基于硼二吡咯亚甲基类(BODIPY)衍生物的合成,光谱及汞离子识别
姜佳丽 卢 华 沈 珍
(南京大学化学化工学院,配位化学国家重点实验室,南京 210093)
基于硼二吡咯亚甲基类(BODIPY)衍生物的合成,光谱及汞离子识别
姜佳丽 卢 华 沈 珍*
(南京大学化学化工学院,配位化学国家重点实验室,南京 210093)
汞离子;硼二吡咯亚甲基;荧光探针
Mercury can accumulate in animal and human body and cause a wide variety of diseases,such as prenatal brain damage and motion disorders[1].Therefore,it is very important to develop highly sensitive and selective sensors for the detection of mercury ion.Currently,remarkable progresses in the design,synthesis and characterization of Hg2+-responsive sensors have been made over the past several years[2].Among the reported sensors,however,many of those sensors display drawbacks for applicability such as,crosssensitivities toward other metal ions,or not easy to synthesize complicated macrocycle receptor and delayed response time.
Boradiazaindacenes (BODIPY)are well known fluorescent dyes with several advantageous characteristics,such as high fluorescence quantum yield,negligible triplet-state formation,easy modificative structure and high stability against physiological conditions and chemical reactions.These properties facilitated their extensive application as labeling reagents,OLEDs,fluorescent switches,chemosensors,and as laser dyes[3].Recently,the fluorescent sensors based on the BODIPY derivatives have been applied as Hg2+sensor[4-5].However,there are only a few reported “turn-on”and colorimetricHg2+sensorsbasedon meso-substitutedBODIPY[3d].
Herein,we report a new meso-substituted BODIPY that show “turn-on” and colorimetric Hg2+-responsive property with high selectivity and sensitivity over awide range of other cations.
1 Experimental
1.1 Reagents and instruments
All reagents were obtained from commercial suppliers and used without further purification unless otherwise indicated.1H NMR spectra were recorded on a Bruker DRX500 spectrometer and referenced to the residual proton signals of the solvent.Mass spectra were measured on a Bruker Daltonics AutoflexⅡTMMALDI-TOF spectrometer.UV-Vis absorption spectra were carried out on a UV-3100 spectrophotometer.Fluores-cence spectra were measured on an Aminco Bowman 2 Luminescence spectrophotometer with a xenon arc lamp as the light source.
1.2 Procedures for metal ion sensing
Stock solutions of the metal ions(100 μmol·L-1)were prepared in deionized water.A stock solution of 1(5 μmol·L-1)was prepared in CH3CN.In titration experiments,each time a 2 mL solution of 1(5 μmol·L-1)was filled in a quartz optical cell of 1 cm optical path length,and the Hg2+stock solution was added into the quartz optical cell gradually by using a micropippet.In selectivity experiments,the test samples were prepared by placing appropriate amounts of metal ion stock solution into 2 mL solution of 1 (5 μmol·L-1).During fluorescence measurements,the excitation wavelength was 488 nm and emission spectra were collected between 500~600 nm.
1.3 DFT calculation
The G03W software package[6]was used to carry out a DFT geometry optimization for 1 using the B3LYP functional with 3-21G basis sets.TD-DFT calculations were then carried out using the same approach.
1.4 Synthesis of BODIPY derivatives 1~3
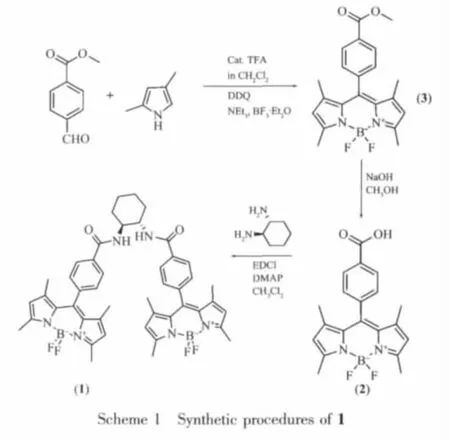
The synthesis of BODIPY derivatives was outlined in Scheme 1.Compound 3 was prepared from acid catalyzed condensation of 2,4-dimethylpyrrole and methyl 4-formylbenzoate according to a published literature[3c].Compound 2 was obtained by the hydrolysis of compound 3.DMAP (cat),EDCI,and(1S,2S)-trans-1,2-diaminocyclohexane were added to the dry CH2Cl2solution of compound 2,the mixture was stirred at room temperature over night,diethyl ether was added,and the organic layer was washed,with 10%hydrochloric acid,water,saturated aqueous NaHCO3,water and brine,respectively and dried with MgSO4and concentrated in vacuo[7].After purification on silicon gel column and recrystallization,compound 1 was obtained as a salmon pink solid.IR (KBr pellet,cm-1):3 426,1 641,1 545,1 511,1 384,1 306,1 193,1 156,1 085,981.λmax(CH3CN)/nm:500(ε/(dm3·mol-1·cm-1)176 800).1H NMR(500 MHz,CDCl3)δ 7.88(d,4 H,J=8.5 Hz),7.36(d,4 H,J=8 Hz),6.91(d,2 H,J=6 Hz),5.97(s,4 H),4.05(s,2 H),2.55(s,12 H),2.27(m,2 H),1.91(m,2 H),1.54(s,12 H),1.49(m,2 H),1.25(m,2 H);MS(MALDI-TOF):m/z 814.53[M]+.
