V2O5·xH2O-BiVO4纳米复合材料的可控合成和可见光响应的光催化性质
李本侠 王艳芬 刘同宣
(安徽理工大学材料科学与工程学院,安徽淮南232001)
V2O5·xH2O-BiVO4纳米复合材料的可控合成和可见光响应的光催化性质
李本侠*王艳芬 刘同宣
(安徽理工大学材料科学与工程学院,安徽淮南232001)
以单斜相V2O5·xH2O纳米线为前驱物,在温和条件下合成出V2O5·xH2O-BiVO4复合光催化剂.为理解产物的物相含量、形貌和光催化性质随合成时间延长而变化的情况,利用X射线衍射仪、场发射扫描电子显微镜、紫外-可见漫反射光谱仪以及光催化性质测试实验对三个典型的V2O5·xH2O-BiVO4样品(分别在反应6、12和24 h获得)进行了研究.分析结果表明:V2O5·xH2O-BiVO4纳米复合材料由V2O5·xH2O纳米线和BiVO4纳米晶组成,并且随合成反应时间的延长,产物中V2O5·xH2O纳米线的含量逐渐减少而BiVO4纳米晶的含量逐渐增加.光催化性质测试结果表明:V2O5·xH2O-BiVO4复合光催化剂在可见光(λ>400 nm)辐射下降解亚甲基蓝时表现出了提高的光催化效率,其中在反应12 h获得的V2O5·xH2O-BiVO4样品体现出最好的光催化活性,这可能是由于其适当的组分含量和特殊的微结构有利于半导体激发和染料激发两种光催化机理的协同作用.
复合光催化剂;化学合成;表征;光降解;有机染料
1 Introduction
Since Fujishima and Honda discovered photo-induced splitting of water on TiO2electrode,1numerous efforts have been devoted to the investigation on metal-oxide semiconductor photocatalysts for environmental remediation,such as air and water decontamination.2-4Although TiO2is currently one of the most widespread photocatalysts for degradation of various pollutants,5,6TiO2is effective only under ultraviolet irradiation(λ<380 nm)due to its large band gap(anatase,3.2 eV),thus severely restricting the use of sunlight for photocatalysis.Hence, it is necessary to open up opportunities for visible-light responsive photocatalysts instead of traditional TiO2,in order to make full use of sunlight as the energy source for pollutant abatement.7-10Recently,a great number of bismuth-containing materials with narrow band gap,such as Bi2WO6,11,12Bi2MoO6,13BiVO4,14,15and Bi2Fe4O9,16have shown admirable photocatalytic properties under visible light.Among them,monoclinic bismuth vanadate(m-BiVO4)has garnered considerable attention as a promising photocatalyst under visible light.With a narrow band gap(2-4 eV),this compound has been extensively investigated as an attractive advanced material for the photocatalytic degradation of organic pollutants17,18and the photocatalytic water splitting.19,20
On the other hand,despite the visible-light absorption of many new photocatalysts,the photocatalytic efficiency remains poor due to the low separation efficiency of electron-hole pairs.21Therefore,the development of visible-light responsive photocatalysts with high charge carrier separation has become one of the most challenging tasks in these days.For such a goal,instead of using a single semiconductor,researchers have switched to composite semiconductor photocatalysts to enhance the photocatalytic efficiency by mutual transfer of charge carriers(electrons and holes)from one semiconductor to another with compatible chemical and electrical properties.22-25In composite semiconductor materials,a heterogeneous interface is constructed between the semiconductors with matching band potentials,and accordingly a contact electric field is built at this heterogeneous interface.26Driven by the contact electric field,photogenerated charge carriers can transport from one semiconductor to another,leading to efficient separation of photogenerated electron-hole pairs.27,28Therefore, rational design of composite semiconductors is an effective strategy for the exploitation of highly efficient photocatalysts for environmental remediation.Many successful examples have been developed in recent years,such as ZnO/In2O3,29V2O5/ ZnO,30Cu2O/TiO2,31ZnO/CeO2,32and so on.Besides,BiVO4-based coupled semiconductors,for example V2O5/BiVO4,28,33WO3/BiVO4,34BiVO4/Bi2O3,35have been reported to exhibit improved photocatalytic or photoelectrochemical performance.
In this work,a series of V2O5·xH2O-BiVO4composite photocatalysts were synthesized using the monoclinic V2O5·xH2O nanowires as the precursor under a mild condition by adjusting the reaction time.The synthesis-time-dependent phase contents,morphologies,and photocatalytic performances of V2O5· xH2O-BiVO4composites were investigated.
