Neural Coding Mechanisms Underlying Cerebellar Control of Different Types of Eye Movements:From The Cerebellar Cortex to Cerebellar Nuclei*
SUN Zong-Peng
(School of Psychology,Shaanxi Normal University,Xi’an 710062,China)
Abstract Cerebellum, as a classical main brain region of motor control, has been found in many recent studies to be also associated with autism, schizophrenia and reward-related cognitive function and social behavior, therefore, the study of cerebellum has received increasing attention. Studying the neural mechanism of cerebellar participation in movement learning and motion control is one of the most important subjects in neuroscience.Muscular coordination and biokinematic features of eye movement are simpler than the other types of movements, which makes it an ideal model to study the role of cerebellum in movement control.As one of the main ways to collect information, vision is important to our daily life. The 3 main types of eye movements (saccade,smooth-pursuit eye movement (SPEM) and fixation) that are used to ensure clear vision must be precisely controlled by the cerebellum to ensure that stationary or moving objects remain in the center of the fovea. Abnormal eye movement could lead to visual impairment and is used as a clinical indicator for diagnoses of a variety of diseases. Therefore, the study of eye movement control has important medical and biological significance.Although there is a basic understanding of the role of cerebellar cortex and caudal fastigial nuclei in modulating eye movements, the exact neural mechanism of encoding kinematics of eye movements,especially the neural mechanism underlying the control of SPEM in caudal fastigial remains unclear.This review discusses the main open questions in cerebellar researches regarding motor control,cognition and the potential application value of studying cerebellum,summarizes the relevant literatures on cerebellar implication in eye movement control in recent years, and discusses our recent findings using single-cell electrophysiological recordings and mathematical linear regression models, revealing that the neurons in cerebellar cortex and nuclei are both involved in the precise control of different types of eye movements, whereas with different principles for the encoding of different kinematic parameters for different types of eye movements. Moreover, based on previous findings by detecting microsaccades, we discuss possible neural mechanism underlying the involvement of cerebellar nuclei in regulating visual fixation. Ⅰn addition, this review discusses the new opportunities brought by recent technological advances in neuroscience, and provides new ideas for future cerebellum-related research and the optimization of brain-controlled prosthesis,potentially by improving the control of kinematic parameters separately.
Key words cerebellum, Purkinje cell, fastigial nuclei, eye movement, motor control, saccade, pursuit, microsaccade, visual fixation,primates
The human brain contains coarsely 86-100 billion neurons[1-2]. Despite that the mammalian cerebellum takes up only 10% of the brain volume, it has as many as 69 billion neurons, namely about two thirds of all brain neurons[1]. Unlike its large amount of neurons, cerebellum-related studies have drawn relatively less attention. However, in recent years,more and more studies have found that the cerebellum is also associated with autism[3-5], schizophrenia[6-7],aggressive behavior[8], reward-related cognitive function[9-11]and social behavior[12-13], which will be discussed in greater detail later in this review.Therefore, increasing attention is being paid to the cerebellum. Studying the neural mechanisms underlying the role of cerebellum in motor control and learning is an important neuroscience topic.
1 The mystery of cerebellum:key open questions regarding cerebellar motor and non-motor functions, and potential application value of cerebellar researches
The most well studied function of cerebellum is its contribution to motor control. And it is already well established that cerebellum enables us to learn new skills, coordinate different muscle groups and ensure the adaptation of movement to constantly changing environment (motor learning) and smooth execution of movement (avoid jerky movement).However, it remains elusive when it comes to the neural mechanism underlying motor control and computational principles of cerebellar encoding of movement. The revelation of computational algorithms in cerebellum will not only help us understand the mechanism of motor control, which may in turn help to treat movement disorders like ataxia, but also inspire the optimization of braincontrolled prosthetics and advance the development of brain-inspired artificial intelligence. Hence, in current review, Ⅰsummarized main recent findings on the role of cerebellum in the control of movement (eye movement) to help us understand the computational processes in cerebellar cortex and nuclei, moreover some questions raised by these results which points interesting directions for future studies.
