Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
Wei Xiao, Qingsong Zhang, Leilei Yu,c, Fengwei Tian,c, Wei Chen,d,e, Qixiao Zhai,c,*
a State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c International Joint Research Laboratory for Probiotics at Jiangnan University, Wuxi 214122 China
d National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
e Beijing Innovation Center of Food Nutrition and Human Health, Beijing Technology and Business University (BTBU), Beijing 100048, China
Keywords:
Vegetarian diet
Gut microbiota
Microbial metabolites
Intestinal physiology
A B S T R A C T
People are increasingly aware of the role of vegetarian diets in modulating human gut microbial abundance and intestinal physiology. A plant-based diet is thought to benefit host health by contributing to establish a diverse and stable microbiome. In addition, microbe-derived metabolites of specific nutrients known to be abundant in vegetarian diets (such as indigestible carbohydrates, arginine, and others) are important to promote effective intestinal immune responses, maintain intestinal barrier function, and protect against pathogens.This review explores the characteristics of the gut microbiome formed by vegetarian diets and the effects of diet-associated nutrients on intestinal microbial abundance. The interactions between the microbe-derived metabolites of vegetarian diet-associated nutrients and intestinal physiology are also discussed.
1. Introduction
Vegetarian diet is one that strictly or partially excludes products of animal origin [1]. Vegetarianism can be further subdivided into 7 categories according to whether seafood, eggs or dairy are also excluded: veganism, lacto-ovo vegetarianism, ovo-vegetarianism,lacto-vegetarianism, semi-vegetarianism, raw veganism, and pescetarianism [2,3]. Table 1 presents the characteristics of 8 dietary patterns and their total scores for the Healthy Eating Index 2010 (HEI)and Mediterranean Diet Score (MDS).

Table 1Characteristics of the 8 dietary patterns and their total scores for HEI and MDS.
With the increasing popularity of vegetarianism, vegetarian diets have become increasingly regarded as healthy and potentially medically beneficial dietary option [4]. Several epidemiological studies support the benefits of vegetarian diets. For example,compared to omnivores, vegetarians have lower body mass index,lower serum cholesterol levels, reduced incidence of diabetes, and lower blood pressure [1,4,5]. Furthermore, the increased intake of plant-derived nutrients, such as dietary fiber, antioxidants, and so on,has been related to a low incidence of cardiometabolic risk and other chronic diseases [1,5,6].
The American Dietetic Association has reported that a wellplanned vegetarian diet can not only lead to significant health benefits,but also meet the nutritional needs of all age groups [3]. Table 2 shows the average daily nutritional intake across both omnivorous and vegetarian diets. Total carbohydrates intake was of the same magnitude in the two diets, but the vegetarian diets contained more non-carbohydrates nutrients than the omnivorous diet [2]. In addition,compared with omnivorous diet, vegetarian diets consume lower total fat and higher unsaturated fat [2]. Furthermore, vitamin C intake by omnivores is lower than that of vegetarians [7]. Similar results were found for vitamin A, folate, calcium, and magnesium, with a high intake being seen in vegetarian diets [2,8]. Additionally, the intake of phytochemical phytosterols in vegetarian diets was higher than omnivorous diet [9,10]. Interestingly, Burkholder-Cooley et al. [9]observed high flavonoids intake in the vegetarian diets, while total polyphenols intake was not as good as that of omnivorous diet. It may be expected that vegetarians consume more fruits and vegetables that were good sources of flavonoids, while non-vegetarians consume more coffee which is another source of phenolic acids belonging to another main type of polyphenols [9].
