Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
Yiteng Qiao, Zhihang Qiu, Fengwei Tian, Leilei Yu, Jianxin Zhao,Hao Zhang,d,e, Qixiao Zhai, Wei Chen,d,*
a State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China
c College of Food Science and Engineering, Shandong Agricultural University, Tai’an, Shandong 271018, China
d National Engineering Research Center for Functional Food, Jiangnan University, Wuxi, Jiangsu 214122, China
e Wuxi Translational Medicine Research Center, Jiangsu Translational Medicine, Research Institute Wuxi Branch, Wuxi, China
Keywords:
Pediococcus acidilactici
Bacteriocin
Immune system
Intestinal flora
Regulation
A B S T R A C T
This study was performed to determine the effects of bacteriocin-producing and non-bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice. Two P. acidilactici strains with antibacterial activity (P. acidilactici CCFM28 and CCFM18) were obtained based on the inhibition-zone assay. The produced components were identified as bacteriocins through protease treatment,pH adjustment and hydrogen peroxide treatment. Bacteriocin-producing and non-bacteriocin-producing P. acidilactici strains (P. acidilactici CCFM28, CCFM18 and NT17-3) caused significant changes in serum immune factors and intestinal flora of normal mice. After 14 days of intervention, the relative abundance of Firmicutes was significantly decreased, but that of Proteobacteria was significantly increased at the phylum level. At the genus level, the administration of three P. acidilactici strains resulted in the downregulation of Blautia and the upregulation of Ruminococcus and Lactobacillus. Furthermore, there were also different regulations on some probiotic strains, such as Bifidobacterium, Coprococcus and Akkermansia, which were closely related to the antibacterial ability of the bacteriocin and the type of strain. The results indicated that the intervention of different P. acidilactici strains could differently change the structure of intestinal flora in normal mice, which provided theoretical guidance for the selective use of bacteriocin-producing strains for health regulation in the future.
1. Introduction
Lactobacillusbacteriocins refer to a type of polypeptide or precursor polypeptide with the antibacterial activity that is ribosomally synthesized in the metabolism of lactic acid bacteria [1-4].It has a promising application prospect in the field of food preservation and disease treatment due to its intolerance to protease,inability to develop drug resistance, less accumulation in the body,good stability and high suitability for industrial production [1-4].
Class IIaLactobacillusbacteriocin, with a common sequence of YGNGVXaaC at the N-terminal, is considered to be the most abundant and fully characterizedLactobacillusbacteriocin with low molecular weight, such as pediocin and enterocin. They can not only strongly inhibit the growth of foodborne pathogens (such asListeria monocytogenes), but also show great inhibition against some spoiled lactic acid bacteria, such asBrochotrixspp.,Clostridiumspp.,Bacillusspp. andStaphylococcusspp.. Compared with other bacteriocins, Class IIa bacteriocins have strong antibacterial activity and good physicochemical properties. Therefore, they are currently recognized as the most promising bacteriocins for various industrial applications in the control of food spoilage bacteria and pathogenic bacteria [5].
There are three main ways for bacteriocins to act in the intestine.First, they can promote the colonization of bacteriocin-producing bacteria in the host. Gillor et al. [6]found that colicin-producingEscherichia colicould still be abundant in the large intestine of mice when the streptomycin was used, while the concentration of non-bacteriocin-producingE. colidecreased by 5 lg (CFU/g) in feces on Day 11. The bacteriocin-producingL. salivariusDPC6005 had a large amount of colonization in the intestinal tract of weaned pigs compared with the other 4Lactobacillusstrains administered at the same time [7]. Furthermore, bacteriocin has the ability to directly eliminate pathogenic bacteria in the body. Compared with non-bacteriocin-producing strains, the bacteriocin-producingL. salivariusUCC118 strain showed a significant inhibitory effect onL. monocytogenesin a mouse model [8]. Bacteriocin can also act as signal peptides to regulate the host immune system and other physiological functions [9]. Meijerink et al. [9]reported thatL. plantarumWCFS1 could cause immune responses in host dendritic cells and peripheral blood mononuclear cells, which was changed when the genes related to the synthesis and secretion of bacteriocins were knocked out.
Antibiotics have played an irreplaceable role in inhibiting the proliferation of pathogenic bacteria and reducing the toxicity of their metabolites to humans and animals [10]. However, the excessive and unreasonable use of antibiotics has raised a concern about the development of resistant bacteria, which may result in the transfer of resistant bacteria and their resistance factors, and hinder the treatment of some diseases [11-13]. Furthermore, there was some huge damage due to the residues of antibiotics in the body, such as allergy, cancer and imbalance of intestinal flora. Therefore, several alternatives have been proposed. The bacteriocins produced by lactic acid bacteria are relatively safe and contains multiple types with different antibacterial spectra [14,15], which can be used forin situregulation of microbial community structure in food fermentation [16]. Furthermore, their coding genes can be modified to improve biological activity [17,18]and stability [19]. Numerous studies have been performed to explore the benefits of bacteriocins against various diseases, such as constipation, inflammation and cancer. It has been shown that bacteriocins can effectively improve the disease status via the regulation of intestinal micro flora. However, the effect of bacteriocins on the intestinal flora of normal mice is still poorly understood.Therefore, it is necessary to investigate the effects of bacteriocinproducing and non-bacteriocin-producingPediococcus acidilacticistrains on physical features and intestinal flora composition.
The aims of this study were: 1) to screen the bacteriocinproducingP. acidilacticistrains; 2) to determine the antibacterial spectrum of bacteriocins; 3) to identify the types of antibacterial substances based on physicochemical characteristics;4) to evaluate the effects of bacteriocin-producing and nonbacteriocin-producingP. acidilacticistrains on the immune system of mice; 5) to analyze the changes in the structure of the intestinal microbial community of mice.