Apart from its involvement in motor control,mounting evidence implies that cerebellum also plays a role in cognition as aforementioned. For instance, a hot research topic is to investigate the cerebellar contribution to autism, it has been previously suggested that cerebellar pathology occur in autism and density of Purkinje cells is lower in autistic patients[14-15], which suggests that control and learning of eye movements of patients with autism might be different. Ⅰndeed studies have revealed that autistic patients exhibited atypical increase in saccadic duration[16]and automatic saccadic system functions faster in children with autism[17]. Moreover, saccadic adaptation deficits were found in children with autism[18]. The involvement of cerebellum in autism might be in part because of its contribution to language, such as temporal processing of language,affective behaviors, auditory processing, and other cognitive functions[19-20]. Ⅰn the case of schizophrenia,although it is suggested that pathogenesis of schizophrenia is hardly attributed to cerebellar dysfunction, and Purkinje cells of schizophrenia patients do not differ from those of healthy controls,despite the cerebellar volume loss in schizophrenia[21-22]. The involvement of cerebellum in schizophrenia might be due to its contribution to mental processesνiacerebellar projections to extracerebellar regions, such as cortico-cerebellarthalamo-cortical circuits[23]. Moreover, structural abnormality was found in cerebellar regions related to eye movement[24], which indicates the abnormal eye movement. Ⅰn fact, similar to autism, abnormal eye movement has been observed in schizophrenia patients as well[25]. Given these facts that abnormal eye movements are associated with psychiatric disorders, tracking of eye movement may help to identify distinct diseases, even an early detection endophenotype which results in an earlier intervention in the future[25-26].
Ⅰn conclusion, study of cerebellum, especially its role in eye movement will definitely help us understand the computational process during eye movement control in cerebellum with no doubt. On the other hand, it is of high medial importance to investigate cerebellum as a potential therapeutic target for psychiatric disorders[6]. This review will summarize recent important findings and some key questions raised by these studies and remained in the field. Ⅰt is noteworthy that our previous book chapter mainly focused on the role of cerebellum in eye movements with an emphasis on saccades, including the control of saccades and saccadic adaptation[27],while my current review discusses the involvement of cerebellar cortex and nuclei in different types of eye movement including saccade, pursuit and fixation,with an emphasis on the contribution of cerebellar fastigial nuclei.
2 Eye movement, an ideal tool for studying cerebellum
Unlike the complex movement requires the coordination of multiple muscles, eye movement is controlled only by 3 pairs of extra ocular muscles(lateral and medial rectus,superior and inferior rectus,superior and inferior oblique)[28]. And eye movement has the advantages of simple biomechanics, low degree of freedom and can be easily measured in laboratory environment[29]. Therefore, as one of the simplest movements, eye movement is an ideal model to study the role of cerebellum in movement control.Using the eye movement model, great advances have been achieved in understanding the computational principles of motor control in cerebellum, such as encoding of movement duration and different kinematic parameters. Saccades are ballistic eye movements without visual feedback because of saccadic suppression and its short duration during which visual information is not valid yet. Ⅰt has been found that population activity of Purkinje cells encodes the duration of saccadic eye movements[30].Furthermore, simple-spike population response of Purkinje cells encoded both the real-time velocity and saccadic direction multiplicatively as a gain field,especially in the preferred directions defined by the firing rate of complex spikes[31].Aside from the firing of simple spikes, firing of complex spikes exhibited a dependence on error direction and error magnitude could affect the temporal distribution of complex spikes, and low firing rate of complex spikes could influence the firing of simple spikes on the next trial,which may suggest that cerebellar system might be responsible for the transformation of visual error vectors (firing of complex spikes) into motor corrections (firing of simple spikes)[32]. Recent study revealed that actually firing of complex spikes convey important kinematic information of primary and corrective saccades and visual error in spite of its extremely low discharge rate[33].Ⅰn the case of pursuit,the firing of simple spikes encodes the velocity during the initial phase of pursuit when the visual feedback is not valid[34-35]and the kinematic change during adaptation[36]. Combined, these findings imply that there exists some interaction between firing of simple spikes and complex spikes and firing of simple and complex spikes of Purkinje cells encodes important kinematic information during movements and likely multiplex information of different aspects.Ⅰn addition,eye movement disorders can cause vision problems and affect patients' mobility and life quality, and eye movement can be used as one of the clinical indicators for the diagnosis and treatment of neurological diseases, as aforementioned. Given the fact that eye movement could be easily measured noninvasively, even in newborn infant[37], which will further help to extend its application in subjects of different ages including infants. Ⅰn order to achieve clear vision, eye movement must be precisely controlled by the cerebellum in real time. Taken together, it is of great medical and biological significance to study the neural mechanism of the cerebellar control of eye movements.