2 Results and discussions
2.1 Spectroscopic properties of 1
The absorption and fluorescence spectra of 1 show the characteristic spectroscopic propertiesofthe BODIPY chromophore with slight stoke shifts.In CH3CN,a strong transition with a maximum at 499 nm(ε=176 800 L·mol-1·cm-1)and a shoulder at shorter wavelength side is observed.The shoulder at 471 nm is assigned to the molecular C-C frame vibration of ca.1300 cm-1,which is typical for cyanine and BDP dyes[8].The absorption spectra of 1 was similar to those classical BODIPY dyes indicated the absence of donoracceptor interaction in the ground state,which was dueto the decoupled structure between the indacene plane and meso-subunit[9].Excitation of 1 yields fluorescence spectrum of mirror image shape with maximum at 517 nm with stokes shift of 18 nm in CH3CN upon excitation at 488 nm.

2.2 Detective property of 1
To evaluate the utility of compound 1 as an ionselective fluorescent probe for Hg2+,the selective properties of 1 toward various metal ions was carried.Upon addition of 20 equiv.Hg2+,the intensity of the emission band at 517 nm increased and 39-fold enhancement was estimated.However,the fluorescence intensity of 1 was almost not influenced by the addition of 40 equiv.of other metal ions such as Ag+,Cr3+,Fe3+,Li+,Na+,K+,Ca2+,Mg2+,Al3+,Pb2+,Fe2+,Co2+,Ni2+,Cu2+,Zn2+,Cd2+and Mn2+,suggesting that compound 1 was a selective fluorescent probe for Hg2+.Fig.2 (RIGHT)illustrated a change in the emission spectra of 1 with increasing amount of Hg2+in CH3CN.The fluorescence spectra also showed 9 nm red-shift with increasing amount of Hg2+.The association constant lgKswas determined to be 4.95 from a nonlinear least-square analysis of fluorescence integrate area versus the concentration of Hg2+.
It was clear that the absorption of 1 was affected by the addition of Hg2+in CH3CN.Fig.3 showed that the absorption maximum of 1 gradually shifted to long wavelength from 499 nm to 514 nm with an isobestic point at 502 nm in comparison to that of the free dye and the color of the solution changed from somewhat light amber of free 1 into pink-red,which could be observed by the naked eye.The spectra shifts could be understood on the basisofthe electron-density redistribution thatoccurred upon excitation:the electron density increased at the meso-position.The complexed meso-group represented a strong electron acceptor,and the dye thus displayed red shifts in absorption,similar modulation ofthe electronic properties of meso-substituted BDP probes have been reported by our group[3d].
TD-DFT calculation was carried out for 1 by wave function B3LYP with 3-21G basis set.Based on TDDFT calculation,the intense low-energy absorption at 499 nm could be attributed to strong state that compositions of HOMO-1 to LUMO,HOMO to LUMO+1 and HOMO-4 to LUMO with contributions 35%,27%and 6%,respectively(Fig.4).Coordination of Hg2+influenced the donor properties of the phenyl substituent and altered the efficiency of non-radiative decay and hence modified the fluorescence properties.