2 Experimental
2.1 Synthesis of V2O5·xH2O-BiVO4composites
All reagents were analytical grade,purchased from Shanghai Sinopharm Chemical Reagent Co.Ltd.and used without further purification.
Firstly,V2O5·xH2O precursor was prepared through a simple hydrothermal method using NH4VO3as the raw material in the presence of sulfuric acid at 180°C for 24 h,similar to that described in our previous paper.36Then,the as-prepared V2O5· xH2O precursor(about 1 mmol)was dispersed by ultrasound in 40 mL aqueous solution of 1 mmol Bi(NO3)3·5H2O to form a suspension,and diluted nitric acid(HNO3/H2O,volume ratio:1: 4)was added drop-wise under stirring until the pH of the solution was about 3.The mixture was sealed in a glass bottle(60 mL),kept static at 80°C for a definite period of time.The final product was collected by a centrifuge,washed with distilled water and absolute alcohol for several times to remove the possible residues and dried at 50°C for 12 h.Typically,V2O5·xH2OBiVO4composites obtained at the reaction stages of 6,12,and 24 h were named as V2O5-BiVO4-6,V2O5-BiVO4-12,and V2O5-BiVO4-24,respectively.
The details of the synthesis of pure BiVO4are presented in the Supporting Information(available free of charge via the internet at http://www.whxb.pku.edu.cn.).
2.2 Sample characterizations
The X-ray diffraction(XRD)patterns were recorded on a Japan Rigaku D/max-rA X-ray diffractometer equipped with graphite monochromatized high-intensity Cu Kαradiation(λ= 0.1542 nm).The field emission scanning electron microscopy (FESEM)images associated with energy-dispersive X-ray spectroscopy(EDX)were performed on JEOL JSM-6700F(Japan) at an accelerating voltage of 20 kV.The specific surface areas of the samples were measured by nitrogen adsorption BET method using a Micromeritics ASAP 2000 system(USA)after the sample was degassed in vacuum at 130°C overnight.The UV-Vis diffuse reflectance spectra(DRS)were recorded on a recording spectrophotometer(Perkin Elmer Lambda 950, USA).
2.3 Photocatalytic property test
The photocatalytic properties of the samples were evaluated by photo-degradation of the pollutant methylene blue(MB)under visible-light irradiation from a 300 W Xe light(CEL-HXF300/CEL-HXUV300,purchased from Beijing Zhongjiao Aulight Science and Technology Co.Ltd.)equipped with a 400 nm cutoff filter.In every experiment,80 mg of photocatalyst was suspended in 100 mL of 5.0×10-5mol·L-1aqueous solution of MB.Prior to irradiation,the suspension was stirred in the dark for 2 h to achieve an adsorption-desorption equilibrium between the photocatalyst and MB molecules.After that, the solution was exposed to the visible-light irradiation under magnetic stirring.At given time intervals,3 mL solution was sampled for analysis of the MB concentration.The photocatalytic degradation process was monitored using a UV-Vis spectrophotometer(Shimadzu UV2550,Japan)to record the characteristic absorption at 665 nm.
3 Results and discussion
3.1 Material characterizations
Fig.1 shows XRD pattern and FESEM image of the V2O5· xH2O precursor.The XRD pattern in Fig.1a is consistent with that of monoclinic V2O5·xH2O(JCPDS 07-0332).FESEM image in Fig.1b indicates that V2O5·xH2O precursor is composed of nanowires on a large scale,with tens of micrometres long, 100-150 nm wide,and 20-30 nm thick.

Fig.1 (a)XRD pattern and(b)FESEM image of V2O5·xH2O precursor
The crystallinity and phase compositions of the three typical composites,V2O5-BiVO4-6,V2O5-BiVO4-12,and V2O5-BiVO4-24,were examined by XRD measurements.All of the XRD patterns in Fig.2 indicate that the two types of crystals are in conformity with monoclinic V2O5·xH2O(JCPDS No.07-0332) and monoclinic structure of BiVO4(JCPDS No.75-2480).In addition,it is notable that the diffraction intensity of V2O5· xH2O component is degressive and that of the BiVO4component strengthens gradually from pattern a to pattern c,which results from the continuous consumption of V2O5·xH2O precursor and generation of BiVO4crystallites,along with the extending of reaction time.Thus,the XRD patterns can be indicative of the relative contents of V2O5·xH2O and BiVO4in the composites.As the reaction time was extended,the amount of V2O5·xH2O was gradually decreased and that of BiVO4was increased in the as-obtained V2O5·xH2O-BiVO4composites.The XRD patterns of pure BiVO4crystallites are shown in Fig.S1 (see the Supporting Information).
