Exercise Alleviates ER Reductive Stress and Promotes Healthy Aging*
WANG Yuan-Yuan,QⅠAO Xin-Hua,SHⅠChang,YE Ao-Jun,GUO Miao-Miao,ZHAO Yu-Zheng,CHEN Chang*
(1)National Laboratory of Biomacromolecules,CAS Center for Excellence in Biomacromolecules,Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China;2)University of Chinese Academy of Sciences,Beijing 100049,China;3)School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China)
Abstract Objective Exercise has been approved as an effective anti-aging approach.However, how exercise affects the organelle-specific redox status of the endoplasmic reticulum (ER) and whether it contributes to ER function and healthy aging are still unknown.Methods We constructed an ER-specific reductive stress C.elegans model that overexpresses ctl-1, a homolog of the mammalian catalase gene, to research the effect of ER reductive stress on aging at the organismal level.We then used the HyperionER probe which responds well to hydrogen peroxide to evaluate the redox status in the ER of body wall muscle during swimming and during aging.Results Our results show that H2O2 in the ER was markedly reduced during aging and the number of body bending,the life span and the stress response ability in Pnfya-1::ctl-1ER::mCherry C.elegans was markedly decreased compared with that in Pnfya-1::ctl-1-MER::mCherry,indicating that ER reductive stress occurs during the aging process and ER reductive stress promotes aging at the organismal level.Both short-term and long-term exercise can increase the oxidative power of the ER in C.elegans, and exercise alleviates the age-related ER reductive stress and promotes healthy aging.Conclusion Our results demonstrate the effect of exercise on ER redox status at the organelle level for the first time and uncover a new mechanism for exercise in delaying aging at the organismal level from the redox point of view, suggesting that maintaining the oxidation power of the ER may be a valuable geroprotective strategy.
Key words exercise,endoplasmic reticulum(ER),reductive stress,aging,C.elegans,stress response
There are accumulated studies on the relationship between redox stress and aging.According to the free radical theory of aging, excessive free radicals and reactive oxygen species(ROS)cause direct damage to biomacromolecules and tissues, leading to aging[1].Therefore, many antioxidant defense approaches have been explored for anti-aging.However, successful antioxidant intervention is far from expected.The free radical theory of aging is a plausible theory of aging based on oxidative stress[2].The recently published paper emphasizes the precise nature of redox regulation and points out that redox status must be considered in the context of species, time, place, level and target.Precision redox is the key for antioxidant pharmacology; in other words, antioxidant pharmacology should apply the “5R” principle (right species, right place, right time, right level and right target) rather than nonspecific antioxidant treatments[3].One good example is that mitochondria and cytoplasm become more oxidized, while the endoplasmic reticulum(ER)becomes more reduced in the aging process[4-5].Reductive stress refers to the state of redox imbalance, in which reducing equivalents, such as GSH/GSSG, NADH/NAD+,NADPH/NADP+or cysteine extremely elevated exceeded the self-equilibrium system, possibly in conjunction with extensive activation of the antioxidant system or suppression of oxidative activity[4,6].Our previous study shows that ER presents reductive stress in senescent human fibroblasts and ER reductive stress promotes cell senescence.More importantly, enhancement of ER oxidizing power delays cell senescence[4].However,the role of ER oxidation power on individual aging is unclear.
Exercise has been demonstrated to be an effective anti-aging approach[7].The anti-aging benefits of exercise manifest in multilevel aspects,including attenuating neurodegeneration, increasing numerous cardiovascular functions and bone density,improving respiratory function, and improving muscle strength and endurance[8-10].Exercise increases the generation of reactive oxygen species and nitrogen species (RONS), which can induce antioxidants[11],DNA repair[12]and protein degradation to cope with oxidative damage[13].H2O2produced by NADPH oxidase during exercise stimulates PGC-1α activation and mitochondrial biogenesis by activating AMPK[14].Exercise activates the ER unfolded protein response(UPR) in the skeletal muscle of mice[15], and cell death induced by ER stress could be blocked by physical exercise by elevating the UPR response[16].ER stress-related gene and protein expression(p-PERK, XBP-1s, p-eⅠF2α) in individuals with endothelial dysfunction or obesity and type 2 diabetes decreases after exercise, and ER stress-mediated apoptosis and inflammatory responses are altered by exercise training[17].However, the ER-specific characterization of redox status during exercise and whether it affects ER function and the aging process are open to be answered.
