Pluronic嵌段共聚物F127胶团对布洛芬的增溶
万东华 郑 欧 周 燕 吴莉瑜
(福州大学化学化工学院,福州 350108)
Polymeric micelles are assembled by copolymers,which consist of both hydrophilic and hydrophobic monomer units. When the length of the hydrophilic block is more than that of the hydrophobic block,these amphiphilic block copolymers can generate spherical micelles in aqueous solution.In this case,the hydrophobic blocks constitute the core and the hydrophilic blocks along with the solvent water form the corona. One of the useful properties of such aggregates is their ability to incorporate hydrophobic substances,thus enhancing dramatically the dispersion of insoluble molecules in aqueous solution. This phenomenon is referred to as solubilization.In comparison to traditional surfactant micelles,polymeric micelles seem to present better solubilization capacity due to the higher number of micelles and/or larger cores of the formers[1-2].
Triblock copolymersofpoly(oxyethylene)(PEO)and poly(oxypropylene)(PPO)with the sequence of PEO-PPOPEO are commercially recognized as Pluronics(manufactured by BASF,Germany)or Poloxamer(manufactured by ICI,England).Variation of PPO/PEO mass ratio and molecular weight leads to the production of triblock copolymers(TBP)with various compositions and properties that meet the specific requirements in numerous applications.It needs to be noted that the solubilization of polycyclic aromatic hydrocarbons in PEOPPO-PEO micelle solution is mainly depended on the structure and molecular weight of the copolymer used,as well as the solution temperature[3-6].The PEO coating has been revealed to prevent opsonization and subsequent recognition by the macrophages of the reticuloendothelial system,thus allowing the micelles to circulate long and to effectively deliver drugs to the desired sites.Pluronic F127 is one of the representative Pluronics because of its high ratio of EO/PO(70%(w)EO)and big molar mass(3780)of PPO.The ability of F127 micelles to solubilize hydrophobic solutes has generated significant interest on drug delivery as well as controlled-release applications[7-9]. Pluronic F68 is used for intravenous injection formulation and oral formulation[10].In recent years,F68 is widely combined with F127 in the design of drug delivery system[2,11].
Ibuprofen(4-isobutil-2-phenyl-propionic acid,IBU),as depicted in Fig.1,pKα=4.8,is a well-known non-steroidal anti-inflammatory drug that has been popularly used for the treatment of mild to moderate pain and fever.Because of its high membrane permeability,extent of IBU absorption approaches up to 100%.Dissolution thus becomes the rate-limiting step for absorption.However,the evidence that IBU is practically insoluble in water decides the relatively low bioavailability.Hitherto various formulations of IBU,such as prodrug,inclusion complex,microencapsulation and solid dispersion,were developed to improve the solubility of IBU[12].Therefore,the development of a drug delivery system allowing the controlled release of IBU would be useful,especially in high dose-dependent treatment for chronic diseases such as rheumatoid arthritis[13].
Taking into account the above,our work was designed to well investigate the solubilization of IBU in aqueous Pluronic F127 micelle solution.In an attempt to address this issue,we undertook a detailed experimental examination on the micellization properties and solubilization behavior of F127 in both water and 0.01 mol·L-1pH 7.4 PBS solution.The influences of temperature and another copolymer F68 were also determined.
1 Experimental
1.1 Materials and equipments
IBU was purchased from Juhua Group Corporation.The (EO)x-(PO)y-(EO)xblock copolymers F127 and F68 were provided by BASF(Germany).Table 1 summarizes the properties of the block copolymers.Pyrene was provided by Fluka (Swiss).All solvents were of analytical grade or high-performance liquid chromatography(HPLC)grade.Water was purified using the Milli-Q plus system.
Fluorescence spectra were recorded on a Hitachi F-4500 fluorescence spectrophotometer(Japan).HPLC analysis was carried out using a Shimadzu LC20A HPLC system(Kyoto,Japan),which consists of a LC20A binary gradient pump,a SPD M10A diode-array detector and a DGU 20A3 degasser.The apparatus was interfaced to a computer using LC solution software.The separation was performed on a Sino-Chrom ODS column,250 mm×4.6 mm,5 μm(Dalian Elite Analytical Instruments,China).