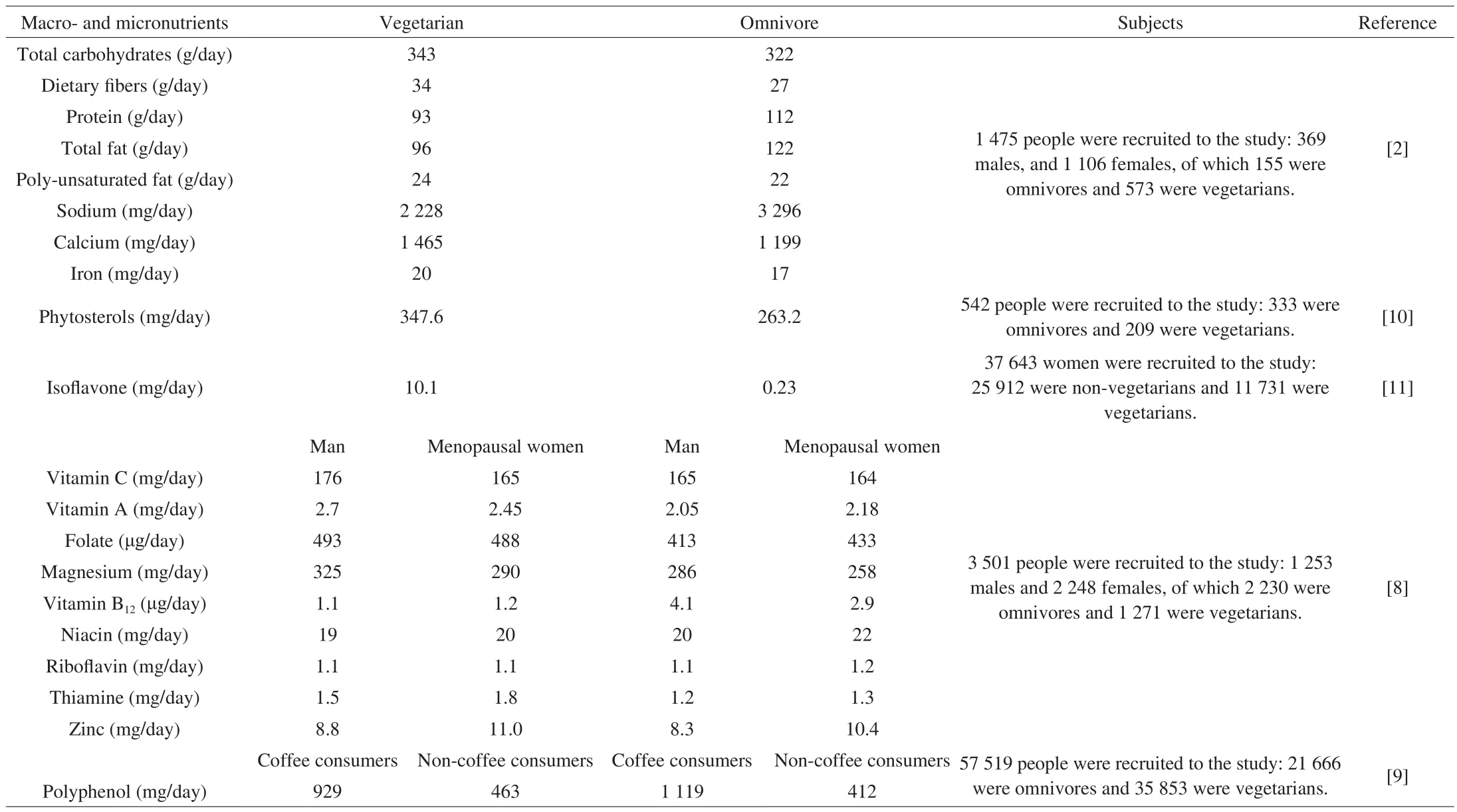
Table 2Comparison of average daily nutritional intake of vegetarians and omnivores.
A vegetarian diet significantly impacts the relative abundance of gut microbiota at the genus, species, and even strain levels [12-15].Moreover, a vegetarian diet may be a potential nutritional therapy for the regulation of intestinal health and protection against inflammatory responses [15]. In recent years, mostly reviews have focused on the vegetarian diets only investigated the impacts of adopting plant-rich diets on the prevention or treatment of chronic diseases or discussed the nutritional value of vegetarian diets [3,16,17]. Few researchers pay attention to the impact of vegetarian diets on the intestinal tract itself. Therefore, this review summarizes the impact of vegetarian diet-derived nutrients on gut microbial abundance. The role of the nutrients and microbial metabolites of the nutrients in the protection against intestinal disease and maintenance of intestinal physiology is also reviewed.