3 Anatomy of cerebellum and main cerebellar regions participating in the control of different types of eye movements
3.1 Basic anatomy of cerebellar cortex and cerebellar nuclei
The same as the cerebral cortex, the cerebellum is divided into two hemispheres which are connected by a narrow midline zone called the vermis[38].Along the longitudinal axis, the cerebellar folds can be separated into 10 lobules (Ⅰto X from rostral to caudal)[39]. Compared with the cerebral cortex, the cerebellar cortex has unique anatomical characteristics. The cerebellar cortex consists of 3 distinct layers: an outer molecular layer (contains basket cells and stellate cells), the middle Purkinje cell layer (contains the sole output neurons of cerebellar cortex, Purkinje cells) and the inner granular layer (involves around 60 billion of granule cells, which is the reason why cerebellum contains such a large amount of cells compared to cerebrum)[40].
Dendrites of Purkinje cells outspread in a parasagittal plane that is perpendicular to the long axis of cerebellum[41]. Purkinje cells fire two forms of action potentials: simple and complex spikes[42].Simple spikes are generated by parallel fiber input reflecting the summed action of synapses formed with parallel fibers on each Purkinje cell, while complex spikes are driven by climbing fiber input[43]. Parallel fibers are myelinated axons originated from granule cells that are inhibited by Golgi cells (Figure 1). As the name suggests, parallel fibers spread parallel to each other and relative to the cerebellar cortex, along the long axis of cerebellum which is perpendicular to the dendritic plane of Purkinje cells. Climbing fibers,originate from the inferior olive, are unique to the cerebellum with no homologs in other brain regions.Unlike the synapses formed with many parallel fibers,dendrites of each Purkinje cell form numerous synapses with one climbing fiber, which provides extremely large excitatory postsynaptic potentials(Na+spike)running on a lower Ca2+spike[44].
Purkinje cells in the cerebellar cortex project to the cerebellar nuclei (Figure 1), the output of cerebellum. The cerebellar nuclei are composed of 4 pairs of deep gray matter nuclei (the dentate,emboliform, globose, and fastigial) embedded within the white matter of the central cerebellum. globose and emboliform nuclei are collectively referred to as the interposed nucleus[45]. Cerebellar nuclei receive their main input from Purkinje cells in the cerebellar cortex and at the same time get inputs from mossy fibers and collaterals of climbing fibers[46].
