Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
Guling Yng, Hiyn Zhong, Xinxin Xi, Zhiwen Qi*, Chengzhng Wng Shiming Li*
a National Engineering Laboratory for Rice and By-products Processing, Food Science and Engineering College,Central South University of Forestry and Technology, Changsha 410004, China
b Institute of Chemical Industry of Forest Products, CAF, Nanjing 210042, China
c Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization, Huanggang Normal University, Huanggang 438000, China
Keywords:
PROTAC
Ligand
Natural products
Target binding
A B S T R A C T
There are numerous evaluations of natural products, of which majority are food bioactives, performed up to date for their various health beneficial activities via targeting specific proteins. However, the direct identification of a targeted protein remains unexplored for natural occurring compounds. Proteolysis targeting chimera (PROTAC) is a type of bifunctional chimeric molecules that can directly degrade the binding proteins targeted by bioactive molecules in an ubiquitin-proteasome pathway. As the agents in protein degradation dependent on ubiquitin ligase, the bifunctional molecule connects the target protein ligand and E3 ligase ligand together via an appropriate linker. It is highly selective and ef ficient to induce the ubiquitin-mediated degradation of targeted binding proteins. Therefore, it has been demonstrated that the PROTAC technology has broad application in the modulation of the target protein level. In this review, we outlined the advances in PROTAC combined molecule compounds, summarized its quantitative structure-activity relationship, and finally reviewed the methods applied in identifying the target proteins of natural products. We hope it will provide an insightful application of PROTAC techniques in the target protein identification of natural products including food bioactive molecules.
1. Introduction
Manyin vitroandin vivobiological property evaluations related to natural products including food bioactive molecules have been performed. The elucidation of molecular pathways of biologically active compounds has resulted in some explanations of action mechanism by cell assays usually. Direct evidence from a proteinligand binding is seldom seen. Recent development of proteolysis targeting chimera (PROTAC) technology becomes one of the most advanced methods to directly address the binding between the bioactive molecule and its target protein (TAP).
Ubiquitin-protease system is the primary pathway of protein degradationin vivo, which can degrade about 80% of the endogenous TAPs [1,2]. PROTAC is designed based on the characteristics of protein ubiquitination, thus can efficiently induce the degradation of the TAPs [3]. PROTAC represents a ternary complex, constituted by the ligand of an ubiquitin-ligating E3 enzyme (L-UBL-E3), a ligand of the interest TAP and the linker connecting L-UBL-E3 and the ligand of target protein (L-TAP, Fig. 1). Specifically, in the PROTAC strategy, upon the binding of the PTOTAC with its TAP, the TAP will be bound to the ubiquitin-conjugating E2 enzyme (E2) and ubiquitin ligase E3 (UBL-E3), followed by ubiquitination and degradation in the proteasomes. Having played a crucial role in promoting the binding of E3 ligase to the TAP, PROTAC will be released after the ubiquitination of the TAP. Hence, PROTAC is regarded as the protein degradation catalyst recycledin vivo, and takes effect by degrading the target cellular proteins [4]. Thus, PROTAC can regulate the amount and activity of the TAPs at the protein level [5]. In the early stage of PROTAC development, PROTAC was covalently bonded with peptides as the TAP ligand. However, the characteristic of a large molecular weight with the peptide PROTAC prevented it from penetrating the cytoplasmic membrane, thus reduced its efficiency of the TAP degradation. Therefore, it is essential to explore novel suitable L-TAP for targeted binding of the TAPs. Currently,several small molecules that have targeted binding capacity toward the proteins have been employed as L-TAP in the preparation of PROTACs.
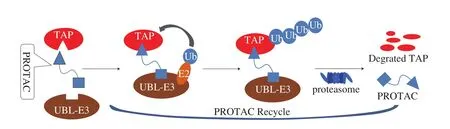
Fig. 1 Mechanism of PROTAC degradation of interest TAPs. (PROTAC molecules are prepared with the L-UBL-E3 and L-TAP through a linker. Then targeted combined with TAP, and degraded by the E3-ubiquitin enzyme, afterward, the PROTAC molecule will be recycled in the degradation of the TAPs.)