2. Effects of vegetarian diet-derived nutrients on the intestinal microbiome
Vegetarian diets can strongly affect the relative abundance of certain genera, and significantly increasing the gut microbial diversity(Fig. 1). These regulatory effects can be summarized as follows:1) increasing the abundance of bacteria that ferment dietary fiber, such asClostridium[15,18,19],Lactobacillus[20,21],Ruminococcus[13,14,21],Eubacterium rectale[13,19], andFaecalibacterium prausnitzii[14,19,22,23],2) reducing the proportion ofBacteroides/Prevotella[23-28], and 3) lowering the abundance ofBifidobacterium[13,18,27,29].In addition, theα-diversity index of the gut microbiome in vegetarians was significantly higher than omnivores (P< 0.05), while there was no obvious statistical difference in theβ-diversity index [30].In addition, multiple studies have reported similar findings that gut microbiotaα-diversity in vegetarians was significantly higher than in omnivores [14,15,22,31].

Fig. 1 The impact of vegetarian and omnivorous diets on the intestinal microbiome. Compared with omnivores, vegetarians have higher intestinal microbiome diversity, higher abundance of Prevotella, Clostridium, Lactobacillus, Ruminococcus, E. rectale, and F. prausnitzii, and a lower abundance of Bacteroides and Bifidobacterium in the intestine. Black arrows before bacterial genus indicate the increased or decreased abundance in vegetarians compared to omnivores.
Vegetarian diets contain fewer ultra-processed foods, characterized by high bioavailability nutrients which are easily assimilated in small intestine [32]. Thus, the nutrients consumed in the vegetarian diets are easily reaching the colon intact, and are well utilized by microorganisms in the colon [33]. For example, a typical vegetarian diet provides the colonic microbiota with nearly 5-15 g of protein, 60 g of fermentable carbohydrates and 5-10 g of lipid [2]. Furthermore, the existence of a connection between the composition of the nutrients and gut microbiota is well established [33]. Vegetarian diets are characterized by a higher intake of dietary fiber, plant protein, flavonoids, vitamin A and calcium, as well as a lower intake of fat. The different contents of macronutrients, along with various micronutrients in vegetarian diets compared with other diets, can partially explain the influences of vegetarian diets on intestinal microbial abundance. The effects of these nutrients rich in vegetarian diets on the abundance of intestinal microorganisms are listed in Table 3.
2.1 Dietary fiber
Although the total amount of carbohydrate intake of vegetarians has been reported to be similar to omnivores, the daily intake of dietary fiber by vegetarians was found to be higher than by omnivores [2].As the major energy and carbon source, dietary fiber plays a key role in the colonization and growth of intestinal microorganisms [53]. For instance, high dietary fiber has been shown to significantly increase the intestinal microbial abundance. Twenty-seven irritable bowel syndrome and 6 healthy subjects were randomly allocated one of two 21-day provided diets, differing only in FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols)content (3.05 g/day vs 23.7 g/day). The results showed that the high FODMAP diet was associated with significantly increased total bacterial abundance [34]. Furthermore, Duncan et al. [36]provided 19 healthy and obese subjects 3 different diets: high carbohydrate content (399 g/day), medium carbohydrate content (164 g/day) and low carbohydrate content (24 g/day) each for 4 weeks. The abundance ofRoseburiaspp. andE. rectalin the gut increased significantly with increasing intake of fermentable carbohydrates. Cotillard et al. [35]allowed 49 obese patients to consume a high dietary fiber diet and found that the number of total microbial gene expression increased significantly.
Sources of dietary fiber include soybean, wheat bran, inulin,pectin, cellulose, resistant starch, etc, and dietary fiber from different sources can selectively stimulate the growth and activity of intestinal microbes. For example, wheat bran and cereal products can significantly increase the abundance ofLactobacillusin the gut [54,55]. Other types of dietary fiber, such as inulin, can also increase the abundance ofLactobacillus. Kim et al. [56]provided 1.5 g of inulin to 14-week infants for 3 weeks and found a significant increase in the level ofLactobacillusandBifidobacteriumin the intestine. Another two studies showed similar results [57-60].Resistant starch 2 and resistant starch 3 can significantly increase the abundance ofR. bromiin subjects [61-63]. In general, the dietary fiber may increase the intestinal microbial abundance,especially the abundance ofRoseburiaspp.,E. rectal, LactobacillusandRuminococcus.The influence of dietary fiber on intestinal microorganisms is similar to that of a vegetarian diet.