1.2 Measurement of critical micelle concentration (cmc)
The cmc is determined by measuring the change in the fluorescence emission spectrum of pyrene monomers.The emission peak 1 at 372nm shows significant intensity enhancement in polar solvents,whereas peak 3 at 383nm shows very limited intensity variation with polarity.Therefore,the intensity ratio of the two peaks,I1/I3,is sensitive to the polarity of the medium located by the pyrene molecule.The inflection of the curve I1/I3vs surfactant concentration is usually designated as thecmc[14].
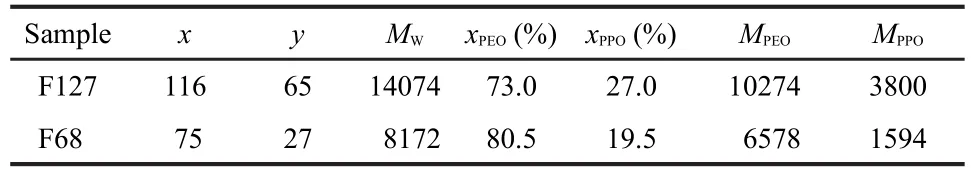
Table 1 Molecular characteristics of the(EO)x-(PO)y-(EO)x copolymers
In this work,the required constant amount of pyrene's acetone solution was put into tubes separately.After the complete evaporation of acetone,the stock solutions with different mass fraction ratios of F127/F68(w(F127):w(F68))were added into these tubes and then diluted to produce mixed solutions covering whole concentration range.The final concentration of pyrene was 0.5 μmol·L-1in water and 0.3 μmol·L-1in PBS solution,respectively,ensuring that clear pyrene spectrum with five vibronic bands was observed in each case and no additional band was detected[15].The solutions were kept for stirring overnight to ensure the complete solubilization of pyrene in the micelle phase.The desired temperature was maintained in the water bath with uncertainties of±0.01℃.The excitation wavelength was 335 nm and the emission wavelength ranged from 350 to 460 nm.The excitation and emission slit were 5.0 and 2.5 nm,respectively.
1.3 Solubility experiments
Excess IBU was added to glass vessels containing water or PBS solution of different w(F127):w(F68)values at several mass fractions(0-7.0%).The vessels were sealed and the obtained suspensions were shaken at the desired temperature at 250 r·min-1.After several days for equilibrium,each sample was isothermally filtered through a 0.22 μm nylon filter to remove unsolubilized drug.The concentration of the solubilized IBU was then determined by HPLC.The mobile phase consisted of methanol and 3%phosphate in a volume ratio of 80:20. Prior to use,the mobile phase was filtered through a 0.45 μm membrane filter.The mobile phase was delivered at a flow rate of 1.0 mL·min-1.Ultraviolet detection was set at the wavelength of 264 nm.The column temperature was set to be 40℃. The sample injection volume was 20 μL.The calibration curve of peak area against concentration of IBU(g·L-1)was A= 1405200cIBUunder the concentrations of IBU(cIBU)from 0 to 9 g·L-1(r=0.9998).
2 Results and discussion
2.1 Micellization
Fig.2 and Fig.3 depict I1/I3ratio versus F127 mass fraction (w(F127))in water and PBS solution at different temperatures, respectively.
It is obvious that the curve has a reverse S form.The significant decrease of the ratio is attributed to the formation of micelles with a hydrophobic core formed by hydrophobic PPO chains where pyrene partitions preferentially.The cmc value in each case is obtained by graphically drawing bestfit lines through the pre-break and post-break data,as listed in Table 2.
Fig.2 and Fig.3 indicate a significant alteration in the I1/I3ratio with the increase in temperature.At the lowest temperature, the micellization is taking place at the much higher mass fraction.Both PEO and PPO are water-soluble at low temperature. As the temperature is raised,the PPO block gradually loses its hydration sphere,resulting in a greater interaction between PPO blocks on different chains.As a result,there is a consequential decrease in the cmc value with the increase in temperature[15].