Unlike traditional inhibitors requiring for binding at the active site of the TAPs, PROTAC-induced protein degradation only requires binding to any site of the TAP [6,7]. Therefore, the PROTAC strategy is expected to have broader application possibilities, particularly for the traditional “undruggable proteins”. As reported, PROTACs based on a potent androgen receptor (AR) antagonist can induce > 95% degradation of AR in AR + prostate cancer cell lines at 0.2-1 nmol/L level [8]. After over 20 years of development, the PROTAC technology has been continuously updated, which shows an excellent capacity in the ubiquitination of the estrogen receptor (ER) [9],tyrosine kinase [10], cyclin-dependent kinase 8 [11]and others.Nevertheless, it remains unclear what the quantitative structureactivity relationship (QSAR) is between the chemical structure of the prepared PROTAC and the degradation efficiency of the TAPs, which are ascribed to the randomness in the design of most PROTACs.The following outlines some studies and applications of the small molecule-based PROTAC to provide references for the rational design of PROTACs.
2. Advantages of the PROTAC technology
At present, more than 3 000 pathogenic natural proteins have been identified, of which only about 400 have been identified as possessing drug targeting properties, and the rest, such as transcription factors,scaffold proteins, and the enzymes without potential target sites, were considered as “undruggable proteins” [7], due to the lack of small molecular binding sites or active blocking sites. For example, Tau protein aggregation is associated with neurodegenerative diseases [12],thus Tau is considered as an “undruggable protein”. Compared with the traditional small molecular compounds’ blocking therapy, the PROTAC strategy exhibits a set of advantages [4,13,14]. First of all,it overcomes the difficulty that most proteins have no binding sites required for traditional drugs. Theoretically, PROTACs can degrade the TAP via ubiquitination pathway as they can bind to any site of the TAPs, and not required to occupy or block the active sites of the TAPs. For instance, some proteins, possessing none binding sites for traditional drugs or inhibitors, can be degraded by PROTACs in the manner of the ubiquitination pathway. Furthermore, there is no direct correlation between the degradation efficiency of the TAPs and the affinity of PROTACs with their TAPs [5]. According to the dataset, a compound-kinase pairing with a higher affinity was not more potently degraded than the one with a lower affinity.Secondly, the PROTAC-ubiquitination dependent degradation can solve the problem of high concentration usage of traditional drugs.It only requires a short period of non-covalent binding between the PROTACs surrogate and the TAP to induce the ubiquitination of the TAP. After that, PROTACs will be released and rebound to new target proteins. Finally, the same PROTAC molecules act in cycles in the degradation of TAPs. Therefore, there is no need to apply a high ligand concentration to maintain its activity. Thirdly, the PROTAC strategy can cope with the issue of traditional drug-induced side effects. Most of the protein families contain numerous subtypes which share highly similar structures, while the functions and the expression levels of different subtypes vary from one to another in different diseases. Notably, random use of the non-specific inhibitors will result in serious toxic effects. Accordingly, the selective degradation of TAPs can be achieved, so long as such small molecules capable of binding to specific isoform proteins can be applied in the synthesis of PROTACs. To date, the most reported PROTACs for the targeted degradation of receptor-interacting protein kinase (RIPK) [5]and bromodomain-containing protein 4 (BRD4) [15,16]have been prepared to apply as highly selective inhibitors which displayed a high specificity to the degradation of TAPs with a specific subtypes.Consequently, PROTACs have a promising application prospect as a therapeutic strategy, especially for “undruggable proteins”.