2.2 Fat
There was also significant diversity in the specific fat levels between the two diets, with unsaturated fat content in the vegetarian diets being significantly higher than that in omnivorous diet [2]. In the study of Fava et al. [38]88 subjects at increased metabolic syndrome risk were randomly allocated one offive 24-week diets: high MUFA/GI;high MUFA/low GI; high CHO/high GI; and high CHO/low GI.In the high monounsaturated fat/high glycemic index group 10% total energy was derived from saturated fatty acids and 20% was derived from monounsaturated fat. They found that the abundance ofF. prausnitziiin subjects consuming high levels of unsaturated fat was significantly increased and the abundance ofBifidobacteriumwas decreased. However, Urwin et al. [39]allowed 123 subjects to consume 300 g of squid meat (rich in unsaturated fat) per day and did not find significant changes in gut microbial abundance.Therefore, the effects of specific fat on gut microbes still need to be further studied.
2.3 Plant protein
Plant protein has been reported to raise the abundance ofLactobacillusand lower the abundance ofBacteroidedsin human gut [40].The earliest study on plant protein affecting intestinal microbes appeared in the 1977s. Hentges et al. [40]compared a high-beef diet to a vegetarian diet which containing only half as much plant protein as the high-beef diet. Using conventional microbial culture methods, Hentges et al. [40]found that the levels ofBacteroidesandClostridiumin the gut of volunteers following the vegetarian diet had significantly reduced. Dominika et al. [41]reported that glycated pea protein, which is resistant to gastrointestinal enzymatic degradation,can alter intestinal microbial abundance, increasing the abundance ofLactobacillusandBifidobacterium. This may be due to that lactic acid bacteria possess more deglycation enzymes making it easy to use glycation of proteins [65]. Additionally, there was a corresponding increase in the levels of the short-chain fatty acids (SCFAs) with the shift of the bacterial abundance. These indicated that increased microbiota was able to utilize plant protein to produce short-chain fatty acids [41]. This ability may be beneficial to affect the intestinal environment and exert a health-promoting effect in humans.
2.4 Phytochemicals
Based on previous reports, the level of flavonoid content is significantly higher in a vegetarian diet than that in an omnivorous diet [9]. Flavonoids cannot be well metabolized and absorbed by the human gastrointestinal tract, which are consequently passed on to the large intestine and be used by certain intestinal microorganisms [66].The effects of different types of flavonoids, including isoflavones,cacao flavanol, red wine polyphenols, and propolis polyphenols, on gut microbiota have been testedin vivostudies. Although the type of flavonoids, intake dose, and intervention time of these studies were different, the results showed high flavonoids intake was associated with increased abundance ofLactobacillus[46,48,49], and reduced abundance ofClostridiumspp. andBacteroides[47,48,50]. Theilmann et al. [67]first revealed a pathway for certain species ofLactobacillusto utilize plant glycosidic (such as flavonoid). They indicated thatL. acidophilusutilized plant glycosides by dedicated phosphotransferase system transporters on the cell membrane, and hydrolyzed plant glycosides through phospho-β-glucosidase into glucose and glycolytic.
Another phytochemical abundant in vegetarian diets that has been reported is phytosterol [10]. Phytosterol is a kind of sterol with similar structure and biochemical properties to cholesterol which is widely found in the roots, stems, and fruits of plants [68]. An animal intervention study showed high doses of phytosterol esters(0.1 g/100 g body weight) significantly exalted Firmicutes and Proteobacteria [52]. However, the specific pathway of phytosterol metabolism by intestinal microorganisms has not been reported.Additionally, Baumgartner et al. [51]given 13 healthy subjects 3 g/day plant stanol ester for 3 weeks, but no significant change in gut microbiota composition was observed.