2. Materials and methods
2.1 Materials
The kits used to measure the levels of IL-6, IL-10, TNF-α,TGF-β and IFN-α were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China). MRS broth, brain heart infusion agar and LB nutrient agar were provided by Qingdao Hope Bio-Technology Co., Ltd (Qingdao, China). TheP. acidilacticistrains used in this study, includingP. acidilacticiCCFM28,P. acidilacticiCCFM18,P. acidilacticiNT17-3,P. acidilactici102H8,P. pentosaceusCCFM550,P. pentosaceusCCFM551,P. pentosaceusCCFM 552,P. pentosaceusCCFM553,P. pentosaceusCCFM554,Lactobacillussp. andBacillus coagulans,were obtained from the culture collection of food microorganisms of Jiangnan University (Wuxi, China). The indicator strains, includingL. monocytogenesCCFM19,Staphylococcusaureus,Micrococcus luteus,Enterococcusfaecalisand 11 types ofLactobacillus, were provided by Jiangnan University (Wuxi, China).
2.2 Screening of bacteriocin-producing P. acidilactici strains
2.2.1 Antibacterial ability determination using the inhibitionzone assay
A total of 4 pathogenic bacteria and 11 types ofLactobacilluswere used to screen the bacteriocin-producingP. acidilacticistrains and analyze their antibacterial spectrum. TheP. acidilacticistrains andLactobacillusstored in 30% (V/V) glycerol broth at –80 °C were first activated three times under anaerobic conditions at 37 °C for 18 to 24 h with MRS broth.L. monocytogenesCCFM19 strain was coated and activated on brain heart infusion agar, and other three pathogenic bacteria (S. aureus,M. luteusandE. faecalis) were cultivated with LB nutrient agar.
The inhibition-zone assay was used to evaluate the antibacterial ability of 9 strains ofP. acidilactici. Briefly,P. acidilacticistrains were cultured at 37 °C for 24 h and then filtered with a 0.22 µm Millipore filter to remove bacterial deposits. Then, the indicator petri dish was prepared by mixing the activated pathogenic bacteria and sterilized media (brain heart infusion agar and LB nutrient agar) at 60 °C in a ratio of 1:99 (V/V). After the media were solidified and punched, 50 µL of supernatants were poured into the small holes,followed by diffusion at 4 °C for 12 h and cultivation at 37 °C for 24 h.The inhibition zone was measured by Vernier calipers.
2.2.2 Preliminary identification of antibacterial substances
P. acidilacticistrains were cultured at 37 °C for 24 h and then filtered with 0.22 µm Millipore filter to obtain supernatants. An aliquot of bacterial supernatants was transferred into 5 mL of sterile tubes and then treated with pepsin and catalase solutions for 2 h. The antibacterial ability of treated supernatants was analyzed by the inhibition-zone assay.Furthermore, the bacterial supernatants with pH at 4 and 6 adjusted by 1 mol/L HCl and NaOH were used to investigate the effect of pH on the antibacterial ability of supernatants [4].
2.3 Effect of bacteriocin-producing and non-bacteriocinproducing P. acidilactici strains on normal mice
2.3.1 Animals and sample collection
Seven-week-old male BALB/c mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and kept in a standard cage at a temperature of (25 ± 2) °C and a humidity of (50 ± 5)% with a 12-h light-dark cycle. The commercial mouse diet and drinking water were freely provided to mice. All protocols for this study were approved and supervised by the Ethics Committee of Jiangnan University, China (JN. No 20171219–20180129 [169]), and the procedures were conducted according to the European Community guidelines (Directive 2010/63/EU).
Two bacteriocin-producing strains (P. acidilacticiCCFM28 andP. acidilacticiCCFM18) and one non-bacteriocin-producing strain(P. acidilacticiNT17-3) were used for intragastric administration to the mice. Brie fly, theP. acidilacticistrains were activated at 37 °C for 48 h and centrifuged at 5 000 ×gfor 15 min. Then, the obtained deposits were cleaned with phosphate-buffered saline three times,dissolved in the sterile skim milk solution and lyophilized into the powder. Prior to intragastric treatment, the bacterial concentration was adjusted to 9 lg(CFU/mL). The cryopreservation experiment indicated that there was no significant change in the number and vitality of all strains within 2 weeks.
2.3.2 Experimental design
All mice were allowed to adapt to the new environment for 7 days and randomly divided into 4 groups (n= 10 for each group):the normal group (skim milk solution), the group treated withP. acidilacticiCCFM28 strain (9 lg(CFU/mL)), the group treated withP. acidilacticiCCFM18 strain (9 lg(CFU/mL)) and the group treated withP. acidilacticiNT17-3 strain (9 lg(CFU/mL)). An aliquot of 200 µL of skim milk solution or bacterial suspension was provided respectively to mice from the normal group and the treatment groups for 14 days, and their weights were measured daily. After 14 days of treatment, the mice were fasted overnight (approximately 16 h) via cervical dislocation, and eyeball blood was then extracted to measure the level of serum immune factors. Furthermore, the entire intestines were removed to analyze the gastrointestinal transit rate, and the colon and cecum contents were extracted for intestinal genome detection.