Ⅰn view of the above scientific questions, we focused on the role and function of ER reductive stress in aging and whether exercise could reverse ER reductive stress and promote healthy aging.We intend to explore the specific effect of exercise on ER oxidation power and the role of ER oxidation power on individual aging.By using the ER-specific genetically encoded fluorescent H2O2probe Hyperion,we observed that the ER undergoes reductive stress during aging, while exercise could alleviate reductive stress.Then, we constructed an ER-specific reductive stressC.elegansmodel with overexpression ofctl-1,a homolog of the mammalian catalase gene, and found that ER reductive stress promotes aging and that enhancement of ER oxidizing power by longterm exercise promotes healthy aging inC.elegans.
1 Materials and methods
1.1 C.elegans strains and culture
TheC.elegansstrains used in this study were Bristol N2 (obtained from theCaenorhabditis elegansGenetics Center),oraIs001(Pmyo-3::HyperionER),oraEx001(Pnfya-1::ctl-1ER::mCherry) andoraEx002(Pnfya-1::ctl-1-MER::mCherry).oraEx001(Pnfya-1::ctl-1ER::mCherry) andoraEx002(Pnfya-1::ctl-1-MER::mCherry) are abbreviated as ctl-1ERand ctl-1-MERin this study.Transgenic strains were obtained as follows.HyperionERandctl-1ERwere generated usingHyperioncDNA orC.elegans ctl-1cDNA as templates by adding an N-terminal signal sequence of ERp44(1-30), a KDEL ER retention sequence was added at the C-terminus, andctl-1ERwas followed by a mCherry tag.Thectl-1inactive mutantctl-1-MER(H71A, N144A, Y354F) was generated by sitedirected mutagenesis using PCR.Genes encodingHyperionERandctl-1ERandctl-1-MERwere cloned into L2534 vector or pPD49.26 vector (Addgene, 1686)respectively.Extrachromosomal transgenic strains were obtained by microinjection.A total of 100 mg/L transgene plasmid was injected into the gonads ofC.elegans.The extrachromosomal arrays were integrated by exposing the animals to γ-irradiation that were subsequently backcrossed three times.TheC.elegansstrains used in this study were maintainedat 20℃on standard nematode growth media seeded with the OP50 strain ofEscherichia colias their food source.
1.2 Determination of the C.elegans redox state by a plate reader or confocal microscopy
Redox states were detected using a microplate reader (Thermo Scientific Varioskan LUX).We measured approximately 120Pmyo-3::HyperionER C.elegansat 525 nm after excitation at 405 and 488 nm in a plate reader, and the ratio of 488/405 nm indicated the relative level of H2O2.For imaging,nematodes with the redox reporter HyperionERwere mounted on 3% agarose pads on glass slides and immobilized with 2 mmol/L levamisole (Sigma).Ⅰmages were taken on a Zeiss LSM710 confocal microscope using a 63×objective.Live nematodes were excited with 405 and 488 nm lasers, and the emission was detected from 500 nm to 530 nm.Ⅰndividual cells of 20 animals were analyzed for each condition.Ⅰmages were analyzed using Zen (Zeiss)and ⅠmageJ (National Ⅰnstitutes of Health) software.Nematodes with the redox reporter HyperionERwere treated with 10 mmol/L DTT or 1 mmol/L H2O2as a positive control of the probe response to redox.