2.5 Micronutrients
Vitamin A intake of vegetarian diets was found to be 25% higher than that of omnivores [8]. A diet rich in vitamin A may lead to the increase ofClostridiumand decrease ofEnterococcusandBacteroides[42]. Children whose diets are deficient in vitamin A have reduced intestinal microbiome diversity and increased abundance ofEnterococcusin their feces [42]. In the study of Lee et al. [69], they found that the vitamin A supplementation in a mice model could significantly increase the abundance ofLactobacillusspp. Hibberd et al. [43]studied mice with a diet lacking vitamin A and found out that the levels ofBacteroidesnoticeably increased in the intestine. In general,high levels of vitamin A will lead to an increase in health-beneficial microbes and a decrease in opportunistic pathogens.
Except vitamin A, vegetarian diets intake 1 465 mg calcium per day, while omnivores consume 1 199 mg per day. Chaplin et al. [45]studied obese mice supplemented with calcium and found increased levels ofBifidobacteriumandLactobacillusin their intestines.Additionally, vegetarian diets are known to contain less vitamin D than omnivorous diets. And a study reported by Mandal et al. [44]indicated that a high vitamin D diet may cause a reduction in microbialα-diversity. While there are few studies on the relationship between micronutrients and intestinal microbes, supplementation of vitamin A and calcium has been shown that there may be some effects to support the beneficial bacteria.
脑卒中位列全球死亡原因第三位,每年会导致59万人死亡[1],是成人慢性重度残疾的主要原因,需要长期的康复治疗[2]。在这一过程中,脑卒中后抑郁(post-stroke depression,PSD)是最常见的精神问题,约有33%的患者在脑卒中发作后发展为PSD,其临床主要表现为情绪低落、思维迟缓,以及言语动作减少等典型症状[3-4]。PSD对脑卒中患者的影响主要表现在2个方面:一方面会进一步恶化认知的恢复、身体功能的康复以及生活质量;另一方面会对患者进行康复治疗的能力有负面影响[1]。
3. Influence of microbe-derived metabolites of vegetarian diet-derived nutrients on intestinal physiology
Adopting a vegetarian diet tends to maintain a favorable intestinal status [70]. For example, studies have been shown that the levels of intestinal inflammation markers intestinal lipocalin-2 and IgE were significantly lower in the gut of vegetarians than in omnivores [15,70].Indeed, microbiome-derived metabolites of vegetarian dietary sources, either produced or transformed by microorganisms, are essential actors affecting the intestinal physiological processes [71,72].These metabolites include SCFAs, lactate, and pyruvate derived from dietary fiber; agmatine, putrescine, spermine, and spermidine yielded from plant proteins; and retinoic acid obtained from metabolizing vitamin A. We propose 3 aspects by which these metabolites may be regulated intestinal physiology: 1) modulating intestinal immunity,2) protecting intestinal barrier integrity, 3) preventing the colonization of pathogens. The effects of these metabolites on intestinal physiology listed in Table 4.

Table 4The effects of microbe-derived metabolites of vegetarian diet-derived nutrients on the intestinal physiology.
3.1 Effects of microbe-derived metabolites on intestinal immune system
SCFAs, including acetate, propionate, and butyrate, are among the most abundant microbial metabolites present in the intestinal lumen [106].They are mainly produced by intestinal microbiota through fermentation and depolymerization of dietary fiber. The high levels of SCFAs are positively correlated with the consumption of dietary fiber and the role of intestinal microbes. As mentioned previously,one of the characteristics of vegetarians’ gut microbiome is the increased abundance of dietary fiber-degrading bacteria.PrevotellaandRuminococcushave the ability to catabolize dietary carbohydrates through succinate pathway to produce acetate and propionate.FaecalibacteriumandEubacteriumcould produce butyrate by acetyl CoA transferase pathway.Lactobacilluscould utilize dietary fiber to synthesize acetate and propionate via 1,2-propanediol pathway [33].Consequently, the levels of these metabolites in the intestines of vegetarians have been reported to be greatly increased [19,21].