2.3.3 Determination of gastrointestinal transit rate
After 14 days of probiotic intervention, the mice were fasted overnight (approximately 16 h) but freely provided with water. Then,an aliquot of 200 µL of activated carbon meal was administered to each mouse in the control group via gavage, and the same volume of the mixture of activated carbon meal and probiotics was provided to each mouse in the treatment group. Thirty minutes later, the mice were killed, and the entire small intestine from the pylorus to the cecum was removed to measure the ink advancement distance and the total length of the small intestine. The gastrointestinal transit rate was defined as the percentage of the ink advancement distance to the total length of the small intestine [20].
2.3.4 Determination of serum immune factors
The levels of IL-6, IL-10, TNF-α, TGF-β and IFN-α in the serum were determined by the enzyme-linked immunosorbent assay (ELISA)instrument according to the manufacturer’s instructions. The obtained eyeball blood was centrifuged at 5 000 ×gfor 15 min under a low temperature to obtain mouse serum. Six serum immune factors were analyzed in 96-well plates with Varioskan LUX multimode microplate reader (Thermo Fisher Scientific Co., Ltd.).
2.3.5 Genome sequencing analysis of intestinal flora
The genomic DNA from the collected fecal samples was extracted using the FastDNA Spin Kit for Soil (MP Biomedical,USA) according to the manufacturer’s instructions. After the obtained microbial genomic DNA was quantified, the V4 region of the 16S rRNA was amplified using the primers (forward primer, 5’-AYTGGGYDTAAAGNG-3’; reverse primer,5’-TACNVGGGTATCTAATCC-3’) by PCR. Different samples were distinguished by a barcode composed of 7 bases that was added to the 5’-end of the upstream primer. The PCR reaction system was carried out in 50 µL of mixture under the following conditions: 95 °C for 7 min, 95 °C for 30 s, 50 °C for 30 s and 72 °C for 50 s, repeat for 30 cycles; and 72 °C for 10 min. After the PCR product was separated with 1.8% agarose gel, the purification and quantification were conducted with the QIAquick Gel Extraction Kit (QIAGEN,Germany) and Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, USA), respectively. Finally, libraries were built using TruSeq DNA LT Sample Preparation Kit (Illumina, USA), and the PCR purified products were sequenced on the Illumina MiSeq 250 platform.
2.3.6 Bioinformatic analysis
After sequencing, the sequences with lengths < 200 bp and primer sequences were removed using the QIIME program. The pair-end reads that overlapped longer than 10 bp and contained no mismatch were assembled according to their overlap sequences. Then, the barcodes and sequencing primers were trimmed from the data, and the sequences were defined as operational taxonomic units (OTUs)when the sequence similarity was greater than 97% . The Ribosomal Database Project (RDP) Naive Bayes classifier was used to classify different OTUs and annotate the taxonomic information for each OTU representative sequence [21,22]. Furthermore, the alpha diversity indexes, including Chao1 index, Observed-species, PD-whole-tree,Shannon index and Simpson index, were calculated to evaluate the diversity of the microbial community using QIIME. The similarity among the microbial communities was analyzed based on unweighted UniFrac distance and visually presented by principal coordinate analysis (PCoA).
2.4 Statistical analysis
All data are presented as mean ± standard deviation for each group. The differences between different groups were analyzed by one-way analysis of variance with Duncan’s multiple range test atP< 0.05.
3. Results
3.1 Screening of strains with antibacterial activity by the inhibition-zone assay
The antibacterial ability of 64 probiotics was evaluated usingL. monocytogenesCCFM19 strain as the indicator bacteria based on the agar plate diffusion method. This method could qualitatively and quantitatively analyze the antibacterial ability of microbial strains based on the inhibition-zone assay. As showed in Table 1, twoP. acidilactici strains exhibited significant inhibitory activity againstL. monocytogenesCCFM19, and the inhibition zone ofP. acidilacticiCCFM28 strain(20.1 mm) was significantly greater than that ofP. acidilacticiCCFM18(12.0 mm) (P< 0.05). However, no apparent antibacterial effect was observed in the fermentation broth of other strains (includingP. acidilactici,P. pentosaceus,B. coagulansand multipleLactobacillus).This indicated that these twoP. acidilacticistrains produced bioactive components that could inhibit the growth ofL. monocytogenes.
The antibacterial spectrum ofP. acidilacticiCCFM28 andP. acidilacticiCCFM18 strains was analyzedin vitrousing a variety of pathogenic bacteria andLactobacillus, withP. acidilactici102H8 andP. acidilacticiNT17-3 strains used as the control group. Apart fromL. monocytogenesCCFM19,P. acidilacticiCCFM28 andP. acidilacticiCCFM18 strains had an inhibitory effect onE. faecalis,and the inhibition zones were 16.1 mm and 12.6 mm, respectively.Furthermore, 4 of 11Lactobacillus(L. thermophiles,L. salivarius,L. helveticusandL. delbrueckii) were differently inhibited by these twoP. acidilacticistrains, and the inhibition radius was in the range of 10.8–16.1 mm forP. acidilacticiCCFM28 strain and 9.2–12.6 mm forP. acidilacticiCCFM18 strain, respectively. However, there was no antimicrobial activity ofP. acidilactici102H8 andP. acidilacticiNT17-3 strains against the tested strains. The above results indicated that the bioactive components produced byP. acidilacticiCCFM28 andP. acidilacticiCCFM18 strains were not broad-spectrum antibiotics, and they only had inhibitory effects on specific strains.