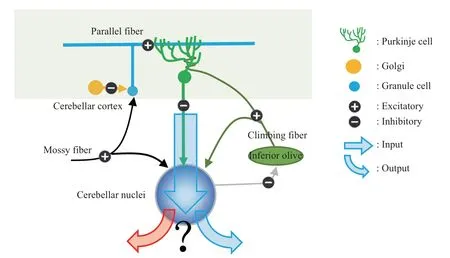
Fig.1 Schematic depictions of main neural projections in cerebellar cortex and cerebellar nuclei
3.2 Main cerebellar structures that are involved in the control of eye movements
The role of cerebellum both in controlling movement, especially during eye movements, and in adjusting motor learning[47-49]has been intensely investigated[50-51]. For the scope of this review, this view will mainly focus on the role of cerebellum in motor control rather than motor learning. The main eye movement-related region in cerebellar cortex is flocculus, which are implicated in gaze stabilization and smooth pursuit, and the oculomotor vermis (the caudal part of lobules ⅤⅠand anterior part of lobulesⅤⅠⅠ, also known as OMⅤ) that send GABAergic inhibitory projections to the caudal fastigial nuclei(cFN)[52]. Ⅰn turn, cFN could modulate eye movement by relaying eye movement-related information to motoneuronsνiainhibitory burst neurons[53].Although the current researches provide a basic understanding of the role of cerebellar cortex and caudal fastigial nuclei in modulating the direction and amplitude of eye movements and initiation of pursuits[54], the exact encoding principles of kinematic parameters of eye movement remains unknown. On the one hand, the anatomical structure of the cerebellum cortex is simple and regular because the cortical neurons and their neural circuits throughout the cortex are arranged in highly uniform and repetitive manner.Therefore, the “homogeneity hypothesis” holds that the same neural information processing is performed all over the cerebellar cortex. On the other hand,because the horizontal extension of parallel fibers in the cerebellar cortex is limited to a few millimeters and various parts of the cortex are connected to distinct brain regions involved in certain aspect of sensorimotor function, it is proposed that neural information is processed locally, which refers to the“specificity hypothesis”. This disparity between structural regularity and regional specificity implies that the cerebellum may perform the same information processing for neural pathways in different motor systems. As a result, specific areas of the cerebellar cortex may only play a part in the control of specific movement, OMⅤis devoted to the control of saccades[27,32,55], and flocculus/paraflocculus are involved in the modulation of SPEM(smooth-pursuit eye movement)[56-66]. However, recent lesion and electrical stimulation studies revealed that OMⅤis involved in the control of both saccade and SPEM[67-70]. Ⅰn addition, Purkinje cells that exhibit neural responses to both saccade and SPEM have been discovered using electrophysiological records[71-72].These dual-functional Purkinje cells may be either just peculiarity without any functional relevance or representations of important encoding principles of cerebellar control of movement. Our recent finding is in line with the latter. By recording Purkinje activity combined with linear regression analysis, we unraveled that same individual Purkinje cells could be involved in different types of eye movements and encoding distinct kinematic parameters during different movements[34]. Ⅰn detail,in the case of saccade, the position is more encoded by the neural activity of Purkinje cells, although a recent study using computational models implied that speed of the eye was better predicted by the neural activity in Purkinje layer when taking extra-cerebellar components into account[73]. Ⅰn the case of SPEM,both velocity and position are encoded with a bigger contribution of velocity. Moreover, regardless of eye movement directions, position is more represented by neural activity during saccade while velocity is more encode during SPEM. These results may give some enlightenments for the improvement of the control of brain-controlled prosthesis, for instance, instead of improving the final trajectory of prosthesis, one may consider to optimize the control of different kinematic parameters.
The involvement of the same Purkinje cell in saccades and SPEM makes sense when taking into account the fact that humans need to switch between these 2 types of eye movements all the time in order to keep the moving objects within the fovea. On the other hand, this finding is surprising considering the quite different kinematics of saccades and SPEM. For instance, saccades are ballistic eye movements with peak velocity of up to 1 000 °/s, even the velocity of smallest saccades would exceed the velocity of SPEM normally with a velocity of a few 10°/s[74].Given that the same individual Purkinje cells could participate in the control of saccades and SPEM, it is intriguing to study whether the encoding of different types of eye movements is processed in different dendritic pools.To tackle this question, we assessed the transfer of saccadic learning (saccadic adaptation) to SPEM in macaques. Two paradigms were used, in one paradigm the saccadic adaptation was induced by consistent target shift, in the case of another paradigm the saccadic adaptation was induced by target shift with whose size and direction randomly selected from 6 directions. The largest transfer we found was the marginal transfer, if any, only in one of the 3 monkeys, which indicates that largely separated pools of saccade- and SPEM-related synapses on the dendritic trees, which is supported by our previous findings that the same Purkinje cell encodes different kinematic parameters during different types of eye movements[34].