3. Existing problems of the PROTAC technology
At present, a large number of research have reported that some PROTACs showed good activities of targeted degradation.Nonetheless, their ultra-high molecular weight leads to poor pharmacokinetics properties, which limited their further application.Additionally, PROTAC incorporates the TAP ligand, joint molecule,and an E3 ubiquitin ligase, the relative molecular weight of this ternary complex is usually over 1 000 Da, which makes it difficult to be absorbed after administration [7,15,17]. More importantly, the off-target probability of PROTAC has become one of the biggest concerns. For example, PROTACs, composed by bestatin eaters and all-trans-retinoic acids (ATRAs), not only degrade retinoic acid receptor but also induce the degradation of AR [13]and ER [14]. As a result, with regard to the synthesis of PROTAC, the specific target binding activity of the small-molecule compounds is another problem that must be paid attention to. This helps to reduce the possibility of off-target effect. Moreover, a certain level expression of some proteins contributes to maintain the basic physiological activitiesin vivo. An excessive degradation of the TAPs may affect the normal physiological activities [13]. Therefore, we should consider all aspects of the design work on PROTACs, includingin vivoabsorption and utilization efficiency, target specificity, and proper concentration in practice to maximize the efficiency of PRTOACs and lower their potential toxicity effects.
4. PROTAC design of bioactive small molecules
PROTAC-ubiquitination design is based on extensive knowledge of QSAR among TAP, L-UBL-E3, and linkers. Variation of any QSAR parameter among the three will result in a significant change of the PROTAC activity. Hence, it is necessary to find a suitable TAP ligand, discover more types of E3 ligases, and clarify linkers in terms of composition, length, and binding sites in the designing of PROTACs. Factors mentioned above will potentially affect the degradation efficiency of TAPs.
4.1 Types of E3 ligases
In recent years, the E3 ligases relating to PROTACs-ubiquitination including mouse double minute 2 homologue (MDM2), cellular inhibitor of apoptosis protein-1 (cIAP1), cereblon, von Hippel-Lindau(VHL) and others [18](Table 1). No specific ligands have been identified for most E3 ligases. For instance, MDM2 ligand of nutlin-3a was used to construct PROTAC, which exhibited AR degradation activity at 10 µmol/L (Fig. 2) [19]. Despite of less active in degrading the TAPs, it has been confirmed that the small molecule based PROTACs is feasible. However, up to date, most identified ligands of MDM2 have been found to show a low degradation efficiency of TAPs due to their large molecular weight. Consequently, there is little research on the construction of PROTACs based on MDM2. Itoh et al. [20]used bestatin, an inhibitor of cIAP1, to develop PROTACs. Results suggested that 75% cellular retinoic acid-binding proteins II of the cells was degraded with the concentration of 10 μmol/L. However, it is often accompanied by an off-target effect because bestatin is also an aminopeptidase inhibitor. Moreover, PROTACs, which were designed with bestatin as E3 ligase ligand, displayed a low degradation efficiency for TAPs and usually requires a higher concentration to maintain its degradation activities [21]. All the above-mentioned drawbacks have restricted the further application of bestatin as an E3 ubiquitin ligand.
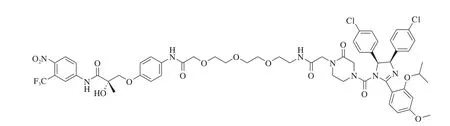
Fig. 2 Structural formula of PROTAC synthesized by an aryl propionamide analog (non-steroidal selective androgen receptor modulator (SARM), selective androgen receptor modulator), polyethylene glycol (PEG) and nutlin.

Table 1Properties of some small molecule PROTACs.
Recently, thalidomide and its derivatives, lenalidomide and pomalidomide (Fig. 3) have been found to bind the E3 ligase cereblon, which has attracted wide attention from researchers.PROTAC ARV-825, a small-molecule compound with pomalidomide as its E3 ligand, can completely degrade BRD4 at a dose of 10 nmol/L for 6 h [22]. Thalidomide, used to prepare a small-molecule PROTAC, degraded 87% BRD4 proteins in human AML leukemia cell line at 100 nmol/L [23]. These findings signify that thalidomide and its derivatives can play a role as E3 ligands in the designing of its corresponding PROTAC. In addition, some small molecules can be competitively bound to VHL. Bondeson et al. [5]developed a small-molecule PROTAC with VHL ligand as E3 ligand. It was observed to exhibit a strong degradation capacity for RIPK2 proteins, with a 50% protein degradation concentration (DC50) of 1.4 nmol/L. In summary,E3 ligase ligands, such as VHL and cereblon, have the advantage of small molecular weight and high degradation efficiency. A majority of PTOTACs with highly targeted degradation efficiency were synthesized using these E3 ligase ligands, such as sirtuin 2 (Sirt2) [24],cyclin-dependent kinase 2/9 (CDK2/9) [25]and TANK-binding kinase 1 (TBK1) [22]. Among them, the targeted degradation efficacy of BRD4 was as low as 30 × 10-12mol/L [26].