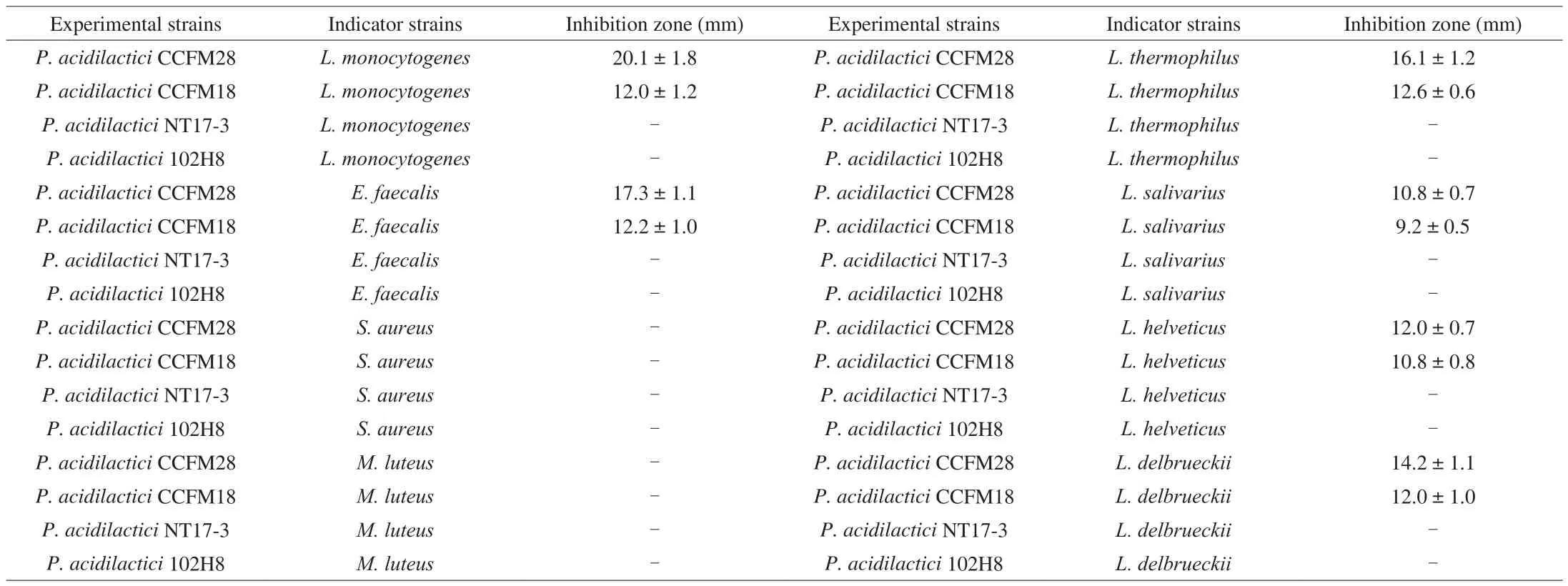
Table 1The antibacterial activity of probiotics based on the inhibition-zone assay.
3.2 Preliminary identification of antibacterial substances
As presented in Fig. 1, the inhibitory activity of the fermentation supernatant ofP. acidilacticistrains had no significant change after pretreatment with catalase. This indicated that the antibacterial effect of twoP. acidilacticistrains was not attributed to hydrogen peroxide.However, the incubation of the fermentation supernatant with pepsin resulted in the loss of inhibitory activity. Therefore, the protein or peptide played a key role in the antibacterial activity of these twoP. acidilacticistrains. Moreover, when the pH of the fermentation supernatant ofP. acidilacticiCCFM28 andP. acidilacticiCCFM18 strains was adjusted from 4 to 6, the inhibition radius changed from 20.1 mm and 12.0 mm to 19.6 mm and 11.5 mm, respectively, with on significant decrease(P> 0.05). This indicated that the organic acids derived fromP. acidilacticistrains were not responsible for the antibacterial activity.

Fig. 1 The antibacterial activity of sterile fermentation supernatant of P. acidilactici strains after pretreatment with pepsin and catalase. (A) Blank control,(B) catalase treatment, (C) pepsin treatment.
According to the above results, we preliminarily concluded that the antibacterial activity produced byP. acidilacticiCCFM28 and CCFM18 strains was achieved by the secretion of bacteriocins.
3.3 The changes in the body weight of mice
The body weight of mice in the normal group and the three treatment groups on Day 14 increased significantly by 12.87% –19.82% (P< 0.05) compared with Day 0. The largest increase occurred in the mice treated withP. acidilacticiCCFM28 strain,followed by mice treated withP. acidilacticiCCFM18 strain.However, there was no significant difference between mice in different treatment groups on Day 0 and Day 14, respectively(P> 0.05). This indicated that the administration of bacteriocinproducingP. acidilacticistrains could promote the growth of mice to a certain extent due to changes in the intestinal flora.
3.4 Effect of P. acidilactici strains on gastrointestinal transit rate of mice
The gastrointestinal transit rate of mice treated withP. acidilacticiCCFM28 strain was the highest (70.91% ), followed by mice treated withP. acidilacticiCCFM18 strain (70.55% ) and mice in the normal group (69.34% ). The administration ofP. acidilacticiNT17-3 strain reduced the gastrointestinal transit rate of mice, compared with the normal group. However, there was no significant difference in the gastrointestinal transit rate of the mice in the 4 groups (P> 0.05).The results indicated that the gastrointestinal transit rate of mice was limitedly enhanced due to the treatment of the bacteriocin-producingP. acidilacticistrains.