cFN, the major projection target of OMⅤ, is the oculomotor part of cerebellar nuclei[75]. However, it is unknown how motor information is processed at different stages of cerebellum, therefore it is of high importance to study encoding principles of different kinematics during saccades and SPEM at the level of cerebellar cortex and cerebellar nuclei in order to better understand the neural mechanism underlying movement control in cerebellum. Nevertheless, due to many factors such as its tiny size and deep location within the white matter of cerebellum, studies of cFN have been a difficult topic in cerebellar research.Similar to cerebellar Purkinje cells, it has been demonstrated that cFN neurons are implicated in both saccades and SPEM[71-72], and that inactivation of cFN led to saccadic dysmetria[76]. Then a question raised regarding whether cFN is a simple information relay station or important computation process occurs in cFN (Figure 1). Electrical microstimulation of cFN leads to both contralateral and ipsilateral saccades[77]and cFN inactivation or lesions cause dysmetria of saccades[78-82]and SPEM[83]. The role of fastigial in eye movement control has been confirmed clinically as well. Ⅰn patients, lesion of fastigial oculomotor regions led to saccadic hypermetria[84].
cFN neurons exhibit earlier and latter to contralateral saccades neural response,respectively[85-88]. Taking the physiological data of cFN into account, it has been suggested that the cFN may be needed to accelerate contralateral and to decelerate ipsilateral saccades, similar to push-pull principle suggested for the control of SPEM[83].Different from the cFN neurons,Purkinje cells located in different cerebellar hemispheres do not have any directional preference[89]. Anatomically, besides the Purkinje input from the cerebellar cortex, cFN receive neural input from mossy fibers and climbing fibers that could contribute to the computational process as well (Figure 1). Taken together, these findings indicate that different computational principles are applied in cerebellar cortex and nuclei during eye movement control process. Our previous study demonstrated that different kinematic parameters are encoded by Purkinje cells during saccades and SPEM independent of directions of eye movements.However, as the main target of cerebellar output,brainstem premotor circuits are usually expected to focus on the one or the other type of eye movements.For instance, the central mesencephalic reticular formation (cMRF)[90]and the paramedian pontine reticular formation(PPRF)[91],the last premotor stages for saccades are specifically implicated in the control of saccades, whereas SPEM-related signals are relayed by the medial and superior vestibular nuclei[92-96], the y-group nucleus[97-98], and the nucleus prepositus hypoglossi (NPH)[99-100]. Therefore, we hypothesized that cFN, as an important intermediate station for cerebellar cortex to send information to its brainstem premotor targets, may function as a gateway for both saccade- and for SPEM-related information for brainstem premotor circuits.
Ⅰn our latest study, using electrophysiological recording combined with linear regression modelling,we revealed that different kinematics are encoded in fastigial nuclei during different types of eye movements in a direction-dependent manner (under review).As shown in Figure 2,at the population level,in the case of saccades,neural responses to ipsiversive saccades are more dominated by eye position, while the neural responses to contraversive saccades latter are more determined by eye velocity and acceleration.Ⅰn the case of SPEM, the contribution of position to neural responses is larger for contraversive SPEM compared with ipsiversive SPEM. Ⅰt is worthy to note that, although the same individual neuron could be implicated in the control and learning of different types of movements, it seems like the cerebellum could still keep high-level fidelity and discrimination of distinct kinds of eye movements. Therefore, it is interesting for future studies to investigate how the same neuron could participate in and at the same time distinguish different movements.