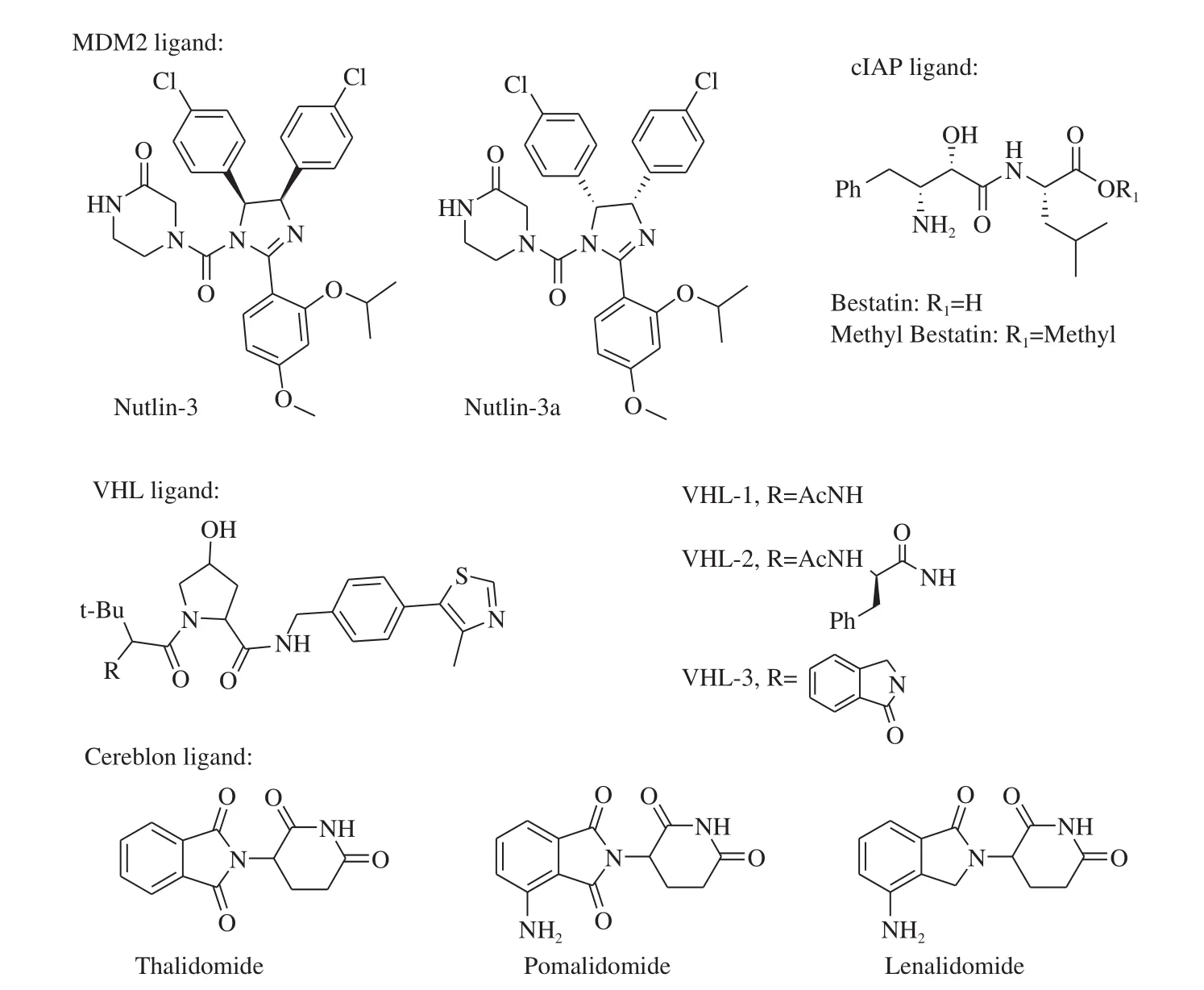
Fig. 3 Major E3 ligase ligands for PROTACs.