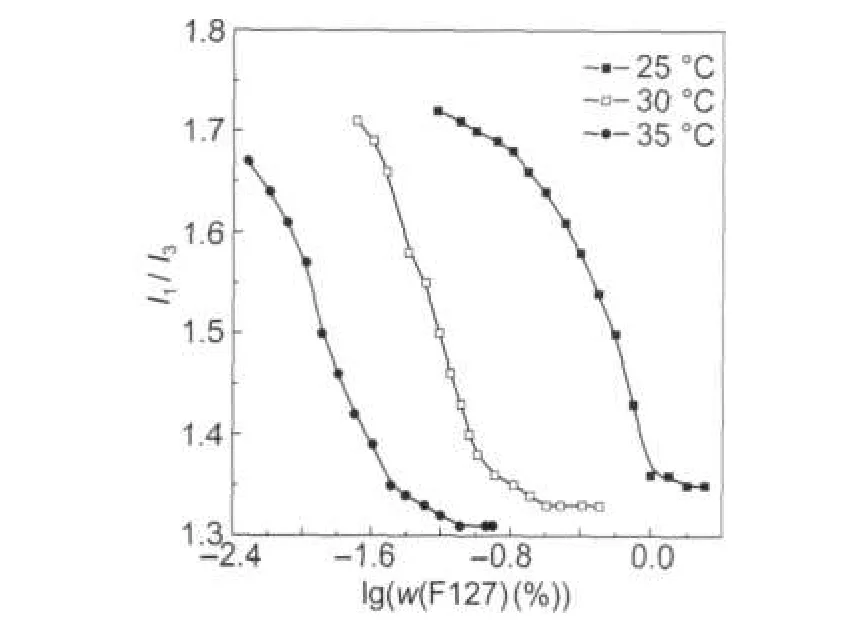
Fig.2 I1/I3versus F127 mass fraction in water at different temperatures
The I1/I3values of pyrene in PPO3000 and PEO300,which can be used as analogous environment of the TBP copolymer micelle core and corona,are 1.28 and 1.83 at 35℃,respectively[16].In these cases,the I1/I3values of pyrene in F127 micelle solution are 1.35,1.33,and 1.31 at 25,30 and 35℃,respectively,as shown in Table 2.This evidence strongly suggests that pyrene should be mainly located in the micelle core.The smaller I1/I3value observed at a higher temperature reflects a less polar microenvironment with growing temperature[17].As previously proposed,the TBP has the core and corona shaped micelle,where the core is mainly occupied by the PPO group. Due to the presence of ether oxygens,the hydrophobic core of TBP micelle is still weakly hydrated[15-16].Thus,on the account of the declined interaction between the PPO blocks and the water molecules at an elevated temperature,the water will be driven out of the core and then the core will become more hydrophobic.
It is observed that the cmc of F127 decreases in PBS solution.The favored micellization is attributed that the solubility of PPO block of F127 is lowed with the addition of the inorganic salt,along with the result of which the cmc decreases[18].The I1/I3values obtained for F127 in PBS solution are similar with those in water.The effect of salts on the polarity of the pyrene microenvironment is minor,suggesting that the salts do not penetrate into the micelle core.