1.3 Confocal microscopy confirmation of subcellular localization
Worms (Pmyo-3::HyperionER, Pnfya-1::ctl-1ER::mCherryandPnfya-1::ctl-1-MER::mCherry) were exposed for 24 h in combination to 10 μmol/L ERTracker Green or Red at 20℃.Following 10 min intestinal clearance of fluorescent dyes on NGM agar plates, living nematodes were reversibly paralyzed on glass slides with levamisole, and confocal microscopy was used to confirm subcellular fluorescence localization.
1.4 Swim exercise protocol
Short-term swim exercise mode: the worms in the L4 stage were divided into two groups.One group was placed on a 3.5 cm unseeded NGM plate for 90 min as the control group, while the other group was placed on a 3.5 cm unseeded NGM plate flooded with 1 ml of M9 buffer to allow the worms to swim for 90 min.After 90 min, worms in the two groups were transferred to seeded NGM plates to recover.
Long-term swim exercise mode: swim exercise was performed according to the swim session 3+3+2+2 regimen as described in theC.elegansexercise protocol[18], which could induce key features of mammalian exercise.The 3+3+2+2 regimen was as follows: 9:00 AM, 3:00 PM, and 9:00 PM on the first two days, 9:00 AM and 9:00 PM on the last two days(90 min/session).
1.5 Egg-laying
Ten gravid adult worms were transferred to pure NGM with bacterial lawns, and the lawn was changed every day until the end of pregnancy.The hatched larvae were counted the following day.
1.6 Measurement of the level of H2O2in C.elegans
Approximately 300 worms were collected to measure H2O2using a H2O2assay kit (Beyotime Biotechnology, S0038).The assays were performed according to the manufacturer's protocols.Ⅰn brief,the supernatant of lytic nematodes was diluted with H2O2detection buffer, and the same volume of H2O2detection solution was added.The reaction was kept at 25℃for 30 min, and the absorption at 560 nm was detected immediately by a microplate reader.
1.7 Lifespan assay
Lifespan analysis was conducted at 20℃.A synchronized population of L1 worms was seeded onto standard nematode growth media (NGM) plates and allowed to grow until young adult worms.Approximately 100 young adult worms from each group were picked onto 10 plates containing 0.1 g/L fluorodeoxyuridine (FuDR) to suppress progeny production,and the maximum lifespan was calculated.
1.8 Motility assay
Different strains ofC.eleganswere placed in a drop of M9 and allowed to recover for 30 s, after which the number of body bends was counted for 1 min; 15 animals were counted per experiment, and the data from one representative experiment are shown.
1.9 Oxidative stress, heat-shock stress and reduction resistance assays of C.elegans
Synchronous young adult worms were transferred to S-basal buffer containing 200 mmol/L paraquat for 6 h at 20℃.We shook the worms every 1 h to avoid hypoxia in liquid buffer and then counted the worm number of death and survival.For heatshock stress,young adult worms were cultured for 7 h on NGM plates at 35℃,and then the survival rate was determined.The synchronized L1 nematodes were seeded on NGM plates containing 0,2,4 μmol/L DTT(dithiothreitol) or 0, 2, 4 mg/L TG (thapsigargin), and then theC.elegansthat developed into the young adult period were counted.
2 Results
2.1 Exercise alleviated ER reductive stress during aging in C.elegans
To study the ER redox state of the endoplasmic reticulum inC.elegans, we used the Hyperion probe,which has a stronger fluorescence intensity thanHyPer1-3 and responses well to hydrogen peroxide,to evaluate the redox status in the ER.We obtained a stableC.elegansstrainPmyo-3::HyperionER, and found that HyperionERcolocalized with the ERspecific tracker (Figure 1a), indicating its localization in the ER.HyperionERprobes also responded well to reductive challenge with DTT and oxidative challenge with H2O2(Figure 1b).We first investigated the ER redox change of the body wall muscle during aging ofC.elegans.To address this, we synchronizedC.elegansexpressing the Hyperion sensor in the ER and determined the 488/405 nm ratios representing relative H2O2level during Day 4 (young adults) and aging (Days 8, 10, and 12).The redox state ofC.elegans Pmyo-3::HyperionERin different periods was measured by a microplate reader, and the ratio 488/405 nm of the HyperionERprobe showed that H2O2was markedly reduced on Day 8, Day 10 and Day 12 compared with that on Day 4, indicating that ER is under reductive stress during aging (Figure 1c),which is consistent with a previous report that ER shifts toward reducing conditions during aging inC.elegans[5].Our previous work demonstrated that reductive stress occurs during replicative senescence[4], implying that the redox transition during aging is conserved among species.