The morphologies of the three typical composites of V2O5-BiVO4-6,V2O5-BiVO4-12,and V2O5-BiVO4-24 were examined by a field-emission scanning electron microscope.The FESEM images are shown in Fig.3.As shown in Fig.3a,the V2O5-BiVO4-6 composite is composed of a large number of nanowires and some particles,in which the nanowires are tens of micrometers long,the same as the morphology of V2O5·xH2O precursor.A magnified SEM image in Fig.3b displays that the particles in the composite have dendritic structures,similar to those of the monoclinic BiVO4microcrystals in the previous reports.14,37The energy disperse X-ray(EDX)analysis attached to SEM further confirms that the nanowires are V2O5·xH2O and the dendritic particles are BiVO4(Fig.S2,Supporting Information).Therefore,it can be deduced that the V2O5-BiVO4-6 composite is composed of a large number of V2O5·xH2O nanowires and a small quantity of BiVO4microcrystals with dendritic structure.Fig.3(c,d)shows the FESEM images of V2O5-BiVO4-12 composite.From Fig.3c,it can be seen that V2O5-BiVO4-12 composite is composed of many microspheres with diameters in the range of 5-10 μm,and the magnified SEM image in Fig.3d clearly reveals that the surfaces of microspheres are compactly covered with nanowires.Compared with V2O5-BiVO4-6 composite,V2O5-BiVO4-12 composite is composed of more BiVO4crystallites and less V2O5·xH2O nanowires.When the reaction time was extended to 24 h,there was only a tiny amount of V2O5·xH2O nanowires remaining in the as-obtained V2O5-BiVO4-24 composite,as shown in Fig.3(e,f).The BiVO4microcrystals with dendritic structures account for almost all of morphology in the composite.Thus,the relative contents of V2O5·xH2Oand BiVO4in the composites are confirmed by the results observed from FESEM images,consistent with the information from XRD patterns.

Fig.2 XRD patterns of the samples(a)V2O5-BiVO4-6,(b)V2O5-BiVO4-12,(c)V2O5-BiVO4-24

Fig.3 FESEM images of the samples(a,b)V2O5-BiVO4-6,(c,d)V2O5-BiVO4-12,(e,f)V2O5-BiVO4-24
The specific surface areas of V2O5·xH2O-nanowire precursor and pure BiVO4crystallites as well as the three V2O5·xH2OBiVO4composites were measured by nitrogen adsorption BET method.The BET measurements showed that the specific surface areas of V2O5·xH2O-nanowire precursor and pure BiVO4crystallites are 26.1 and 7.5 m2·g-1,respectively.Otherwise, the specific surface areas of V2O5-BiVO4-6,V2O5-BiVO4-12, and V2O5-BiVO4-24 are 20.3,16.0,and 11.7 m2·g-1,respectively.The differences in specific surface areas of these samples are mostly ascribed to the sizes and dimensions of the particles.The low specific surface area of the BiVO4crystallites is due to the relatively bigger particle size.The incorporation of V2O5·xH2O-nanowire component contributes to an increase in the specific surface areas of the V2O5·xH2O-BiVO4composites.
3.2 Formation mechanism of V2O5·xH2O-BiVO4composites
In this work,V2O5·xH2O-BiVO4composites were fabricated by a two-step method,in which the V2O5·xH2O-nanowire precursor was firstly prepared by a simple hydrothermal method described in our previous paper.36Then V2O5·xH2O-nanowire precursor was used to fabricate V2O5·xH2O-BiVO4composites by a solid-solution reaction between V2O5·xH2O precursor and Bi3+ions in acidic solution at a low temperature(80°C).The formation of the V2O5·xH2O-nanowire precursor was based on the H+intercalating and splitting process,which has been discussed specifically in the previous paper.36Regarding the formation of V2O5·xH2O-BiVO4composites at the three typical reaction stages,the schematic illustration for the growth process is presented in Fig.4.The reaction was carried out in acidic solution to avoid the hydrolysis of Bi3+ions.After the V2O5·xH2O nanowire precursor was dispersed in acidic solution of Bi(NO)3by ultrasound,BiVO4crystal nucleuses would slowly form via the reactionbetweenV2O5·xH2O precursorandBi3+ionsas Eq.(1):

It has been demonstrated that monoclinic BiVO4is inclined to form the microcrystals with branched morphology in acidic solution because of its structural characteristics and the induction of protons.37As the reaction proceeded,BiVO4crystal nucleuses grew into dendritic BiVO4crystallites,and thus the V2O5· xH2O-BiVO4composite obtained at the reaction time of 6 h was composed of a large number of V2O5·xH2O nanowires and a small quantity of dendritic BiVO4crystallites.When the reaction time was extended,more BiVO4crystallites formed at the consumption of V2O5·xH2O precursor,and meanwhile,V2O5· xH2O nanowires intertwined with BiVO4crystallites to form the BiVO4microspheres covered by V2O5·xH2O nanowires,as shown by the FESEM images of V2O5-BiVO4-12 sample. When the reaction continued for 24 h,most of the V2O5·xH2O nanowires converted into BiVO4crystallites that grew up into square-branched structures.Such a strategy is convenient and efficient to obtain the V2O5·xH2O-BiVO4composite photocatalyst with different component contents only by setting the reaction time.
