed. Aluminum hydride is currently the most promising metal hydrides adding in energetic materials, but the stabilization of aluminum hydride should be paid attention to and improve its stability.
(2)New materials modification methods should be developed to tune the existing hydrogen-storage materials. At present, the main methods include nanocrystallization, elements doping and coating. However, it should be noted that the hydrogen content of the modified metal hydride materials should not be lower than the hydrogen content of magnesium hydride (7.6%), otherwise the goal cannot be achieved from the perspective of adding hydrogen.
(3)The interaction between metal hydrogen-storage materials and other components in energetic materials, should be studied deeply so that the performance of energetics materials containing metal hydrogen-storage materials could be commanded. For example, although magnesium hydride has high thermal stability, its compatibility with energetic materials is problematic and should be taken seriously.