It is noteworthy that only a few E3 ligase recruiters have been successfully employed for TAP degradation, including cereblon, VHL,MDM2, cIAP, DCAF15, DCAF16, RNF4, and RNF114 among others [27].Certain proteins were resistant to PROTAC-ubiquitin dependent degradation as particular components of the ubiquitin-proteasome system were absent in the relevant cells [28]. Therefore, expanding the scope of functional E3 ligases for PROTACs is beneficial to extend the application of TAP degradation. Recently, it has been demonstrated that the compound CCW 28-3 is capable of degrading BRD4 in the proteasome- and RNF4-dependent manners [29].Nimbolide, a natural product fromAzadirachta indica, has been confirmed as an E3 ligase recruiter of RNF114 [30,31]. DCAF16 has been recognized as an E3 ubiquitin ligase [32]. Previous studies have also proved that immunomodulatory imide drugs and indisulam as well as its derivates can bind with cereblon and DCAF15 [33].
4.2 Effects of linkers on the degradation efficiency of PROTAC
The linker is another indispensable constituent of PROTAC.Notably, the chemical structure, length, as well as the binding sites of the linker will affect the activity of PROTACs. A series of PROTACs was designed with the linker lengths of 9, 12, 16, 19, and 21 carbon atoms, respectively [34]. The results revealed that the degradation activity on the ER was the strongest when the linker length was 16 carbon atoms. Besides length, the chemical composition of the linker was also an influencing factor on the PROTAC activity. For example, the BRD4 degradation activity of the PROTAC (ARV-825)consisted of polyethylene glycol, showed 10 times higher activity than that of the PROTAC (dBET116) consisted of poly-saturated alkanes [15]. Moreover, it was identified that the PROTAC with the linker’s junction site at C-7 of estradiol had the highest activity in the degradation of estrogen receptor-alpha (ERα) compared with at O-17 or C-16 site of estradiol [35]. In conclusion, the linker’s chemical composition, length, and its binding sites of the target ligands play vital roles in PROTAC’s degradation efficiency.
4.3 Small molecule ligand of the TAP
Since the discovery that PROTAC constructed with nutlin as the ligand of SARM could trigger the TAP degradation [19], a large number of small-molecule compounds have been applied in constructing PROTAC, for instance, bestatin methyl ester (MeBS)(Fig. 3) specifically binding to ATRA and MV1 selectively combining with the BIR3 domain of cIAP1. A few years ago, it was found that some small-molecule ligands were capable of competitively binding to VHL, and the molecular weights of these PROTACs were less than 400 Da [36,37]. This study provided strong support for the synthesis of small-molecule PROTACs based on the E3 ligase of VHL. It was evidenced that estrogen-related receptor α (ERRα) played a major role in regulating cellular energy balance. In 2015, a small nonpeptide VHL type PROTAC, with high affinity and high specificity,was reported to successfully target and degrade ERRα [14], which acted as a pivotal part in regulating the balance of cell energy.PROTAC_ERRα was conjugated with the VHL ligand of thiazolidinone, and the ERR-α level in MCF7 breast cancer cells was observed decreasing in a VHL dose-dependent manner. At a concentration of 100 nmol/L, 50% ERRα was down-regulated by PROTAC_ERRα. Meanwhile, another VHL E3-based small-molecule degradation agent was designed. Which targeted the receptor-interacting protein kinase 2 (RIPK2), serving as an important mediator of the innate immune signal. The RIPK2 ligand was linked to VHL30 by a 12-atom PEG chain, and this PROTAC possessed a high degradation efficiency. It degraded 95% RIPK2 at a concentration of as low as 10 nmol/L, while no degradation activity was detected in its differential isomer PROTAC_RIPK2_epi. These results demonstrated that the E3 ligase ligand was quite crucial in the degradation process. Researchers proved the efficiency of a single PROTAC molecule in mediating the degradation of multiple RIPK2 protein substrates by a radio-labeling assay, and the unique catalytic properties of PROTAC were also validated. Eventually, the limitation of equilibrium occupancy of small molecule inhibitors was overcome through the distinct “event-driven” pharmacological approach of PROTACs [38].