3.3 UV-Vis diffusion reflectance spectra
The color of V2O5-BiVO4-6 composite containing more V2O5·xH2O component is brownish yellow,while the color of V2O5-BiVO4-24 composite containing more BiVO4component is bright yellow.The optical properties of the as-prepared V2O5· xH2O-BiVO4composites as well as V2O5·xH2O precursor and pure BiVO4crystallites were probed by UV-Vis diffuse reflectance spectroscopy(DRS).Fig.5 shows the UV-Vis DRS of different photocatalysts.The relationship between absorption coefficient(α)and incident photon energy(hν)can be described as Eq.(2)for direct electron transition.


Fig.4 Schematic diagram for the growth process of V2O5· xH2O-BiVO4nanocomposites
where A is a constant,and Egis the direct band gap energy.As shown in Fig.5b,the band gaps of V2O5·xH2O precursor and pure BiVO4crystallites,estimated from the intercept of the tangents to the plots,are 2.26 and 2.49 eV,respectively.The presence of V2O5·xH2O component makes the absorption edges of the composites shift to long wavelength region.The optical absorptions of the V2O5·xH2O-BiVO4composites start at about 600 nm,corresponding to the absorption edge of V2O5·xH2O. Therefore,the visible-light responsiveness of the V2O5·xH2OBiVO4composites is expected to find important application in photocatalysis,since more sunlight can be effectively used for photocatalytic reactions.

Fig.5 (a)UV-Vis diffuse reflectance spectra of V2O5·xH2Onanowires,BiVO4crystallites,and V2O5·xH2O-BiVO4composites; (b)band gaps(Eg)of V2O5·xH2O nanowires and BiVO4crystallites estimated from the absorption edge
3.4 Photocatalytic performances and photocatalytic mechanism
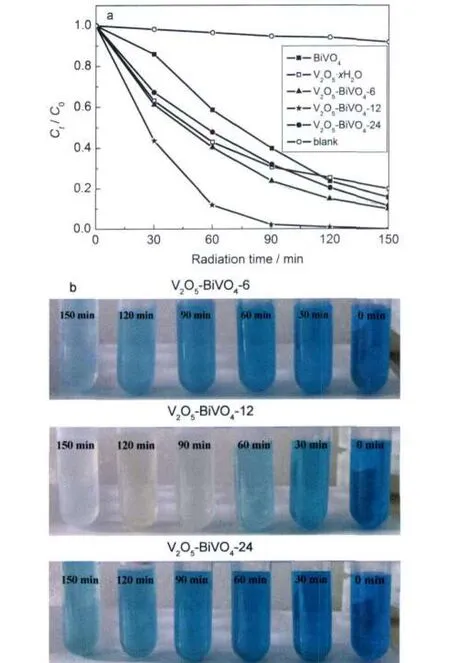
Fig.6 (a)Photodegradation of MB(5×10-5mol·L-1,100 mL) using different photocatalysts under visible light,(b)photograph images of the sampled dye solutions during 150 min irradiation under visible light
The photocatalytic performances of the as-prepared V2O5· xH2O-BiVO4composite photocatalysts were evaluated by testing the photodegradation of methylene blue(MB)under visible-light irradiation from a 300 W Xe lamp.Prior to irradiation,the photocatalytic reaction systems were magnetically stirred in the dark for 2 h to reach the adsorption/desorption equilibrium of MB on the particle surface of the photocatalyst. After this treatment,the MB concentration measured was used as the initial concentration(C0).Fig.6a displays the time course of the decrease in MB concentration using different photocatalysts and under the irradiation of the visible light(λ>400 nm), where C0and Ctare the initial concentration after the equilibrium adsorption and the reaction concentration of MB,respectively.A blank test(MB without any photocatalyst)under irradiation exhibited no obvious change in the concentration of MB.After 150 min of irradiation,we can see that the concentrations of MB in the systems with different photocatalysts are diverse.Especially,the MB concentration at this time in the system containing the V2O5-BiVO4-12 photocatalyst is distinct-ly lower than those in the systems containing other photocatalysts.The decreases of MB concentrations in the presence of pure BiVO4and pure V2O5·xH2O precursor both are smaller than those with V2O5·xH2O-BiVO4composites