3.5 The changes in the serum immune factors of mice
Six cellular immune factors, including pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and anti-inflammatory cytokines(TGF-β1, IL-10, IFN-α), were measured to investigate the effect ofP. acidilacticistrains on the immune system of mice, and the results were shown in Fig. 2. TNF-α is considered to be the earliest and most important cellular mediator in response to inflammation.It can activate neutrophils, regulate the metabolic activity of human tissues, and promote the synthesis and release of other cytokines and lymphocytes. Compared with the normal group, the level of TNF-α in the serum of the mice was downregulated due to the administration ofP. acidilacticistrains, where the mice treated withP. acidilacticiNT17-3 strain had the lowest TNF-α content (449.65 ng/L). IL-6 is able to induce B cells and T cells to differentiate and produce antibodies, and regulate the body’s immune response. The treatment of threeP. acidilacticistrains resulted in the upregulation of the level of IL-6 in the serum of mice (6.97% –14.24% ), and only the change inP. acidilacticiCCFM18-treated group was significantly different(P< 0.05). IL-1β is also a pro-inflammatory cytokine and show a different change in three treatment groups. A slight decrease (0.64% and 1.61% ) in the level of IL-1β occurred in the mice treated with bacteriocin-producingP. acidilacticiCCFM28 andP. acidilacticiCCFM18 strains, while the value increased by 11.72% due to the treatment ofP. acidilacticiNT17-3 strain. Three anti-inflammatory cytokines, including TGF-β1, IL-10 and IFN-α, play an important role in the regulation of cell growth, differentiation, inflammatory response and immune function. The level of TGF-β1 decreased by 6.50% compared with the normal group while the content of IL-10 and IFN-α increased by 12.78% and 14.00% , respectively when theP. acidilacticiCCFM28 strain was provided. However, these three parameters in the serum of mice were upregulated due to the treatment ofP. acidilacticiCCFM18 andP. acidilacticiNT17-3 strains.
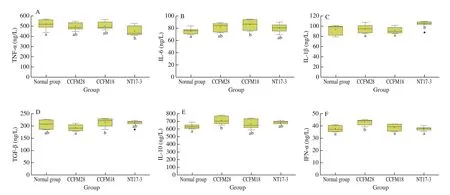
Fig. 2 The serum immune factors of mice in different groups. Normal group: no treatment; CCFM28: treatment with P. acidilactici CCFM28 strain; CCFM18:treatment with P. acidilactici CCFM18 strain; NT17-3: treatment with P. acidilactici NT17-3 strain. “” represents the anomalous value.
3.6 Effect of P. acidilactici strains on the alpha and beta diversities of fecal microbiota
The genome of fecal microbiota was sequenced based on the Illumina MiSeq 250 platform to explore the effect ofP. acidilacticistrains on the microbial community of the mouse intestinal tract.A total of 491 950 high-quality 16S rRNA gene sequences were generated from 50 fecal samples, and the average sequence read was 9 839 for each sample. At a 97% sequence identity cutoff, the clustering of the sample sequences with the representative sequences yielded 488–788 OTUs.
The alpha diversity index describes the abundance and diversity of the microbial community in an individual sample, such as Chao1 index, Coverage, Observed species, Shannon index, Simpson index and PD_whole_tree. The Coverage that reflected the coverage rate of each sample library was greater than 90% for 4 groups, indicating that the sequencing result in this study had high authenticity and credibility. According to Table 2, the total number of species(Chao1 index) increased significantly from 5 333.08 in the normal group to 8 715.17, 15 939.45 and 14 225.42 in the mice treated withP. acidilacticiCCFM28 strain,P. acidilacticiCCFM18 strain andP. acidilacticiNT17-3 strain, respectively (P< 0.05). However, no obvious changes occurred in the microbial diversity of mice. It could be seen from the Shannon index and the Simpson index that the administration of threeP. acidilacticistrains led to a slight decrease in the diversity and uniformity of the microbial community, with no significant difference (P> 0.05). Therefore, although there was an evident increase in the number of species in the three treatments, the diversity and uniformity slightly decreased.

Table 2Alpha diversity indexes of microbial community.
The beta diversity reveals the differences and similarities in the microbial community among different samples. Fig. 3 showed the PCoA based on the unweighted uniFrac matrix of the beta diversities in the gut microbiota of mice treated with the threeP. acidilacticistrains. The first principal components in the distribution map were 22.8% (PC1) and 15.6% (PC2). The microbial communities of the three groups were not clearly separated, indicating that there were only limited changes in the gut bacteria of mice due to the treatment of threeP. acidilacticistrains.
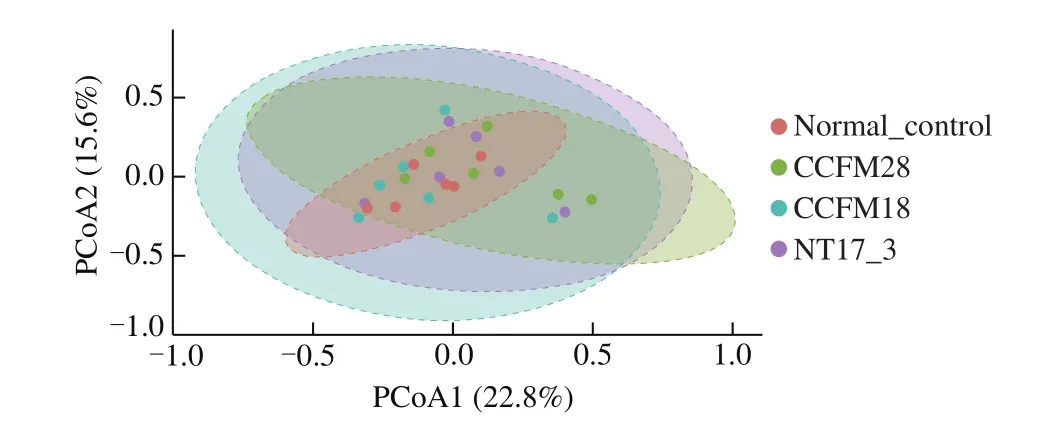
Fig. 3 Principal component analysis of microbial communities in mice feces.