4.4 PROTAC design and optimization
At present, most PROTACs are prepared with the molecules characterized by specific binding properties and low molecular weight. Based on these ideas, the BRD4 degradation agent and CDK9 degradation agent were synthesized, respectively [25,26]. In the meantime, some new type of PRTOACs has also been presented.A potential PROTACs library with various linker lengths/types was generated, linking positions of L-TAP, and L-UBL-E3 (Fig. 4a) [39].This design alleviated the repetitive QSAR screening work of PROTACs. A cereblon homo-PROTAC was also designed and prepared [40]. Both L-UBL-E3 and L-TAP of the PROTAC were the same ligands of cereblon. Accordingly, the application of this homo-PROTAC induced the bidirectional polyubiquitination of cereblons. As a result, both targeted binding cereblons were degraded by proteasomes (Fig. 4b). In photo-PROTACs, a photosensitive group was incorporated to the PROTAC’s UBL-E3, TAP, or the linker. When irradiated with an external light of specific wavelength,the photosensitive group of the photo-PROTAC would be cleaved,thus the inactivated photo-PROTAC was converted into an active PROTAC [41](Fig. 4c).
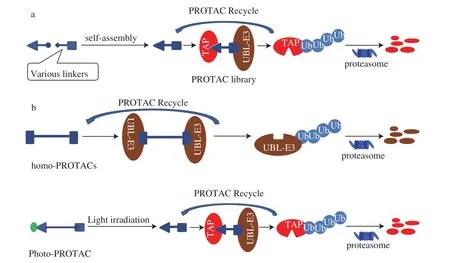
Fig. 4 Principle of the newly appeared PROTACs.
5. The TAP of natural products
Natural products possess special advantages, such as unique structures, good biocompatibility and multiple functionalities among others [70], compared with the chemical synthesized molecules.Therefore, it is of great significance to identify the interaction between bioactive natural products and their potential TAPs, which can clarify the mechanism of action, predict its potential efficacy and the side effects. Accordingly, the corresponding measures should be taken in the structure transformation or the application mode of natural products based on their binding properties.
At present, biotinylated affinity chromatography is the most common method in the target identification of natural products [71]. With the method, the specific combination between trapoxin and its targeted histone deacetylase (HDAC) was discovered [72,73]. Griffith et al. [74]proved that the biotinylated fumagillin could bind to the methionine aminopeptidase of MetAP2 in a covalent irreversible pattern,which inhibited the methionine hydrolase activity of MetAP2.Besides, the TAPs of withaferin A from Solanaceae family [75],adenanthin from the leaves ofIsodon adenanthus[76], triptolide fromTripterygium wilfordii[77], and berberine fromBerberisfamily plants [78]were also confirmed through the modification of biotinylation. However, affinity chromatography assay requires the specific biotinylated modification of natural products, which may affect the original bioactivity, or block the binding of the natural products with its TAPs due to steric hindrance, thus leading to the adverse effect to the final identification result. Therefore,further biochemical and cellular experiments are warranted to verify their binding properties. Owing to the low expression level of some TAPs, A target amplification and recognition method has been developed based on the phage display technology [79], by which, some potential TAPs of camptothecin were speculated [80].Protein microarray is another high-throughput screening method applied in screening the TAPs of natural products [81].UMP/CMP Kinase 1 was confirmed to be the TAP of doxorubicin by this method. Extreme heavy workload in constructing a phage display library or protein microarray for the whole proteome, and different secretion of parts of proteins under these methods are the limiting factors of the wide application of these assays. There were other methods applied in the TAPs’ identification of natural products, such as cellular thermal shift assay (CETSA) [82], target identification by chromatographic co-elution(TICC), and others. But it also has hurdles for a broad application of these methods due to some limitations. Recently, the intermolecular interaction of inverse docking prediction technology is adopted to identify the targets of natural products [83]. However, due to the insufficient information of the molecular structures of the TAPs, the prediction accuracy of molecular docking assay still needs improved.In conclusion, the TAPs of natural products cannot be simply and efficiently identified based on the existing methods. It is necessary to establish a new method in identifying the TAPs of natural products.