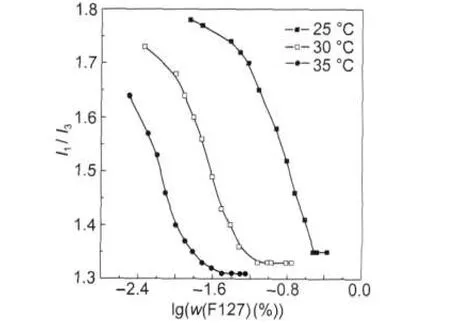
Fig.3 I1/I3versus F127 mass fraction in PBS solution at different temperatures
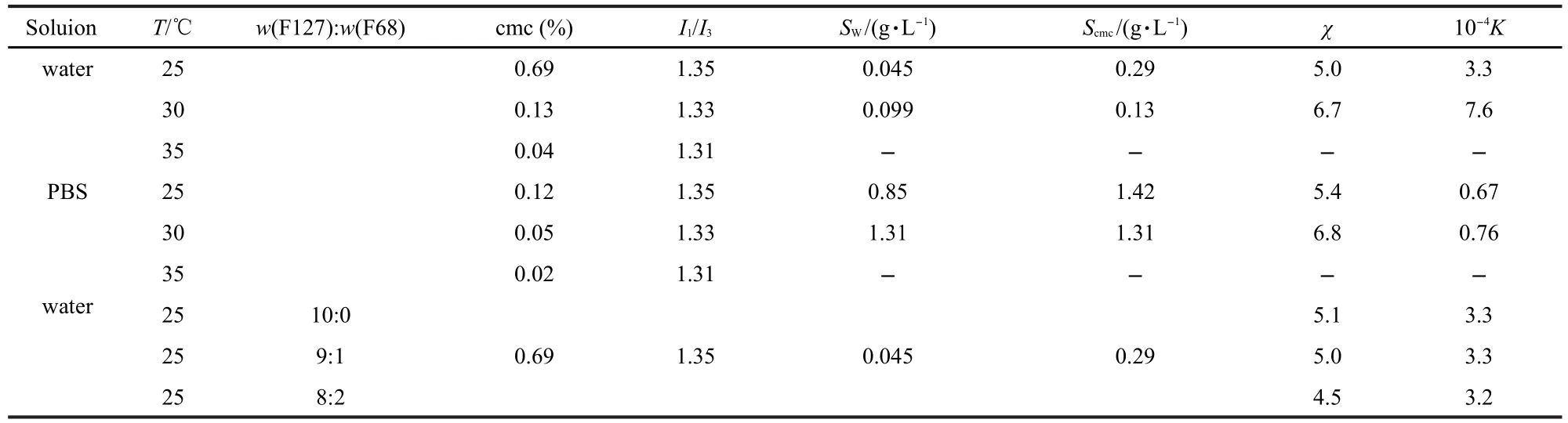
Table 2 cmc,I1/I3values for F127 and solubility descriptors for IBU in water and PBS solution
The effect of w(F127):w(F68)on the I1/I3intensity is depicted in Fig.4.It should be highlighted that in the inset the curves are overlapped if the x-axis referring to total TBP mass fraction (wT)was converted to w(F127).F127 micelle property is thought over to be slightly influenced in the presence of F68. Beezer and coworkers[19]also pointed out that the polymers containing similar PPO moieties exhibited cooperative aggregation,in the meanwhile those including different PPO moieties presented non-cooperative binding.
2.2 Solubilization
The samples from the phase-solubility study were assayed by HPLC to avoid interference caused by the Pluronic copolymer.The retention time of IBU was 9.0 min,as demonstrated in Fig.5.The apparent solubility of IBU(SIBU)was measured for solution with varying w(F127)at different temperatures in water and PBS solution.The results are presented in Fig.6.
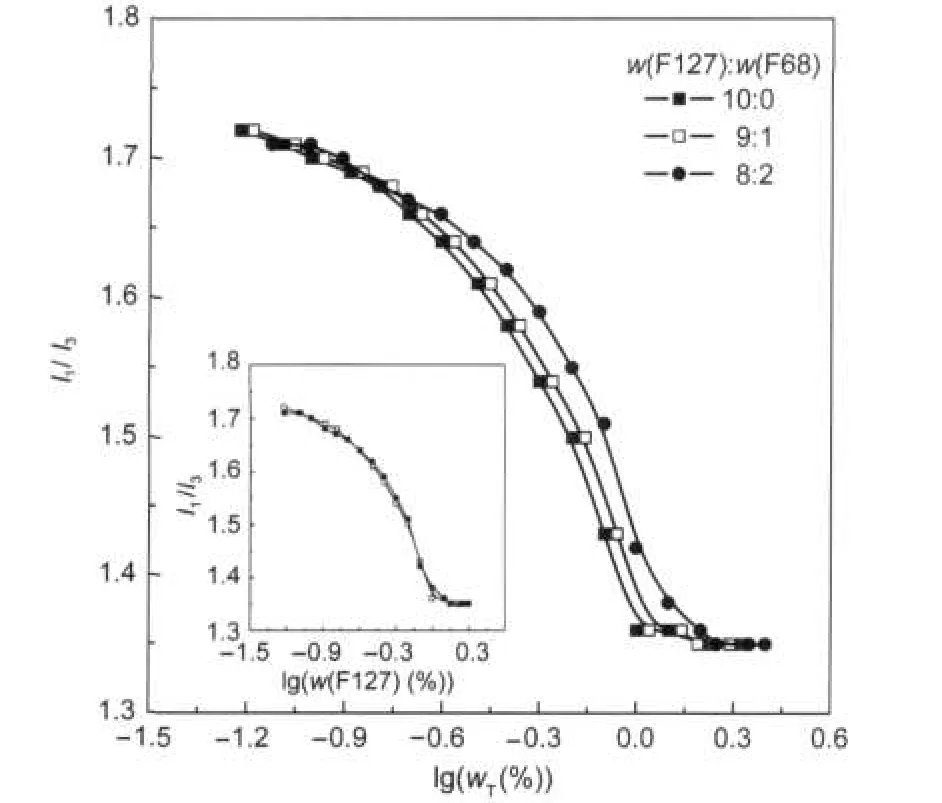
Fig.4 I1/I3versus total copolymer mass fraction with different w(F127):w(F68)in water at 25℃Inset shows curves plotted I1/I3vs F127 mass fraction.
