甲醛N-叔丁基腙与环状N-酰基三氟酮亚胺的亲核加成反应
祝小艳,聂 晶
(天津大学理学院,天津300072)
偶氮化合物在有机化学领域有着非常重要的作用,不仅可以作为含氮亲电试剂用于有机合成[1],也是一类用途广泛的自由基引发剂[2]。偶氮化合物可以作为食品色素、染料等,还可以作为药物前体,在人体酶的作用下释放出有药物活性的物质[3]。然而,由于脂肪族偶氮化合物受热易分解[4],高效简便地合成此类化合物的方法一直受到化学家的关注。已有的报道大部分是利用芳基重氮盐结合非活泼的烯烃以及烯烃衍生物,通过自由基反应来获得脂肪族偶氮化合物[5-8]。通过甲醛单取代腙与其它亲电底物反应是合成脂肪族偶氮化合物的另一条有效途径,但相关报道还很少[9]。近年来,Lassaletta小组利用甲醛N-叔丁基腙与α-羰基酸酯及硝基烯烃的不对称加成反应,得到一系列手性脂肪族偶氮化合物[10,11]。然而,通过甲醛单取代腙与酮亚胺的加成反应来合成脂肪族偶氮化合物的反应尚未见文献报道。
鉴于此,作者在碱作用下通过甲醛N-叔丁基腙与环状N-酰基三氟酮亚胺的亲核加成反应,合成了新型三氟甲基取代偶氮化合物。由于所得偶氮化合物在酸性条件下容易发生异构化反应形成腙,它是重要的合成中间体,可以转化为相应的醛类化合物和腈类化合物[12]。因此,该研究不仅扩展了腙参与的亲核加成反应的类型,并且三氟甲基的引入往往会赋予母体化合物特殊的化学、物理、生理性质[13],具有重要的理论意义和潜在的应用价值。
1 实验
1.1 试剂与仪器
甲醛(37%)、三氟乙酸(99%),天津光复精细化工研究所;叔丁基肼盐酸盐(97%),上海韶远化学科技有限公司;氢氧化钠、碳酸钠,市售分析纯;环状N-酰基三氟酮亚胺(Ⅰ)按文献[14]制备;甲醛 N-叔丁基腙(Ⅱ)按文献[15]制备;二氯甲烷、四氢呋喃、甲苯、乙腈、甲醇,均经过重蒸处理。
SGW X-4型显微熔点仪,AVATAR 360FTIR型光谱仪,microTOF-QⅡ型质谱仪,Varian 400MHz型核磁共振仪(内标为Me4Si)。
1.2 甲醛N-叔丁基腙(Ⅱ)与环状N-酰基三氟酮亚胺(Ⅰa)的亲核加成反应(图1)

图1 甲醛N-叔丁基腙与环状N-酰基三氟酮亚胺的亲核加成反应Fig.1 Nucleophilic addition of formaldehyde N-tertbutyl hydrazone to cyclic N-acyl trifluoromethylketimine
在10mL的Schlenk瓶中加入化合物Ⅰa(19mg,0.05mmol)、Et3N(7.0μL,0.05mmol)、CH2Cl2(2mL),室 温 搅 拌 下 加 入 化 合 物 Ⅱ (14μL,0.1 mmol),室温搅拌12h,经TLC检测反应完全,通过硅胶柱[洗脱剂为:乙酸乙酯∶石油醚(60~90℃)=1∶5(体积比)]纯化得到白色固体Ⅲa 15.7mg,熔点47~50℃,收率58%。
1.3 加成产物Ⅲa的异构化反应
在三氟乙酸(TFA)作用下,对加成产物Ⅲa进行进一步的转化,得到Ⅲa的异构化产物N-叔丁基腙(Ⅳa),如图2所示。