as photocatalysts after visible-light irradiation for 150 min.As for the V2O5· xH2O-BiVO4composite photocatalysts,V2O5-BiVO4-12 sample is found to exhibit the highest photocatalytic activity,and about 97.8%of MB is photodegraded from the aqueous solution after visible-light irradiation for 90 min,100%after 150 min.Additionally,the synthesis-time-dependent photocatalytic activity was also proved by the photograph images(Fig.6b), which showed the color-change sequence of MB solutions during 150 min irradiation under Xe lamp light and in the presence of different photocatalysts.As shown in Fig.6b,the blue color of MB was decolorized almost after 90 min irradiation in the system containing V2O5-BiVO4-12 as the photocatalyst. Whereas,both of the solutions containing V2O5-BiVO4-6 and V2O5-BiVO4-24 still presented light blue after 150 min irradiation,suggesting that some MB molecules remained.In the presence of a photocatalyst,the absorption intensity of MB gradually decreased without any shift in the absorption wavelength after increasing irradiation time(Fig.S3,Supporting Information),suggesting the degradation of MB molecules.
The photocatalysis of semiconductors generally involves two mechanisms.The first is based on the excitation of the semiconductor,which involves excitation of the semiconductor by light irradiation to form photogenerated electrons in the conduction band(CB)and holes in the valence band(VB),and the subsequent chemical reactions with the surrounding media after the photogenerated charges move to the particle surface.38It is obvious that the separation efficiency of the photogenerated electron-hole pairs and the lifetime of the charge carriers in photocatalyst are crucial for the photocatalytic performance.39,40According to the band-edge positions of V2O5and BiVO4reported previously,28,33the charge transport in V2O5·xH2O-BiVO4composite photocatalyst is probably explained on the basis of the difference of band potentials between V2O5crystallites and BiVO4crystallites,as illustrated in Fig.7.This potential difference induces a contact electric field at the interface of V2O5· xH2O-BiVO4composite.When electrons and holes are photogenerated inV2O5·xH2O and BiVO4crystallites,the excited electrons in the conduction band of BiVO4crystallites are transferred to the conduction band of V2O5·xH2O.Whereas the excited holes on the valance band of BiVO4are difficult to transfer to the valance band of V2O5·xH2O because their valance band potentials are very close.Thus,the separation of photogenerated electron-hole pairs in BiVO4crystallites is promoted and the recombination of electron-hole pairs is suppressed,which greatly facilitatesandenhancesthephotocatalyticreaction.
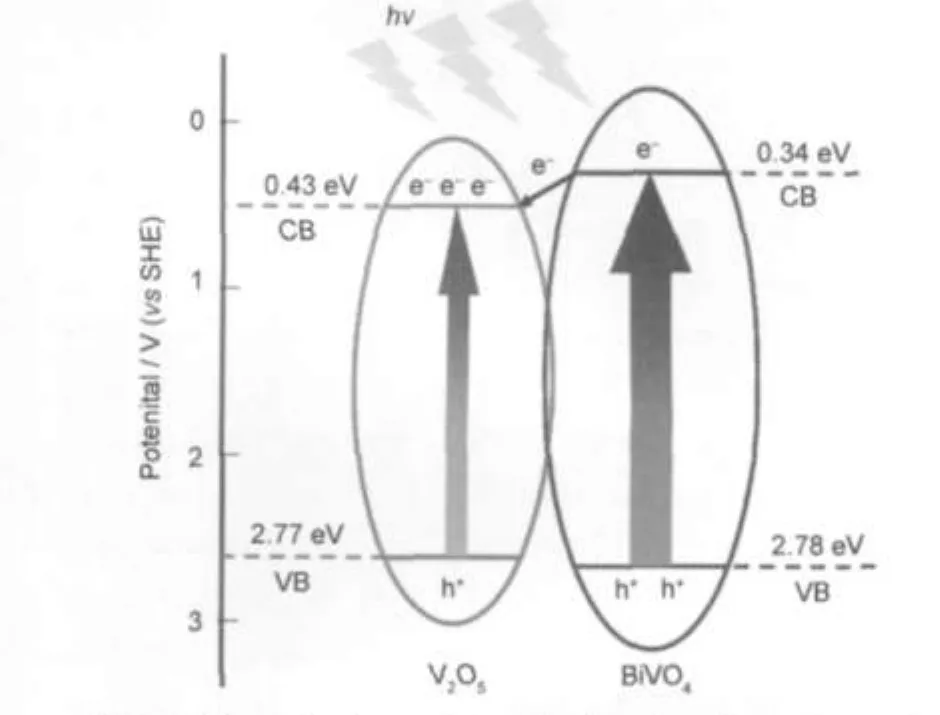
Fig.7 Schematic diagram showing band configuration and electron-hole separation at interface of V2O5·xH2O-BiVO4 composite under visible light