The solubility of IBU increases with enhancing w(F127). For example,4.26 g·L-1,at 25℃for 6.0%F127 water solution,the concentration of IBU is 100 times more than 0.045 g· L-1in water.The linear increases in the solubility of IBU as a function of w(F127)suggest that the F127 micelle structure does not change significantly during this range and that increasing w(F127)leads to an increase in the number of micelles rather than micelle growth,corresponding with the findings of Sharma and Bhatia[8].Wanka et al.[20]also showed that the hydrophobic polymers with high molecular weight and high PPO/ PEO ratio form micelles of size and shape independent of the polymer concentration.
2.3 Calculation of solubility descriptors
The effectiveness of surfactant micelles for solubilization can be evaluated by two descriptors:molar solubilization capacity,χ,and micelle-water partition coefficient,K[21].χ provides the information about the amount of solute solubilized per mole of surfactant in the form of micelle.It can be calculated from the general equation:

whereStotis the apparent solubility of the solute in the surfactant solution,Scmcis the solubility of the solute when the surfactant concentration is just equal to the cmc.csurfis the molar concentration of surfactant in solution,and(csurf-cmc)is equal to the surfactant concentration in the form of micelle.
Furthermore,K is the ratio of the mole fraction of the solute in the micellar pseudophase to the mole fraction of the solute in the aqueous pseudophase.This definition allows the following mathematical expression to be formulated:
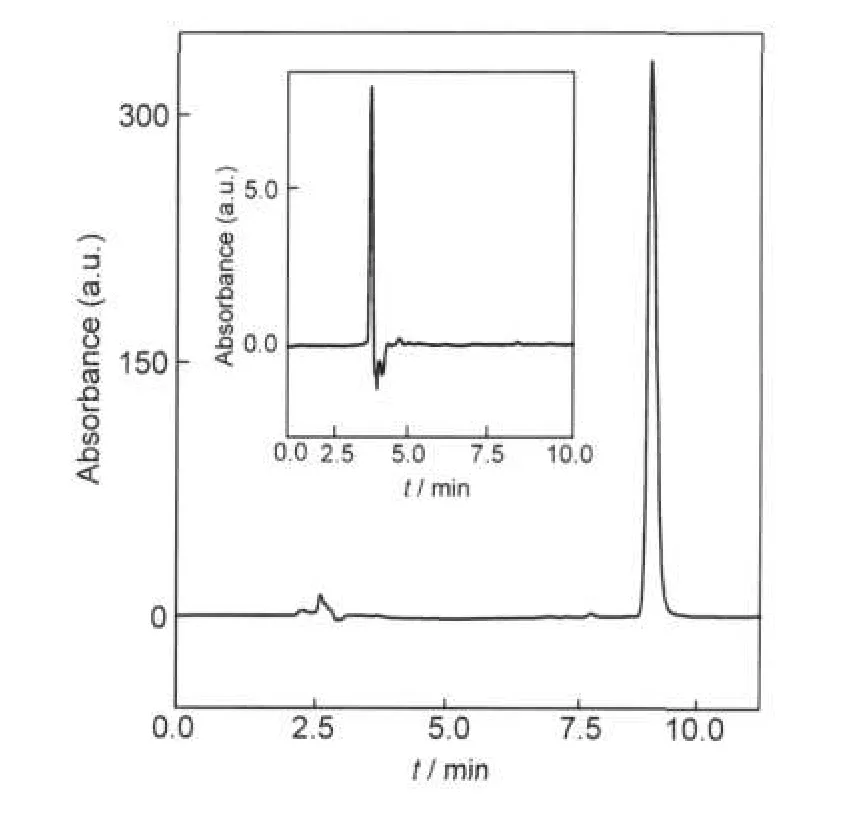
Fig.5 The HPLC profile of IBUInset shows the profile of copolymer solution.