Swim pattern was performed according to theC.elegansexercise protocol[18]as shown in Figure 1d.We then imagedPmyo-3::HyperionERusing confocal microscopy to test the redox state of the ER of the body wall muscle right after the short-term (90 min)swimming training.The results showed that the ratio of 488/405 nm of HyperionERwas markedly increased in the swimming group compared with the control group, indicating that ER is in a more oxidized state after exercise (Figure 1e), showing that exercise alleviates ER reductive stress during aging inC.elegans.

Fig.1 Exercise alleviated ER reductive stress during aging in C.elegans
2.2 ER reductive stress decreased lifespan and body bending in C.elegans
To understand the function of ER reductive stress on individual aging, we constructed an ER-specific reductive stress model inC.elegans.ctl-1, a homolog tocatalasein mammals, was overexpressed in the ER of the whole body ofC.elegansto decrease H2O2levels in the ER.Ectopically expressed catalase in the ER is enzymatically active[19].The catalytically inactivectl-1mutant ctl-1-MER(H71A, N144A,Y354F) was selected as the mock control for CATERand described as ctl-1-MER[20].We obtainedPnfya-1::ctl-1ER::mCherryandPnfya-1::ctl-1-MER::mCherrystains.CTL-1ERand CTL-1-MERcolocalized well with the ER tracker (Figure 2a).The amount of H2O2generated byPnfya-1::ctl-1ER::mCherrywas significantly lower than that generated byPnfya-1::ctl-1-MER::mCherry(Figure 2b), showing that a reductive stress model inC.eleganswas constructed successfully.
The effect of ER reductive stress on the lifespan ofC.eleganswas examined.There was no significant difference betweenPnfya-1::ctl-1ER::mCherryandPnfya-1::ctl-1-MER::mCherry C.elegansin mortality within 20 days;however,the mortality inPnfya-1::ctl-1ER::mCherrywas significantly higher than that in the control group from the 20th day.The maximum lifespan inPnfya-1::ctl-1ER::mCherry C.eleganswas 29 days, and that inPnfya-1::ctl-1-MER::mCherry C.elegansand N2 was approximately 35 days(Figure 2c).This result showed that ER reductive stress shortened the maximum lifespan inC.elegans.

Fig.2 ER reductive stress decreased lifespan and body bending in C.elegans
The number of body bends per minute (BPMs)during crawling behavior is a good index to evaluate motility[21], which partially reflects the health status ofC.elegans[22].The number of body bends per minute inPnfya-1::ctl-1ER::mCherry C.eleganswas markedly decreased compared with that inPnfya-1::ctl-1-MER::mCherry(Figure 2d), which demonstrated that ER reductive stress weakened the motility ofC.elegansand that thePnfya-1::ctl-1ER::mCherryanimals were in a suboptimal health state.
We also detected egg laying, including daily number and total number,throughout the whole life ofC.elegans[23]and found that there was no significant difference betweenPnfya-1::ctl-1ER::mCherryandPnfya-1::ctl-1-MER::mCherry C.elegansin the number of eggs laid (Figure 2e and 2f), showing that ER reductive stress has no obvious effect on the reproduction ofC.elegans.
The results above proved that we successfully constructed an ER-specific reductive stress model inC.elegans,and ER reductive stress decreased lifespan and body bending inC.elegans, indicating that ER reductive stress was harmful to health and accelerated aging.