The other mechanism is based on the excitation of dye.41,42The process includes the excitation of dye molecules by visible light and the subsequent electron injection or electron transfer from the excited dye(dye*)to the conduction band of semiconductor.Then,the electron is trapped by surface adsorbed O2to generate various reactive oxidation species.The cationic dye radical(dye*+)subsequently self degrades or is degraded by reactive oxidation species.Considering that the conductionband-edge positions of V2O5·xH2O and BiVO4are very low, electrons can be transferred from the excited MB molecules (MB*)to the conduction band of V2O5·xH2O-BiVO4.Direct electron transfer from MB*to the V2O5·xH2O semiconductor is favorable,43because the potential of the conduction band of V2O5·xH2O is lower than that of BiVO4.Moreover,V2O5·xH2O component with larger specific surface area allows good access of dye molecules to its surface in the photocatalytic system. Therefore,degradation of MB in the present case is determined by the cooperative effect of both the excitation of semiconductor and the excitation of dye.
The synthesis-time-dependent photocatalytic performance of V2O5·xH2O-BiVO4composite essentially reflects the effect of the proportion of BiVO4and V2O5·xH2O in the composite on the photocatalysis.As demonstrated in the present work,the V2O5-BiVO4-12 sample shows distinctly more enhanced photocatalytic activity than the other V2O5·xH2O-BiVO4composites, presumably because the component proportion of V2O5-BiVO4-12 is favorable for the cooperative effect of two photocatalysis mechanisms.In V2O5-BiVO4-12 photocatalyst,the separation of photogenerated electron-hole pairs in BiVO4crystallites is achieved,and meanwhile the V2O5·xH2O component is enough for the absorption and excitation of the dye molecules as much as possible.As for V2O5-BiVO4-6 and V2O5-BiVO4-24 samples, exiguous BiVO4or V2O5·xH2O component is detrimental to either the separation of photogenerated electron-hole pairs or the excitation of dye molecules,resulting in the inferior photocatalytic efficiency.
4 Conclusions
V2O5·xH2O-BiVO4composite photocatalysts were synthesized by using the monoclinic V2O5·xH2O nanowires as the pre-cursor under a mild condition.The synthesis-time-dependent phase contents,morphology,and photocatalytic performance of V2O5·xH2O-BiVO4composites were investigated.XRD and SEM measurements reveal that V2O5·xH2O-BiVO4composites are composed of the monoclinic BiVO4crystallites and V2O5· xH2O nanowires.As the reaction time was extended,more and larger BiVO4crystallites formed at the expense of V2O5·xH2O nanowires.The morphology of BiVO4crystallites evolved over the reaction time.UV-Vis DRS shows that all the V2O5· xH2O-BiVO4composite photocatalysts have strong absorption in visible-light region.The results of photocatalytic performance indicate that V2O5·xH2O-BiVO4composite photocatalysts exhibit the increased photocatalytic efficiency for the degradation of MB under the visible light(λ>400 nm).Moreover, the V2O5·xH2O-BiVO4composite photocatalyst obtained at the reaction stage of 12 h exhibits the best photocatalytic degradation activity.The enhanced photocatalytic performance of V2O5·xH2O-BiVO4composite was determined by the cooperative effect of the two photocatalysis mechanisms involving the excitationof thesemiconductorandtheexcitationof dye.
(1) Fujishima,A.;Honda,K.Nature 1972,238,37.
(2) Lu,F.;Cai,W.P.;Zhang,Y.G.Adv.Funct.Mater.2008,18, 1047.
(3)Yang,D.J.;Liu,H.W.;Zheng,Z.F.;Yuan,Y.;Zhao,J.C.; Waclawik,E.R.;Ke,X.B.;Zhu,H.Y.J.Am.Chem.Soc.2009, 131,17885.