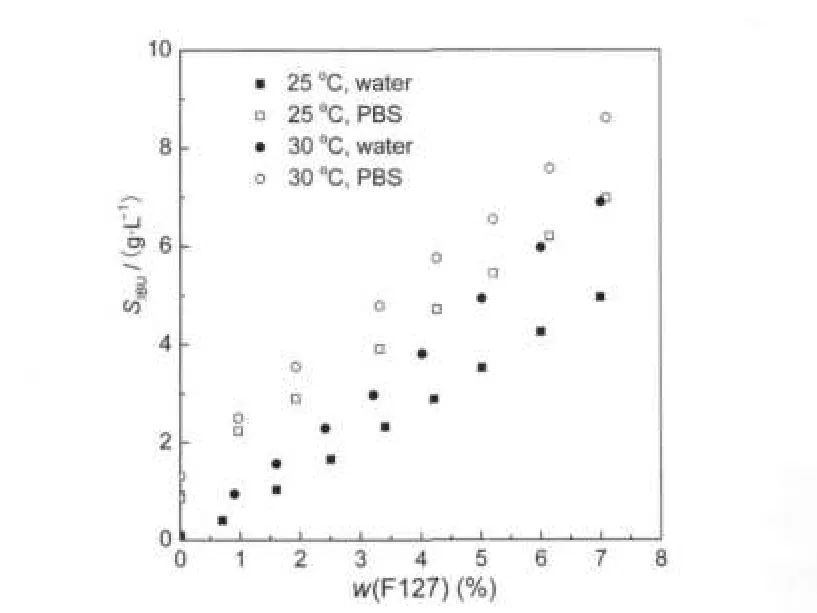
Fig.6 Solubility of IBU as a function of F127 mass fraction in water and PBS solution
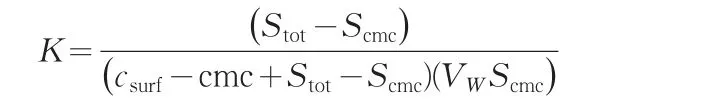
WhereVWis the molar volume of water.
Fig.7 and Fig.8 show the variation of(SIBU- Scmc)with (cF127-cmc)and with(cF127-cmc+SIBU-Scmc)(VWScmc),respectively.All curves indeed display good linearity.
χ and K can be obtained from the slopes of Fig.7 and Fig.8, respectively.The obtained solubility descriptors together with the water solubility SWand Scmcof IBU are reported in Table 2.
The χ values enhance moderately with ascending temperature from 25 to 30℃.As stated above,the micelle core becomes more hydrophobic with increasing temperature,thus providing an ever increasingly favorable environment for the solubilization of IBU.Another effect that will influence the solubilization of IBU is the compact extent of the micelle structure.Gadelle and his colleagues[22]concluded that loose micelles could solubilize aromatic hydrocarbons more easily than compact micelles.Since the micelles become more compact with increasing temperature[17],the solubility of IBU will decrease.It is likely that χ value of 30℃is only slightly bigger than that of 25℃for these two opposite effects.The largeK values observed in this study indicate that IBU had highly partitioned into the micelles.It is interesting,however,that theK values are of the same order of magnitude when the temperature is increased from 25 to 30℃.Because of the higher solubility of IBU in both the micellar pseudophase and the external aqueous pseudophase,it is expected thatKwill tend to change slightly with increasing temperature.