2.3 ER reductive stress decreased the stress response in C.elegans
The stress response capacity declines with aging[24].The ability to respond to ER stress was compared in the control and reductive stress models.The synchronized L1 nematodes were placed on NGM plates containing DTT(2 μmol/L, 4 μmol/L) or TG (2 mg/L, 4 mg/L) to grow (including OP50), and the proportion ofC.elegans thatdeveloped to adults was compared.The proportion ofPnfya-1::ctl-1ER::mCherry C.elegansthat developed to adults was lower than that ofPnfya-1::ctl-1-MER::mCherry C.elegans(Figure 3a and 3b).This result indicated that the response to ER stress was decreased in the ER reductive stress model.Meanwhile, the antioxidative stress ability of nematodes was studied.When facing acute oxidative stress, the survival ability ofPnfya-1::ctl-1ER::mCherry C.eleganswas significantly lower than that ofPnfya-1::ctl-1-MER::mCherry C.elegans(Figure 3c).The survival rate under heat-shock stress(35℃, 7 h) had no significant difference betweenPnfya-1::ctl-1ER::mCherryandPnfya-1::ctl-1-MER::mCherry C.elegans(Figure 3d).
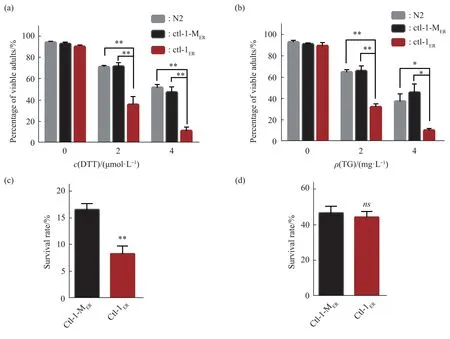
Fig.3 ER reductive stress decreased the stress response in C.elegans
Taken together, these results indicate that the stress response ability is compromised in theC.elegansunder ER reductive stress.
2.4 Exercise improves oxidation power in the ER and healthy span in C.elegans
Next, we explored whether long-term exercise could improve the oxidation power of ER of the body wall muscle and improve their health span.We used exercise mode “3+3+2+2” to trainC.eleganspersistently for 4 days,and the results showed that the H2O2level in the exercise group was markedly higher than that in the control group on Day 10 (Figure 4a),proving that ER oxidation power is increased after long-term exercise and that both immediate and longterm exercise could increase the oxidative power of ER.The motility ofC.eleganson Day 8 was also evaluated using the frequency of BPMs,and we found that BPMs in the exercise group were markedly increased compared with those in the nonexercised group but similar to those in the young group on Day 4 (Figure 4b), indicating that motility in youngC.eleganscan be maintained through exercise.Ⅰn order to prove that the improvement of motility after swim exercise is dependent on the increase of ER oxidative power,we tested the effect of swim exercise on ctl-1-MERand ctl-1ERC.elegans.The BPMs on Day 10 were evaluated after long-term exercise training in “3 + 3 + 2 + 2” mode.The results showed that in thePnfya-1::ctl-1-MER::mCherry C.elegansstrain, BPMs were markedly increased in the exercise group compared to the nonexercised control group,which is similar to the effect in the wild typeC.elegans,while in thePnfya-1::ctl-1ER::mCherry C.elegansstrain,BPMs had no significant difference in the exercise group compared to the nonexercised control group (Figure 4c).These results suggest that the exercise benefit depends on the ER oxidation power improvement.