SCFAs-mediated immune regulation is mainly accomplished via inhibiting histone deacetylases (HDACs) and activating G proteincoupled receptors (GPRs) present in intestinal epithelial cells (IECs)and other immune cells [73]. Butyrate could directly affect the amount and function of adaptive Treg cells and IECs through inhibiting HDACs, promoting the secretion of anti-inflammatory factor IL-10 [74]. Furthermore, the anti-inflammatory properties of butyrate could be achieved by inhibiting the activation of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), NF-κB, IL-6 and IL-12 [73]. Butyrate as the main carbon source for colon cells can ameliorate colitis by providing energy for intestinal epithelial cells [75,76]. Another known effect of butyrate is to indirectly promote the secretion of IgA by B cell [77]. Acetate exhibited the ability to affect IECs and neutrophils to inhibit the secretion of inflammatory cytokines C5aR, CXCR2, TNF-α, IL-6, and NF-κB through activating GPR43 [78,79]. Propionate exhibited antiinflammatory properties through inhibition of the pro-inflammatory factors IL-6, IL-1β, and TNF-α. Additionally, propionate has been reported to upregulate the expression of islet-derived protein type 3 to ameliorate colitis in mice [80,81].

Fig. 2 The effects of SCFAs on intestinal immune function. Butyrate facilitates the differentiation of Foxp3+ Tregs by inhibiting HDAC activity. Besides, butyrate directly affects macrophage and IECs to inhibit the secretion of the TNF-α, NF-κB, IL-6 and IL-12. Butyrate provides energy for IECs. Furthermore, butyrate indirectly promotes the secretion of IgA by B cell. Acetate induces IL-10-producing Tregs cells by suppressing HDAC function through GPR43. In addition, acetate affects IECs and neutrophils to inhibit the secretion of C5aR, CXCR2, TNF-α, IL-6, and NF-κB through activating GPR43 and GPR41. Propionate upregulates the expression of islet-derived protein type 3 by GPR43 and GPR109.
Additionally, lactate, which is also metabolized by dietary fiber,has been shown to prevent the activation of NF-κB and the expression of TNF-α in THP-1 cells. Watanabe et al. [93]speculated that this may be caused by suppressing the LPS/TLR4 signaling pathway. In anin vitrostudy, lactate exhibited the modulatory capacity by downregulating the expression of proinflammatory molecules IL-1β and TNF-α without affecting the expression of enterocyte function [94].Furthermore, the downregulation of proinflammatory responses by lactate through modulating the activation of the dendritic cells has also been evidenced [95].
Vitamin A is necessary for maintaining immune function, which mainly depends on immune cell differentiation and proliferation [98].Certain gut microorganisms convert dietary vitamin A to bioactive retinoic acid through retinol dehydrogenase 7 (Rdh7) [99]. In an animal experiment, genetically engineered mice lacking Rdh7 exhibited lower levels of retinoic acid, leading to the decreased level of IL-22 which is an important cell signal that coordinates the intestinal antibiotic response [99]. Additionally, Veldhoen et al. [98]demonstrated that retinoic acid derived from dietary vitamin A generated by CD103+dendritic cells and IECs playing a role in differentiation of T cells and B cells.
Although the total protein content in vegetarian diets is lower than non-vegetarian diets, the arginine consumption of vegetarian subjects is significantly higher than non-vegetarian subjects [106].The catabolism of arginine can produce putrescine and spermidine by decarboxylase, and spermine as part of the polyamine synthesis pathway. Putrescine has been reported to reverse the effect of difluoro methyl ornithine on IECs by maintaining a normal cell growth [100]. Furthermore, arginine combined withBifidobacteriaLKM512 can inhibit the expression of inflammatory cytokines IL-1β,MIP-2, and TNF-α by upregulating the production of putrescine in the intestine [101]. Besides, spermine exerts anti-inflammatory effects by suppressing the release of IL-12 and TNF-α, and increasing the production of IL-10 [102].
3.2 Effects of microbe-derived metabolites on intestinal barrier integrity
The intestinal barrier is essential for maintaining intestinal physiological health because the disorder of the intestinal barrier function leads to the development or aggravation of the intestinal disease [107]. Microbe-derived metabolites mainly regulate the intestinal barrier integrity by modulating the function of tight junctions between the IECs. Besides, certain metabolites promote the production of mucus and improve intestinal mucosa [108].