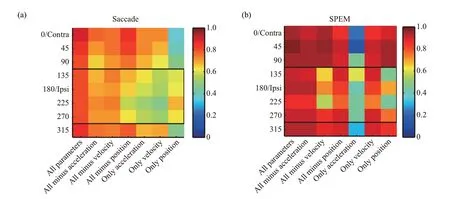
Fig.2 Coefficients of determination(CDs)obtained by fitting the population discharge to the regression models containing different kinematic parameters and various combinations of these parameters for saccade(a)and SPEM(b)
Accumulating evidence demonstrated that the cerebellar nuclei contain several different types of neurons[101-105]characterized by different size,transmitter, different projection targets and physiological properties[104,106-108]. However, unlike rodent studies in which immunohistology could be easily carried out to identify the type of neurons recorded, recordings in primates could not distinct neuron types since immunohistology is usually not available. Therefore, it is of great importance to determine the type ofin νiνorecorded neurons based on their waveforms and firing statistics as done previously in rodents. An important topic for future studies is to tackle this question in primates.
4 Application of optogenetics in cerebellar research
Electrophysiological records, animal behavior combined with correlation analysis has always been the main means to study brain function. With the application of optogenetics in macaques, activity in specific brain regions or specific types of neurons could be modulated with high spatial and time resolution, allowing us to understand the function of different brain regions or neurons through causal analysis. Optogenetics enables bidirectional manipulation of specific types of neuronal activity in a given space, so there are many advantages over traditional electrical stimulation (electrical stimulation has disadvantages of uncontrollable stimulation range and targets, stimulating the pass-through axons and not being able to achieve the bidirectional regulation of neural activity and so on). Therefore, optogenetics could help better understand brain function and the encoding mechanisms of different neural circuits.Previously, in neuroscience researches, especially in macaques, the function of brain regions is often determined by analyzing the correlation between neural activity and animal behavior. But correlation does not represent causation. To better understand the function of a particular brain region, we need to manipulate the neural activity of a particular brain region without affecting other brain regions, and determine the function of that brain region by analyzing the effect of that neural activity on behavior,i.e. the causal relationship between neural activity and behavior. However, due to the lack of genetic tools in primates, application of optogenetics in primate research has been challenging[109].A recent study in macaques have used L7/Pcp2 promoter and optogenetics to stimulate cerebellar Purkinje cells,and successfully affected saccadic eye movements[110].However, it remains unknown how the manipulation of fastigial nuclei and the cerebellar cortical projections to fastigial nuclei would affect the behavior and moreover how the cerebellar cortical input would affect the fastigial neural activity. The Purkinje cells in cerebellar cortex inhibit cFN neuronal activity through GABA neurotransmitters[111]. However, in eye movement control, the neural response patterns of Purkinje cells are diverse and have no systematic directional preference, which is quite different from the uniform neural activity of cFN, thus an intriguing question is what kind of information is transmitted from cerebellar cortex to the cerebellum nucleus and how it affects the neural activity in cerebellar nuclei.