In summary,a highly selective and sensitive fluorescent and colorimetric chemosensor based on meso-substituted BODIPY for Hg2+have been designed according to our reported modulation method.Coordination of Hg2+influences the electronic propertiesof meso-position and alter the efficiency of non-radiative decay,hence show red-shifted absorption spectrum and enhanced fluorescence.This findings support our designed idea that meso-substituted BODIPY can be usedasfluorescentandcolorimetricsensors.


[1](a)Grandjean P,Wei H P,White R F,et al.Environ.Res.,1998,77:165-172
(b)Yuan M J,Li Y L,Zhu D B,et al.Org.Lett.,2007,9:2313-2316
(c)Boening D W.Chemosphere,2000,40:1335-1351
(d)O′Rourke B,Backx P H,Marban E.Science,1992,257:45-248
[2](a)Nolan E M,Lippard S J.Chem.Rev.,2008,108:3443-3480(b)Domaille D W,Que E L,Chang C J.Nat.Chem.Biol.,2008,4:168-175
(c)Zhang J F,Kim J S.Anal.Sci.,2009,25:1271-1281
[3](a)Loudet A,Burgess K.Chem Rev.,2007,107:4891-4932
(b)Ulrich G,Ziessel R,Harriman A.Angew.Chem.,Int.Ed.,2008,47:1184-1201
(c)Shen Z,Roehr H,Rurack K,et al.Chem.Eur.J.,2004,10:4853-4871
(d)Yu Y H,Descalzo A B,Shen Z.et al.Chem.Asian J.,2006,1:176-187
(e)Yu Y H,Shen Z,Xu H Y,et al.J.Mol.Struc.,2007,827:130-136
(f)Wang Y W,Li M,Shen Z.Chin.J.Inorg.Chem.,2008,24:1247-1252
(g)Xu H Y,Shen Z,Okujima T,et al.Chin.J.Inorg.Chem.,2006,22:801-807
(h)Wang Y W,Descalzo A B,Shen Z,et al.Chem.Eur.J.,2010,9:2887-2903
[4](a)Yuan M J,Li Y L,Li J B,et al.Org.Lett.,2007,9:2313-2316
(b)Yuan M,Zhou W,Liu X,et al.J.Org.Chem.,2008,73:5008-5014
(c)Lu H,Zhang S S,Liu H Z,et al.J.Phys.Chem.A,2009,113:14081-14086
(d)Lu H,Xiong L Q,Liu H Z,et al.Org.Biomol.Chem.,2009,7:2554-2558
(e)Wang J,Qian X.Org.Lett.,2006,8:3721-3724
[5]Zhang X,Xiao Y,Qian X.Angew.Chem.Int.Ed.,2008,47:8025-8029
[6]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 03,Gaussian,Inc.,Wallingford CT,2004.
[7]Fuchs S,Berl V,Lepoittevin J P.Eur.J.Org.Chem.,2007,7:1145-1152
[8](a)Qin W,Baruah M,Van der Auweraer M,et al.J.Phys.Chem.A,2005,109:7371-7384(b)Descalzo A B,Xu H J,Xue Z L,et al.Org.Lett.,2008,10:1581-1584
[9]Kollmannsberger M,Rurack K,Resch-Genger U,et al.J.Phys.Chem.A,1998,102:10211-10220
[10]Gorelsky S I.SWizard program,revision 4.6,http://www.sgchem.net/
Synthesis,Spectroscopic Properties and Hg2+Recognition Based on a Boron Dipyrromethene Dye(BODIPY)
JIANG Jia-LiLU Hua SHEN Zhen*
(School of Chemistry and Chemical Engineering,State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093)
The synthesis and sensing properties of a new BODIPY derivative 1 are outlined.1 shows fluorescence“turn-on” and colorimetric responses with high selectivity toward Hg2+over the other metal cations.Coordination of Hg2+influences the electronic properties of the receptor at meso-position and alter the efficiency of nonradiative decay,hence increase the fluorescence intensity and red shift the absorption spectrum.
Hg2+;boron-dipyrromethene;fluorescent sensor
O614.24+3;O613.8+1
A
1001-4861(2010)06-1105-04
2010-01-08。收修改稿日期:2010-03-24。
国家自然科学基金面上基金项目(No.20971066)和教育部新世纪优秀人才支持计划(No.NCET-08-0272)资助。
*通讯联系人。E-mail:zshen@nju.edu.cn,Tel:025-83686679
姜佳丽,女,23岁,硕士研究生;研究方向:分子荧光探针的合成和性质研究。