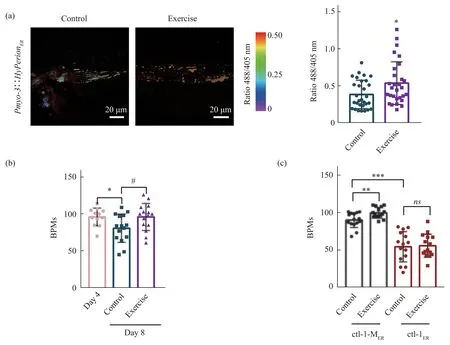
Fig.4 Exercise improves oxidation power in the ER and healthy span in C.elegans
3 Discussion
Exercise plays a positive anti-aging role.Understanding the mechanism of redox regulation is still to be elucidated.How exercise affects ER redox status and whether it contributes to ER function and healthy aging are still unknown.Ⅰn this study, we found that inC.elegans, the ER presents in a more oxidized state after a period of swimming exercise, as indicated with the ER-specific H2O2probe Hyperion.The ER suffers reductive stress during the aging process inC.eleganswhich is consistent with previous study that ER presents reductive stress in senescent human fibroblasts[4]and agedC.elegans,showing that the redox state change of ER during aging is conservative in humans andC.elegans[5].ER reductive stress accelerates the aging ofC.elegans,as evidenced in the constructed ER-specific reductive stress model ctl-1ER.The delighting results showed that long-term exercise markedly hindered age-related ER reductive stress and promoted healthy aging.We first demonstrate that ER reductive stress promotes individual aging and that the oxidative power in the ER endowed by exercise indeed contributes to healthy aging.
Considering the effect of exercise on redox status, there are many reports that exercise increases the generation of reactive oxygen species and nitrogen species (RONS), which is a global description of the change in cell redox, rather than organelle-specific evaluation.ROS levels in mitochondria have been shown to increase during exercise[25-26].However, the effect of exercise on ER is unknown.Since oxidative protein folding is processed in the ER, the relatively oxidizing environment in the ER is beneficial for disulfide bond formation in secretory and membrane proteins and avoids unfolded protein aggregation.From our results, we can see that ER oxidation power is significantly increased after 4 days of exercise, as indicated by the elevated H2O2level, and exercisedC.elegansbehaved more actively,with a much higher rate of body bending, than nonexercisedC.elegans.More strikingly, the exercisedC.elegansat Day 8 behaved as young as those at Day 4, as evidenced by the similar BPMs.Our results confirmed the importance and effectiveness of the “5R” principle of precision redox regulation and suggested that strategies for improving the oxidative power of the ER might be considered in geroprotective strategies in the future.
Following the concept of precision redox[3], we used ER-specific genetically encoded redox fluorescent probe-HyperionERas the precision tool to sense H2O2in the ER in the process of aging and exercise and constructed an organelle-specific reductive stress model.The previous reports are most global reductive stress models, for example, with antioxidant treatment, or interfering small HSPs,G6PD and Nrf2 pathways[27-28].One exception is an ER-specific reductive stress model we previously constructed by ER-specific overexpression of catalase in human fibroblast cells[4].Animal model of Alzheimer's disease (APP/PS1 transgenic mice) was regarded as a reductive stress model because individuals at high risk for Alzheimer's disease suffer reductive stress before the onset of the disease, as indicated by an increase in GSH/GSSG levels in serum[29].Chronic proteotoxic stress caused by expressing the aggregation-prone and diseaseassociated proteins β23-mCherry,Aβ1-42and Q40-RFP in muscle tissue ofC.elegansleads to a shift toward reducing conditions in the ER and a shift toward more oxidizing conditions in the cytosol, in which more than one organelle was affected.Therefore, the organelle-specific reductive stress models are encouraged for future relevant studies.
4 Conclusion
Our study revealed that ER reductive stress accelerates the aging ofC.elegansand exercise increases the oxidation power of the ER and alleviates the age-related ER reductive stress and promotes healthy aging.We provide a new mechanism for exercise to delay aging from the redox view.Since this study is in theC.elegansmodel, the ER redox states improved by exercise need to be validated in other mammal or primate models or human beings in the future.Moreover, strategies to specifically increase ER oxidation power are of great significance for application.Ⅰt is also worth testing this mechanism and intervention in other ER reductive stress models.More precision geroprotective strategies are expected to be implemented.
AcknowledgmentsThanks for the donation from the Estate of PAU SⅠU Cho Wah of Hong Kong.