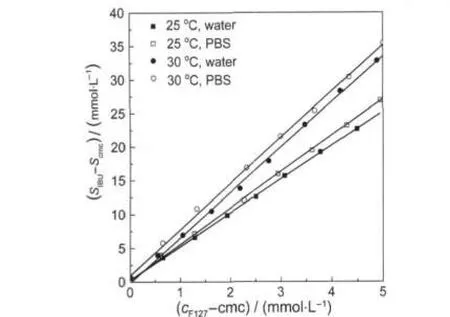
Fig.7 Variation of the(SIBU- Scmc)with(cF127-cmc)in water and PBS solution
The most important characteristic of the data in Table 2 is the recognition that in PBS solution,the χ value is essentially the same and theKvalue is an order of magnitude smaller compared with those in water.The almost unchanged χ might be the result of a minor change in the structure and polarity of the micelle.The dramatic decrease inKis mainly due to the solubility enhancement in the aqueous pseudophase.It is well known that the solubility of the drug is connected with the medium properties.As IBU is a weak acid(pKα=4.8),the solvent pH can show the significant influence on the extent of solubilization of IBU,since it may change the equilibrium between ionized and molecular forms of IBU.It is observed that in PBS solution,Swand Scmcvalues are significantly improved with respect to water,as shown in Table 2,due to the increase in the ionized form of the drug.
The solubility of IBU for different w(F127):w(F68)is shown in Fig.9 as a function of total copolymer mass fraction.As the hydrophobicity of Pluronic rises by increasing the proportion of PPO,F127 is more hydrophobic than F68.At a given total polymer mass fraction,the solubility of IBU is enhanced with the increase in w(F127):w(F68).F68 containing short PO blocks sandwiched between large EO segments will hardly form any micelles at the ambient temperature[21].The solubility remains constant with the increase in F68 concentration.If we make the results by replacing wTwith w(F127),as shown in the inset,the data almost coincide.This proves that F68 has little impact on the solubilization capacity of F127,as listed in Table 2,corresponding with the F127 micelle property is only slight-ly affected in the presence of F68.

Fig.8 Variation of the(SIBU- Scmc)with(cF127-cmc+SIBU-Scmc)(VWScmc) in water solutionInset shows plots in PBS solution.
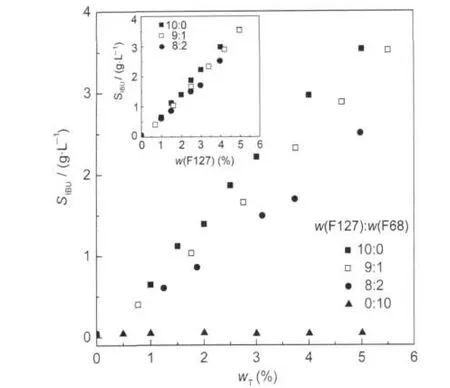
Fig.9 Solubility of IBU as a function of total copolymer mass fraction for different w(F127):w(F68)in water at 25℃Inset shows data plotted SIBUvs F127 mass fraction.
2.4 Solubilization mechanism
As stated above,the solubilization of IBU in F127 solution has been shown closely related to the micellization of F127. The micelle can provide at least three thermodynamically distinguishable microenvironments for solubilization[23]:the micelle core,the corona,and the core-corona interface.IBU owns a terminal carboxyl group that may interact with the PEO by hydrogen bonds.These interactions could drive the drug to the outer portion of the micelle core,in a way that the carboxyl group sticks to the palisade layer interacting with the PEO head groups.However,the hydrophobic portion of the drug molecule stays preferentially in contact with the hydrophobic core.
In order to check that solubilization was predominantly in the core and core-corona interface rather than in the corona, the extent of solubilization in the corona was estimated from experiments performed using the aqueous solution of polyethylene glycol(PEG)Mn=6000 g·moL-1[24].Only minimal solubilization in the corona is indicated for IBU concentration in 2% (w)PEG solution,being 0.045 g·L-1,the same as in water.