3.7 Effect of P. acidilactici strains on the composition of fecal microbiota at the phylum level
As shown in Fig. 4, the administration of threeP. acidilacticistrains led to a certain change in the microbial community of mice.Firmicutes (68.62% ) was the most dominant phylum in the microbial community of mice in the normal group, followed by Proteobacteria(16.00% ), Bacteroidetes (8.79% ) and Actinobacteria (4.46% ).There were also other phyla with low relative abundance (1% ),such as Tenericutes and Verrucomicrobia. Although the intestinal flora of mice in theP. acidilactici-treated groups was dominated by Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria, the relative abundance had a significant difference. After 14 days of treatment, the relative abundance of Firmicutes significantly decreased from 68.62% to 51.86% , 62.47% and 50.71%in mice treated withP. acidilacticiCCFM28 strain,P. acidilacticiCCFM18 strain andP. acidilacticiNT17-3 strain, respectively (P< 0.05). In contrast, a significant increase (from 16.00% to 27.35% –30.85% ) in the relative abundance of Proteobacteria occurred in theP. acidilactici-treated groups, compared with the control group. Moreover, the microbial community of mice treated byP. acidilacticiCCFM28 strain showed an increase in the level of Bacteroidetes and a decrease in the relative abundance of Actinobacteria. However, these two phyla had opposite changes in the gut flora of other treatments, respectively.
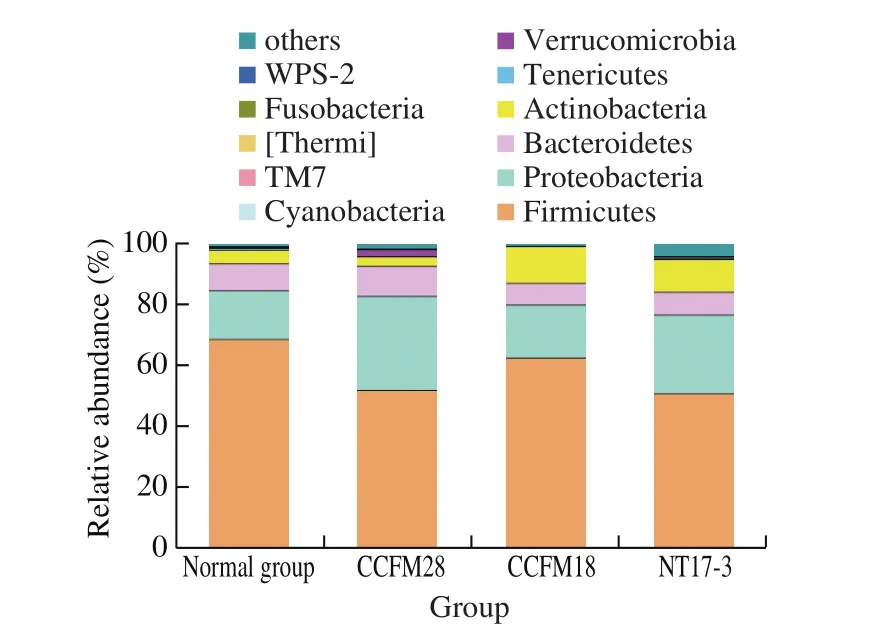
Fig. 4 Relative abundance of main phyla in the microbial community of mice in different treatment groups.
Some studies reported that the changes in Firmicutes and Bacteroides have a great relationship with obesity and metabolic diseases [23,24]. Firmicutes is the most numerous and diverse Grampositive bacteria in the intestine, which is mainly composed of Clostridia, Bacilli and Mollicutes. Compared with the control group,the relative abundance of Firmicutes in the intestinal flora of obese mice increased significantly, while the level of Bacteroides decreased significantly. Therefore, the proportion of Firmicutes and Bacteroides could reflect the health of the individual. In this study, the ratio of Firmicutes to Bacteroides in the intestinal flora of normal mice was 7.81, which significantly reduced to 5.23 due to the intervention ofP. acidilacticiCCFM28 strain, indicating that this probiotic might contribute to the host’s intestinal health and metabolic balance.The administration of non-bacteriocin-producingP. acidilacticiNT17-3 strain also decreased the ratio of Firmicutes to Bacteroides(6.75). However, this phenomenon did not occur in theP. acidilacticiCCFM18 strain-treated group.
Therefore, the intervention of bacteriocin-producing and nonbacteriocin-producingP. acidilacticistrains greatly affected the microbial community structure of intestinal flora of mice at the phylum level. This effect might come from the direct inhibition of probiotics or bacteriocins on specific microbes or the feedback effects on other phyla.
3.8 Effect of P. acidilactici strains on the composition of fecal microbiota at the genus level
The microbial community of intestinal flora of mice in the normal group was mainly dominated byBlautiaand unknown genera (belonging to Clostridiaceae, Enterobacteriaceae and Ruminococcaceae), with a total of 50.64% classified into these genera (Fig. 5). However, the most dominant genera became unknown genera (belonging to Clostridiaceae, Enterobacteriaceae and Ruminococcaceae) andBacteroidesforP. acidilacticiCCFM28-treated group. Apart from two unknown genera (belonging to Clostridiaceae and Enterobacteriaceae),BifidobacteriumandLactobacilluswere found to be the most predominant genera inP. acidilacticiCCFM18-treated group andP. acidilacticiNT17-3-treated group, respectively. The changes of these dominant genera were further analyzed to explore the effect ofP. acidilacticistrains on the composition of fecal microbiota. Enterobacteriaceae is a Gramnegative bacillus, which is considered as an important type of bacteria that inhabits the human intestine. The relative abundance of the genus from Enterobacteriaceae significantly increased from 10.53% in the control group to 23.98% , 13.51% and 15.33% in threeP. acidilacticitreated groups, respectively (P< 0.05). The most significant increase in theP. acidilacticiCCFM28-treated group might be attributed to the suppression of the relative abundance of other flora in the intestinal tract.