SCFAs have been reported to promote intestinal epithelial repairment through the activation of NOD-like receptor protein 3, and the mechanisms may be involved in Ca2+mobilization and membrane hyperpolarization [82]. Butyrate plays a key role in maintaining the stability of hypoxia-inducible factor (HIF) by regulating the oxygen concentration in colonic epithelial tissue. While HIF is a critical transcription factor that modulates barrier function, mucin production, and so on [83]. Additionally, the reduction of microbederived butyrate results in the disruption of IECs junctional integrity,which is restored after local administration of exogenous butyrate [84].Furthermore, butyrate and acetate have been shown to stimulate goblet cell mucus secretion to repair damaged intestinal mucosa [85,86]. Propionate can upregulate the expression of tight junction proteins (zonula occludens-1, occludin, and E-cadherin) to ameliorate the dextran sodium sulfate-induced colitis [81].
Microbe-derived lactate has a protective effect on severe intestinal damage in mice caused by radiation exposure and chemotherapy treatment. Lactate has been reported to stimulate the Wnt/β-catenin signal pathway on paneth and intestinal stromal cells through a lactate-specific receptor GPR81, thereby promoting the proliferation of intestinal stem-cell [96].
Polyamines are involved in multiple signaling pathways of tight junction protein expression in IECs [106]. In the study of Guo et al. [104],spermidine depletion reduced the amount of occludin, zonula occludens-1, and zonula occludens-2, but did not affect the mRNA of these tight junction proteins. Furthermore, increased cellular polyamines upregulated the expression of stromal interaction molecule 1 and inhibited the expression of stromal interaction molecule 2, thereby controlling transient receptor potential-1-mediated Ca2+signaling [109].Additionally, microbe-derived spermine is a nutrient for the intestinal mucosa, and this metabolite has the ability to enhance the expression of villous cells and crypt cells [103].
3.3 Effects of microbe-derived metabolites on preventing pathogen colonization
Colonization resistance through microbial-derived metabolites helps prevent harmful intestinal pathogens from colonizing and expanding in the intestine [110]. SCFAs can directly inhibit the growth of intestinal pathogens or indirectly regulate the intestinal environment to mediate colonization resistance. For example,multiple studies have reported that the depletion of SCFAs in the intestine drives expansion of gram-negative pathogens [87,88].Butyrate has been reported to restrict the oxygen in the intestinal epithelium by stimulating the PPAR-γ, thereby limiting the aerobicSalmonella typhimuriumaccess to oxygen and inhibiting their colonization [89,90]. Furthermore, butyrate plays a key role in driving monocytes to enter the macrophage differentiation process and enhancing the antibacterial activity of macrophages [91]. Propionate protects against the Salmonella invasion by repressing the expression of genes encoded withinSalmonellaPathogenicity Island 1 [92].
Pyruvate is usually derived from the fermentation of dietary fiber by intestinal microorganisms and can be further catabolized into lactate [33]. In the study of Morita et al. [97], microbe-derived pyruvate and lactate enhanced the immune response by inducing GPR31-mediated intestinal CX3CR1+cells, which provides high resistance to intestinalSalmonellainfection. Besides, Iraporda et al. [94]hypothesized thatLactobacillusspp. can inhibit pathogenic bacteria colonization by reducing the pH in the intestinal lumen by metabolizing dietary fiber to produce lactate.
4. Conclusions
To date, research has found that vegetarian diets can be an effectual strategy to develop a more diversified ecosystem of intestinal microorganisms and maintain favorable intestinal physiology.This is thought to be primarily due to the specific macronutrient(carbohydrates, protein, and fat), micronutrient (vitamin A, calcium),and phytochemicals (polyphenols and phytosterols) composition associated with the diet. Correspondingly, the gut microbiome has been found to affect nutrient absorption and metabolism, and is metabolites directly or indirectly regulating subsequent host intestinal physiology. In conclusion, an in-depth understanding of the interaction between vegetarian diets, intestinal microbiota and its metabolites, and intestinal healthy aspects, exemplified here, is conducive to found a new approach based on personalized diets to prevent or treat a series of intestinal diseases.
Funding
This work was supported by the National Natural Science Foundation of China Program [No. 31871773 and No. 31820103010];Projects of Innovation and Development Pillar Program for Key Industries in Southern Xinjiang of Xinjiang Production and Construction Corps [2018DB002]; National First-Class Discipline Program of Food Science and Technology [JUFSTR20180102].
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
- 食品科学与人类健康(英文)的其它文章
- Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
- Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria
- Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: biological variation and effects of postmortem ageing and storage