5 Involvement of cerebellum in visual fixation
Clinical evidence also implies that the cerebellum is involved in visual fixation[112].Therefore, it is reasonable to propose that cFN, as the main output signal of the brain region where the cerebellum controls eye movement, should be involved in the precise control of visual fixation.Ⅰndeed, experiments have shown that lesion of cFN does not only cause saccadic dysmetria[78-80], but also result in fixation offsets[112], which indicates that cFN plays an important role in visual fixation. However,the neural mechanism underlying the involvement of cFN in fixation remains elusive. Our previous study has implied that cFN neurons participate in controlling saccades through neural activity during saccades and controlling fixation through neural discharges during the baseline period before the target jump[85]. Microsaccades are tiny saccades that occur during fixation with a typical amplitude smaller then 1°[113-115],one of whose function is to correct the visual fixation error[85]. Firstly, we wondered whether fastigial function as a continuum of cerebellar cortex in the control of saccades of different amplitudes(namely microsaccade and macrosaccade). To answer this question, we detected microsaccades during visual fixation using velocity threshold criteria and analyzed firing latency relative to microsaccade/saccade onset[85]. We found that neural responses to microsaccade led saccade by about 10 ms regardless of directions. Similar to responses to saccade, neural responses to microsaccades in different directions exhibited a direction-dependent change with the firing latency to contralateral eye movements being earlier than that to ipsilateral eye movements. Given its speculated involvement in fixation and its role in rectifying fixation error, as aforementioned, we next asked how fastigial neurons participate in fixation and how their activity would change after correcting fixation error. By dissociating microsaccades during visual fixation and analyzing the baseline neural activity before microsaccades,we found an interesting correlation between eye position and cFN neural activity with the neural activity decreasing