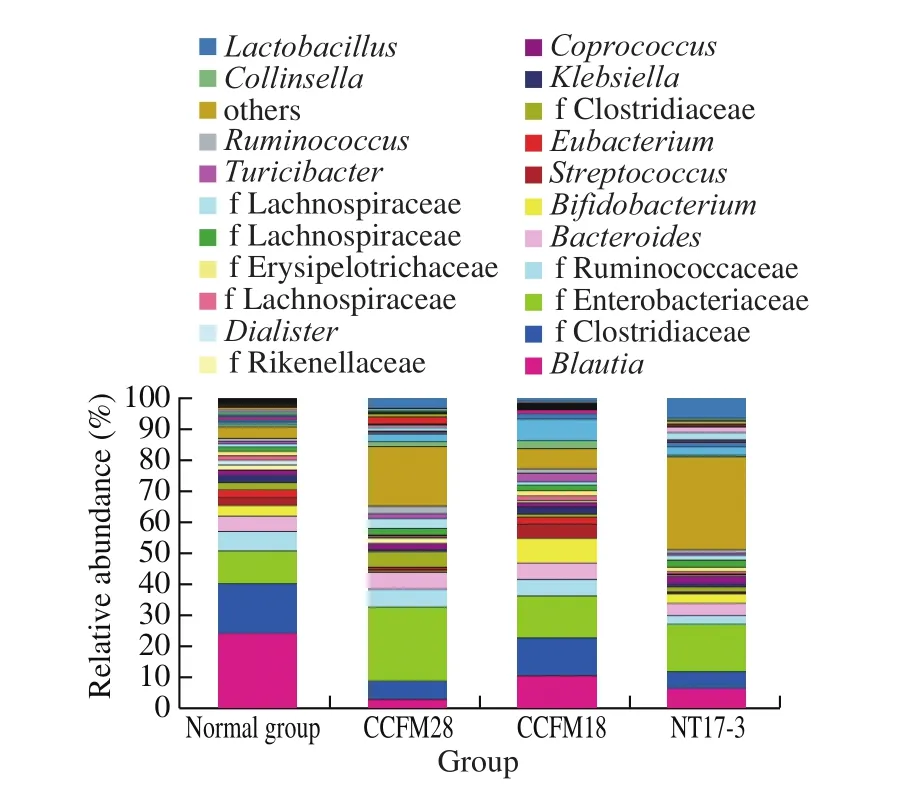
Fig. 5 Relative abundance of main genera in the microbial community of mice in different treatment groups.
It was reported thatBlautiawas related to irritable bowel syndrome. The administration of threeP. acidilacticistrains resulted in a significant decrease in the relative abundance ofBlautia(from 24.10% to 2.79% –10.45% ) (P< 0.05). The strongest antibacterial activity produced byP. acidilacticiCCFM28 strain caused the greatest decline in the level ofBlautia. A significant increase in the level ofRuminococcus(33.17% –161.87% ) occurred in the mice provided with threeP. acidilacticistrains, which contributed to the production of short-chain fatty acids and the inhibition of pathogens in the intestine. The administration ofP. acidilacticiCCFM28 strain caused the greatest increase, followed byP. acidilacticiCCFM18 strain.Lactobacillus(belonging to Firmicutes) is an anaerobic or facultative anaerobic Gram-positive bacteria that can inhibit the growth of pathogenic bacteria, enhance human immunity, protect the gastric mucosa, improve the intestinal tract function and prevent cancer [25]. There was a significant increase (from 0.15% to 1.81% –8.83% ) in the level ofLactobacilluswhen threeP. acidilacticistrains were provided (P< 0.05). Although the growth of 4 kinds ofLactobacilluswas inhibitedin vitroby the sterile fermentation supernatant ofP. acidilacticistrains, the relative abundance ofLactobacillusgenus was increased in the intestine. The reason might be that the application ofP. acidilacticistrains changed the internal competition ofLactobacillus. Furthermore, some probiotics, such asBifidobacterium,CoprococcusandAkkermansia, were differently regulated by two bacteriocin-producingP. acidilacticistrains [26].For example, onlyP. acidilacticiCCFM18 strain could increase the relative abundance ofBifidobacteriumand failed to upregulate the level ofCoprococcusandAkkermansia. Therefore, although bothP. acidilacticistrains could produce bacteriocins, they differed in the regulation of intestinal flora (especially at the genus level). This might be caused by the antibacterial ability of bacteriocins produced by twoP. acidilacticistrains.
According to the above results, we found that the administration of bacteriocin-producing and non-bacteriocin-producingP. acidilacticistrains resulted in a significant change in the intestinal flora of normal mice at the genus level. This effect was closely related to their antibacterial ability. Therefore, the regulation of probiotics on the intestine and immunity system was greatly affected by the type and antibacterial ability of bacteriocins via direct and indirect ways. The obtained results provided a basis for the selective use of bacteriocin-producing strains in host health regulation in the future.
4. Discussion
Since the discovery of Nisin, researchers have discovered and characterized a large number of bacteriocins. The bacteriocins synthesized by lactic acid bacteria are mainly divided into three categories: Class I bacteriocin (a polypeptide produced by the ribosome and modified post-translationally, such as nisin, enterocin AS-48 and subtilosin A), Class II bacteriocin (without posttranslational modification after ribosome production, such as pediocin PA-1, lactococcin Q and lacticin Q), Class III bacteriocin (without modification after ribosome production, such as bacteriolysins) [27].