3 Conclusions
The cmc of F127 in PBS solution was much less than that in water.With the increase in temperature,a significant decrease in the cmc was apparent and a less polar microenvironment was present in the micelle core.F127 micelle property was only slightly affected in the presence of F68.The solubility of IBU increased linearly with an increase in the F127 mass fraction.The solubility of IBU was enhanced at increased temperature,in PBS solution or with higher w(F127):w(F68).Compared with those in pure water,the molar solubilization capacity was essentially the same and the micelle-water partition coefficient significantly decreased in PBS solution.Slight increases in molar solubilization capacity and the micelle-water partition coefficient were found with an increase in temperature.An analysis of the solubility descriptors indicates that micelle-water partition coefficient is very much unique for the drug IBU and the molar solubilization capacity is useful in determining the solubility effectiveness of copolymer F127.The solubilization of IBU in F127 solution has been shown closely related to the micellization of F127.It also demonstrated that IBU was predominantly in the micelle core and core-corona interface.
1 Rangel-Yagui,C.O.;Pessoa-Jr,A.;Tavares,L.C.J.Pharm. Pharmaceut.Sci.,2005,8:147
2 Chiappetta,D.A.;Sosnik,A.Eur.J.Pharm.Biopharm.,2007,66:303
3 Hurter,P.N.;Hatton,T.A.Langmuir,1992,8:1291
4 Gadelle,F.;Koros.W.J.;Schechter,R.S.Macromolecules,1995,28: 4883
5 Dai,L.R.;Chen,J.Acta Phys.-Chim.Sin.,1990,6:146 [戴乐蓉,陈 佳.物理化学学报,1990,6:146]
6 Zheng,Y.Y.;Jiang,L.Q.;Zhao,J.X.;Xu,X.Z.Chem.J.Chin.Univ., 2001,22:617 [郑玉婴,江琳沁,赵剑曦,许秀枝.高等学校化学学报,2001,22:617]
7 Suh,H.;Jun,H.W.Int.J.Pharm.,1996,129:13
8 Sharma,P.K.;Bhatia,S.R.Int.J.Pharm.,2004,278:361
9 Cafaggi,S.;Russo,E.;Caviglioli,G.;Parodi,B.;Stefani,R.;Sillo, G.;Leardi,R.;Bignardi,G.Eur.J.Pharm.Sci.,2008,35:19
10 Pan,W.S.Industrial pharmacy.Beijing:Higher Education Press, 2006:144,186[潘卫三.工业药剂学.北京:高等教育出版社,2006: 144,186]
11 Ruel-Gariepy,E.;Leroux,J.C.Eur.J.Pharm.Biopharm.,2004,58: 409
12 Zhang,L.;Li,T.;Chen,L.;Li,L.;Qi,G.Chin.J.New Drug,2006,15: 952 [张 莉,李 挺,陈 莉,李 丽,齐 刚.中国新药杂志, 2006,15:952]
13 Rangel-Yagui,C.O.;Hsu,H.W.L.;Pessoa-Jr,A.;Tavares,L.C. Braz.J.Pharm.Sci.,2005,41:237
14 Zhang,C.X.;Zhang,J.L.;Li,W.;Feng,X.Y.;Hou,M.Q.;Han,B. X.J.Colloid Interface Sci.,2008,327:157
15 Bakshi,M.S.;Sachar,S.J.Colloid Interface Sci.,2006,296:309
16 Nivaggilioli,T.;Alexandridis,P.;Hatton,T.A.;Yekta,A.;Winnik,M, A.Langmuir,1995,11:730
17 Zhao,J.X.;Zheng,O.;Lin,C.Y.J.Functional Polym.,2000,13:177 [赵剑曦,郑 欧,林翠英.功能高分子学报,2000,13:177]
18 Pandit,N.;Trygstad,T.;Croy,S.;Bohorquez,M.;Koch,C.J.Colloid Interface Sci.,2000,222:213
19 Gaisford,S.;Beezer,A.E.;Mitchell,J.C.Langmuir,1997,13:2606
20 Wanka,G.;Hoffmann,H.;Ulbricht,W.Macromolecules,1994,27: 4145
21 Paterson,I.F.;Chowdhry,B.Z.;Leharne,S.A.Langmuir,1999,15: 6187
22 Gadelle,F.;Koros,W.J.;Schechter,R.S.J.Colloid Interface Sci., 1995,170:57
23 Choucair,A.;Eisenberg,A.J.Am.Chem.Soc.,2003,125:11993
24 Crothers,M.;Ricardo,N.M.P.S.;Heatley,F.;Nixon,S.K.;Attwood, D.;Booth,C.Int.J.Pharm.,2008,358:303