(4) Li,B.X.;Wang,Y.F.J.Phys.Chem.C 2010,114,890.
(5)Song,X.M.;Wu,J.M.;Tang,M.Z.;Qi,B.;Yan,M.J.Phys. Chem.C 2008,112,19484.
(6)Zhang,H.;Chen,G.;Bahnemann,D.W.J.Mater.Chem.2009, 19,5089.
(7)Shang,M.;Wang,W.Z.;Sun,S.M.;Ren,J.;Zhou,L.;Zhang, L.J.Phys.Chem.C 2009,113,20228.
(8) Zhang,A.P.;Zhang,J.Z.Acta Phys.-Chim.Sin.2010,26, 1337.[张爱平,张进治.物理化学学报,2010,26,1337.]
(9) Xiao,X.;Zhang,W.D.J.Mater.Chem.2010,20,5866.
(10)Shang,M.;Wang,W.Z.;Xu,H.L.Crystal Growth Des.2009, 9,991.
(11) Wu,J.;Duan,F.;Zheng,Y.;Xie,Y.J.Phys.Chem.C 2007,111, 12866.
(12)Ma,D.K.;Huang,S.M.;Chen,W.X.;Hu,S.W.;Shi,F.F.; Fan,K.L.J.Phys.Chem.C 2009,113,4369.
(13)Bi,J.H.;Wu,L.;Li,H.;Li,Z.H.;Wang,X.X.;Fu,X.Z.Acta Mater.2007,55,4699.
(14) Zheng,Y.;Wu,J.;Duan,F.;Xie,Y.Chem.Lett.2007,36,520.
(15)Shang,M.;Wang,W.Z.;Ren,J.;Sun,S.M.;Zhang,L. CrystEngComm 2010,12,1754.
(16) Ruan,Q.J.;Zhang,W.D.J.Phys.Chem.C 2009,113,4168.
(17)Shang,M.;Wang,W.Z.;Zhou,L.;Sun,S.M.;Yin,W.Z. J.Hazard.Mater.2009,172,338.
(18)Jiang,H.Y.;Dai,G.X.;Meng,X.;Ji,K.M.;Zhang,L.;Deng, J.G.Appl.Catal.B-Environ.2011,105,326.
(19) Iwase,A.;Kudo,A.J.Mater.Chem.2010,20,7536.
(20) Wang,D.E.;Jiang,H.F.;Zong,X.;Xu,Q.;Ma,Y.;Li,G.L.; Li,C.Chem.Eur.J.2011,17,1275.
(21) Zhang,X.;Udawa,K.;Liu,Z.;Nishimoto,S.;Xu,C.;Lu,Y.; Sakai,H.;Ave,M.;Marakoi,T.;Kujishima,A.J.Photochem. Photobiol.A 2009,202,39.
(22) Li,B.X.;Wang,Y.F.J.Phys.Chem.Solids 2011,72,1165.
(23) Du,H.;Wang,S.;Liu,L.L.;Liu,Z.X.;Li,Z.;Lu,N.;Liu,F.S. Acta Phys.-Chim.Sin.2010,26,2726.[杜 欢,王 晟,刘恋恋,刘忠祥,李 振,卢 南,刘福生.物理化学学报,2010,26, 2726.]
(24)Chen,P.;Gu,L.;Cao,X.B.CrystEngComm 2010,12,3950.
(25)Shang,M.;Wang,W.Z.;Zhang,L.;Sun,S.M.;Wang,L.; Zhou,L.J.Phys.Chem.C 2009,113,14727.
(26)Lu,M.Y.;Song,J.H.;Lu,M.P.,Lee,C.Y.;Chen,L.J.;Wang, Z.L.ACS Nano 2009,3,357.
(27)Xu,F.;Volkov,V.;Zhu,Y.M.;Bai,H.Y.;Rea,A.;Valappil,N. V.;Su,W.;Gao,X.Y.;Kuskovsky,I.L.;Matsui,H.J.Phys. Chem.C 2009,113,19419.
(28)Su,J.;Zou,X.X.;Li,G.D.;Wei,X.;Yan,C.Wang,Y.N.; Zhao,J.;Zhou,L.J.;Chen,J.S.J.Phys.Chem.C 2011,115, 8064.
(29)Wang,Z.Y.;Huang,B.B.;Dai,Y.;Qin,X.Y.;Zhang,X.Y.; Wang,P.;Liu,H.X.;Yu,J.X.J.Phys.Chem.C 2009,113, 4612.