In recent years, the studies on bacteriocins mainly focus on the following three aspects: 1) screening of bacteriocin-producing lactic acid bacteria and evaluation of antibacterial ability; 2) application of bacteriocins in food preservation and disease treatment as an alternative to antibiotics; 3) bioengineering of bacteriocins. However,there are still few studies on the effect of bacteriocin-producing lactic acid bacteria on the structure of intestinal flora, physiological activity and immune indicators of normal mice. In this study, bacteriocin-producing lactic acid bacteria were screened from 64 probiotics using the agar plate diffusion method, withL. monocytogenesCCFM19 strain as the indicator bacteria. TwoP. acidilacticistrains(P. acidilacticiCCFM18 andP. acidilacticiCCFM28) had significantly inhibitory activity againstL. monocytogenesCCFM19,and the protein or peptide was responsible for the antibacterial activity according to the results of protease treatment, hydrogen peroxide treatment and pH adjustment. Therefore, threeP. acidilacticistrains (P. acidilacticiNT17-3 used as the control) were used to investigate the effects of bacteriocin-producing and non-bacteriocinproducingP. acidilacticistrains on the physiological activity, immune characteristics and intestinal flora of normal mice. The results showed that although the intervention of bacteriocin-producingP. acidilacticistrains could partly increase the body weight and gastrointestinal transit rate of normal mice compared with the normal group, no significant differences were found (P> 0.05). This indicated that the bacteriocin-producingP. acidilacticistrains could not affect the physiological activity of normal mice. Furthermore, threeP. acidilacticistrains caused the up-regulation of IL-6, IL-10 and IFN-α and the down-regulation of TNF-α in mouse serum. However,the levels of IL-1β and TGF-β1 were regulated differently. Therefore,the treatment of the threeP. acidilacticistrains influenced the immune status of normal mice.
After 14 days of intervention, the microbial community structure of intestinal flora in normal mice had some changes, where the total number of species increased, while the diversity and uniformity slightly decreased. At the phylum level, the relative abundance of Firmicutes significantly decreased by 24.42% , 8.96% and 26.10% forP. acidilacticiCCFM28 strain,P. acidilacticiCCFM18 strain andP. acidilacticiNT-13 strain-treated groups, respectively (P< 0.05). Comparatively,the level of Proteobacteria significantly increased by 8.46% –92.83% compared with the normal group (P< 0.05). Umu et al. [28]reported that the microbial community structure of gut flora in normal mice was not strongly affected byLactobacillusproducers of various Class II bacteriocins (i.e., SakA, PedPA-1, enterocins [Q and L50],plantaricins [EF and JK]and GarML), which was inconsistent with the results of this study. The phenomenon could be caused by different experimental strains, different mouse lines and different treatment methods. Furthermore, a significant decrease was observed in the ratio of Firmicutes to Bacteroides in the intestinal flora ofP. acidilacticiCCFM28 strain-treated group, butP. acidilacticiCCFM18 strain failed to achieve this effect. This indicated that the difference in the antibacterial spectrum and antibacterial ability of bacteriocins produced by lactic acid bacteria might have different effects on the structure of the intestinal flora of mice. At the genus level, there were also some differences in the regulation of intestinal flora, such as the effect onBifidobacterium,CoprococcusandAkkermansia. The obtained results provide a basis for the selective use of bacteriocin-producing strains in the regulation of host health in the future.
5. Conclusion
Here, the bacteriocin-producingP. acidilacticistrains were screened, and their effects on the immune system and intestinal flora of normal mice were investigated. TwoP. acidilacticistrains(P. acidilacticiCCFM28 and CCFM18), had significant antibacterial activity againstL. monocytogenesand several types ofLactobacillus.The produced components were identified as bacteriocins according to protease treatment, pH adjustment and hydrogen peroxide treatment. ThreeP. acidilacticistrains (two bacteriocinproducingP. acidilacticistrains and one non-bacteriocin-producingP. acidilacticistrain) caused significant changes in serum immune factors and intestinal flora of normal mice. After 14 days of intervention, the relative abundance of Firmicutes was significantly decreased, but that of Proteobacteria was significantly increased at the phylum level. At the genus level, the administration of threeP.acidilacticistrains resulted in the downregulation ofBlautiaand the upregulation ofRuminococcusandLactobacillus. Furthermore,there were also different regulations on some probiotic strains, such asBifidobacterium,CoprococcusandAkkermansia, which was closely associated with the antibacterial ability of the bacteriocin and type of strain. The results indicated that the intervention of differentP. acidilacticistrains could differently change the structure of intestinal flora in normal mice, which could guide us to selectively use bacteriocin-producing strains for host health regulation in the future.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics Statement
All protocols for this study were approved and supervised by the Ethics Committee of Jiangnan University, China (JN. No 20171219–20180129 [169]), and this experiment was conducted according to the European Community guidelines (Directive 2010/63/EU).
Acknowledgements
This work was supported by the National Natural Science Foundation of China Program (32021005); the Natural Science Foundation of Jiangsu Province (BK20200084); Projects of Innovation and Development Pillar Program for Key Industries in Southern Xinjiang of Xinjiang Production and Construction Corps(2018DB002); National First Class Discipline Program of Food Science and Technology (JUFSTR20180102); the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
- 食品科学与人类健康(英文)的其它文章
- Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
- Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria
- Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: biological variation and effects of postmortem ageing and storage