PROTAC is a technology based on the affiliation of a ligand for a TAP then degradation of the TAP. The precondition to form a PROTACT molecule from the diverse structure of natural products is that a covalent bond can be formed between the natural product molecule and the linker in the PROTAC molecule. For instance, a natural product with a free hydroxyl, a primary or secondary amino group or carboxylic acid group can form a covalent bond with a linker, thus this natural product can form PROTAC molecules to evaluate its binding capacity toward the TAP. Ursolic acid (UA) is a natural triterpenoid widely distributed in herbs, fruits, and vegetables.To verify its target binding protein, in the previous study [84], we prepared six UA PROTAC units via a chemical combination of UA and L-UBL-E3 of MDM2 with different linkers. The results revealed that compound 1B, connected with a 3-polyoxyether possessed remarkablein vitroantitumor activity (with the IC50value of 0.23-0.39 μmol/L against A549, Huh7, HepG2). Western blot results demonstrated that the administration of compound 1B induced significant degradation of MDM2, and promoted the expression of P21 and PUMA proteins, and thus inhibited the proliferation and promoted the apoptosis of A549 cells. There are numerous natural products containing hydroxyls or amino groups, such as polyphenols,organic acids, some alkaloids alcohols, amino and carboxylic acids among others. Therefore, PROTAC technology can be applied in majority of bioactive natural products in theory.
6. Conclusion
This paper summarized the advances of PROTAC which takes advantage of the inherent ubiquitination systemin vivoin the degradation of TAPs. The technology has started from large molecular weight peptide PROTACs to current many small molecule PROTACs. The weaknesses of low activity and poor trans-membrane properties of the PROTAC were solved by the replacement of peptides with small molecules. The emergence of small-molecule PROTAC has drawn much attention, and a growing number of proteins were successfully degraded via this technology. Furthermore,PROTAC has been validated to possess the unique characteristics of high efficacy, high specificity, sub-stoichiometry, and specific targeting of the traditional “undruggable proteins”. However, many existing drawbacks in the design and synthesis of PROTACs need to overcome. Future research directions include further reducing the relative molecular mass, improving the bioavailability and tissue distribution of PROTACs, and specifying more suitable target ligands as well as diverse E3 ligases. In general, the PROTAC strategy is expected to be a new target identification method, which will create a possibility of solving currently intractable human diseases and have far-reaching implications for future medical development.
Nowadays, there were few reports on the application of PROTAC in identifying the target proteins of natural products. This is mainly due to the newly developed PROTAC surrogate technology.After years of development, much progress has been made in the structure-activity relationship (SAR) between PROTAC units and ubiquitination degradation of the TAPs. Moreover, the emergency of new technologies, such as PROTAC library, has greatly reduced the screening workload of SAR in the application of PROTAC. Hence, it exhibits a good application prospect of TAP identification of natural products based on the PROTAC strategy.
Declaration of competing interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgments
This research work was supported by the key scientific research projects of Hunan Provincial Department of Education of China(No. 19A513), the National Nonprofit Institute Research Grant of CAFINT, China (No. CAFYBB2018GA001) and Grant from Hubei Province, China (GRANT number 2019ABA100).
- 食品科学与人类健康(英文)的其它文章
- Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
- Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria
- Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: biological variation and effects of postmortem ageing and storage