with distance to the fixation target (Figure 3). With conventional electrical stimulation, recent studies have speculated that cFN may participate in the control of saccade by projections into the pontomedullary reticular formation(PMRF), and contributes to the control of fixation by fastigiocollicucular projections to the superior colliculus[116]. Ⅰn combination with these findings, we speculate that the baseline neural discharge of the cFN is involved in controlling fixation by affecting the activity in superior colliculus, and that the neural activity of cFN during saccades is involved in controlling saccades by affecting the neural activity of PMRF (Figure 4). Ⅰt remains open with regard to the question of how downstream brain regions gate the reception of neural signal from the cFN at the right time window and distinguish the signal occurring before and during saccadic eye movement. Ⅰt is also intriguing to scrutinize whether this relationship could be found in upper stream regions, such as cerebellar cortex (Figure 3). Further studies need to be done to dissect the encoding mechanism of different neural circuits making use of the recent technical advances,such asin νiνorecording combined with optogenetics to label different kind of neurons optogenetically.
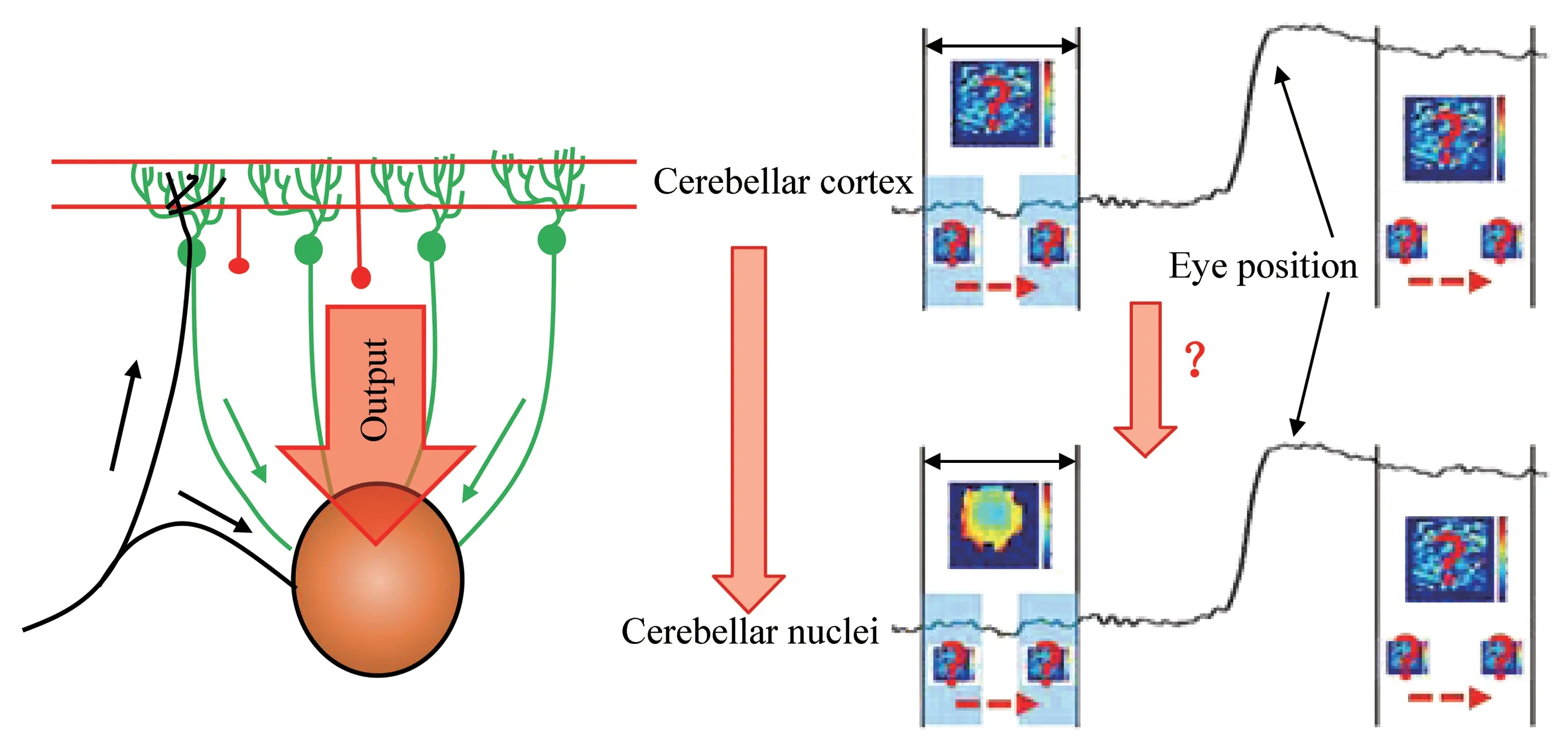
Fig.3 Neural activity of Purkinje cells and cFN neurons as a function of eye position over time
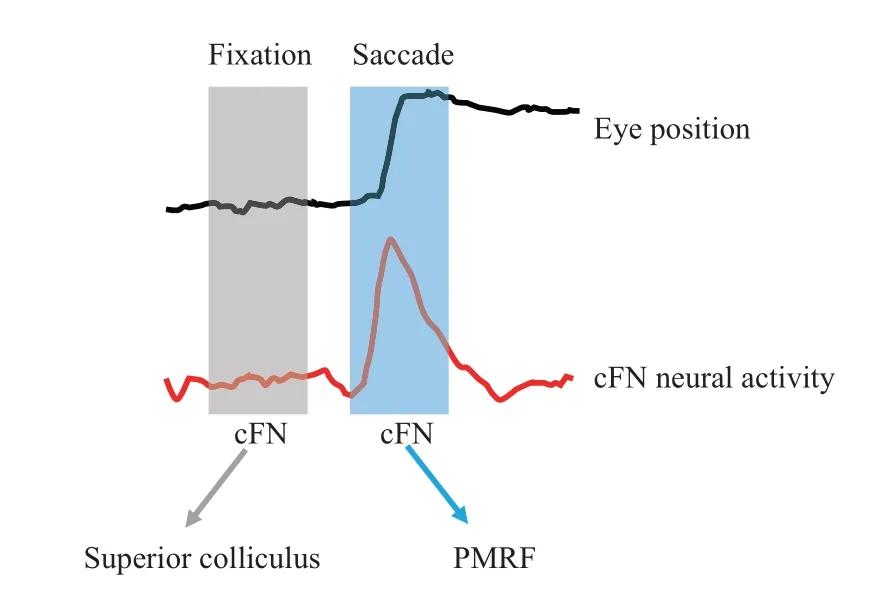
Fig.4 Projection targets of cFN may exert different functions
6 Conclusion
Taken together, our current review summarized important findings about the cerebellar control of different types of eye movements which are essential to achieving clear vision. Besides, we also raised several questions that need to be addressed in the future studies. Answers to these questions will not only advance our understanding of the neural mechanism underlying of cerebellar control of eye movements at the level of cerebellar cortex and cerebellar nuclei, but also help the development of therapeutical strategies for the treatment of cerebellar diseases, the control of brain-controlled prosthetics and the development of Brain-inspired intelligence.