(30)Zou,C.W.;Rao,Y.F.;Alyamani,A.;Chu,W.;Chen,M.J.; Patterson,D.A.;Emanuelsson,E.A.C.;Gao,W.Langmuir 2010,26,11615.
(31)Lalitha,K.;Sadanandam,G.;Kumari,V.D.;Subrahmanyam, M.;Sreedhar,B.;Hebalkar,N.Y.J.Phys.Chem.C 2010,114, 22181.
(32) Ma,T.Y.;Yuan,Z.Y.;Cao,J.L.Eur.J.Inorg.Chem.2010,716.
(33) Jiang,H.Q.;Nagai,M.;Kobayashi,K.J.Alloy.Compd.2009, 479,821.
(34) Su,J.Z.;Guo,L.J.;Bao,N.Z.;Grimes,C.A.Nano Lett.2011, 11,1928.
(35)Guan,M.L.;Ma,D.K.;Hu,S.W.;Chen,Y.J.;Huang,S.M. Inorg.Chem.2011,50,800.
(36) Li,B.X.;Xu,Y.;Rong,G.X.;Jing,M.;Xie,Y.Nanotechnology 2006,17,2560.
(37) Zhao,Y.;Xie,Y.;Zhu,X.;Yan,S.;Wang,S.X.Chem.Eur.J. 2008,14,1601.
(38) Fujishima,A.;Zhang,X.T.;Tryk,D.A.Surf.Sci.Rep.2008, 63,515.
(39) Wu,Y.;Tamaki,T.;Volotinen,T.;Belova,L.;Rao,K.V.J.Phys. Chem.Lett.2010,1,89.
(40) Zhai,X.H.;Long,H.J.;Dong,J.Z.;Cao,Y.A.Acta Phys.-Chim.Sin.2010,26,663. [翟晓辉,龙绘锦,董江舟,曹亚安.物理化学学报,2010,26,663.]
(41) Li,B.J.;Cao,H.Q.J.Mater.Chem.2011,21,3346.
(42) Clifford,J.N.;Palomares,E.;Nazeeruddin,M.K.;Thampi,R.; Grätzel,M.;Durrant,J.R.J.Am.Chem.Soc.2004,126,5670.
(43)Kamat,P.V.Chem.Rev.1993,93,267.
July 15,2011;Revised:October 10,2011;Published on Web:October 18,2011.
Adjustable Synthesis and Visible-Light Responsive Photocatalytic Performance of V2O5·xH2O-BiVO4Nanocomposites
LI Ben-Xia*WANG Yan-Fen LIU Tong-Xuan
(College of Materials Science and Engineering,Anhui University of Science&Technology,Huainan 232001, Anhui Province,P.R.China)
V2O5·xH2O-BiVO4composite photocatalysts were synthesized using monoclinic V2O5·xH2O nanowires as a precursor under mild conditions.To determine the synthesis-time-dependent phase content,morphology,and photocatalytic performance of the products,three typical V2O5·xH2O-BiVO4samples were obtained at 6,12,and 24 h.These samples were investigated using X-ray diffraction,fieldemission scanning electron microscopy,UV-visible diffusion reflectance spectroscopy,and a photocatalytic property test.The results indicate that the V2O5·xH2O-BiVO4nanocomposites are composed of V2O5·xH2O nanowires and BiVO4nanocrystals.As the reaction time was extended,the amount of V2O5·xH2O nanowires decreased gradually and that of the BiVO4nanocrystals increased in the composite.The results of photocatalytic performance indicated that the V2O5·xH2O-BiVO4composite photocatalysts exhibited enhanced photocatalytic efficiency for the degradation of methylene blue(MB)under visible-light irradiation (λ>400 nm).The V2O5·xH2O-BiVO4sample obtained at 12 h exhibited the best photocatalytic activity,which is probably because of the appropriate proportion of components and the special microstructures of the sample that were favorable for a synergistic effect between the two photocatalysis mechanisms involving the excitation of the semiconductor and the excitation of the dye,respectively.
Composite photocatalyst;Chemical synthesis;Characterization;Photodegradation; Organic dye
10.3866/PKU.WHXB20112946
O643
*Corresponding author.Email:bxli@aust.edu.cn;Tel:+86-554-6668649.
The project was supported by the National Natural Science Foundation of China(21001003),Natural Science Foundation of Anhui Province,China (10040606Q15),and Natural Science Research for Colleges and Universities ofAnhui Province,China(KJ2010A101).
国家自然科学基金(21001003),安徽省自然科学基金(10040606Q15)及安徽高校省级自然科学研究重点项目(KJ2010A101)资助