图2 加成产物Ⅲa的异构化反应Fig.2 Isomerization of the adductⅢa
在10mL的Schlenk瓶中加入加成产物Ⅲa(105mg,0.3mmol),加入CH2Cl22mL溶解,将体系降至0℃,再滴加TFA(36mg,0.3mmol),搅拌12h后,将饱和NaHCO3溶液加入到反应体系中,然后以乙酸乙酯萃取,无水硫酸镁干燥,最后柱纯化[洗脱剂为:乙酸乙酯∶石油醚(60~90℃)=1∶3(体积比)],得到白色固体Ⅳa 132mg,熔点67~72℃,收率94%。
2 结果与讨论
2.1 反应条件的优化
在环状N-酰基三氟酮亚胺用量为0.05mmol、甲醛N-叔丁基腙用量为1.0mmol、碱用量为0.05 mmol、溶剂用量为2mL的条件下,考察碱和溶剂对亲核加成反应的影响,结果见表1 。
由表1 可看出,当使用三乙胺(Et3N)和三乙烯二胺(DABCO)时,分离收率不高;当使用碱性更强的1,8-二氮杂二环[5.4.0]十一碳-7-烯(DBU)时,反应主要生成其它副产物,分离收率很低;当使用强碱弱酸盐如Na2CO3、K2CO3时,分离收率有较大提高,其中使用Na2CO3时收率最高;当不加碱时,分离收率也能达到83%。参考甲醛腙与三氟甲基酮的加成反应[16],分析认为环状N-酰基三氟酮亚胺C=N双键上连有拉电子能力较强的三氟甲基,提高了C=N双键上C原子的亲电性,同时醛腙中NH和C=N双键发生共轭,使得C=N双键中C原子具有亲核性[17],因此,即使不加碱时反应仍能进行。

表1 碱和溶剂对亲核加成反应的影响Tab.1 Effect of bases and solvents on nucleophilic addition
由表1 还可看出,不同溶剂对分离收率的影响不同。以Na2CO3为碱,当使用甲醇作溶剂时,完全形成了甲氧基与Ⅰa加成的副产物,分离收率为0%;由于底物Ⅰa在乙醚中溶解性较差,因此分离收率较低,为71%;当使用四氢呋喃作溶剂时,反应体系较复杂,副产物的生成降低了分离收率,仅为58%;当使用二氯甲烷、甲苯和二甲苯为溶剂时,分离收率都能达到90%以上(4#、8#和12#)。因此,确定最佳反应条件为:100%Na2CO3作为碱,2mL二氯甲烷作为溶剂,室温反应12h。
2.2 反应底物拓展
在最优反应条件下,对亲核加成反应的底物适应性进行研究,反应式如图3所示,结果见表2 。

图3 化合物Ⅱ与化合物Ⅰa~Ⅰn的亲核加成反应Fig.3 Nucleophilic addition of compoundⅡto compoundsⅠa~Ⅰn
由表2 可以看出,无论环状N-酰基三氟酮亚胺中芳环上的取代基是吸电子基团(Ⅰa~Ⅰd)还是给电子基团(Ⅰf~Ⅰh),都能以中等到好的收率(88%~99%)得到目标产物;即使芳环上没有取代基时(Ⅰe)也能以93%的收率获得加成产物Ⅲe;芳环上有2个卤原子取代基(Ⅰi~Ⅰj)时,产物(Ⅲi、Ⅲj)的收率高达95%以上;将底物中N原子上的保护基改为2,4,6-三甲基苄基(Ⅰk)时,能以接近当量的收率(99%)得到目标产物Ⅲk;即使选用位阻较大的1-萘甲基作为保护基(Ⅰl),产物的收率仍能达到97%。由此可见,保护基团的空间位阻作用对该亲核加成反应收率的影响并不明显。但是,当选用溶解性较差的底物Ⅰm时,反应速率减慢,反应时间需延长至2d,同时会伴随有Ⅰm与水加成的副产物生成,产物的收率有所降低(86%);若将底物中的三氟甲基替换成二氟甲基时,产物的收率明显下降(Ⅲn)。这是由于,底物Ⅰ中C=N双键邻位取代基的拉电子能力越强,C=N双键中C原子的亲电性就越强,越容易被亲核试剂Ⅱ进攻;而Ⅰn中二氟甲基的拉电子能力较弱,因此反应收率降低。
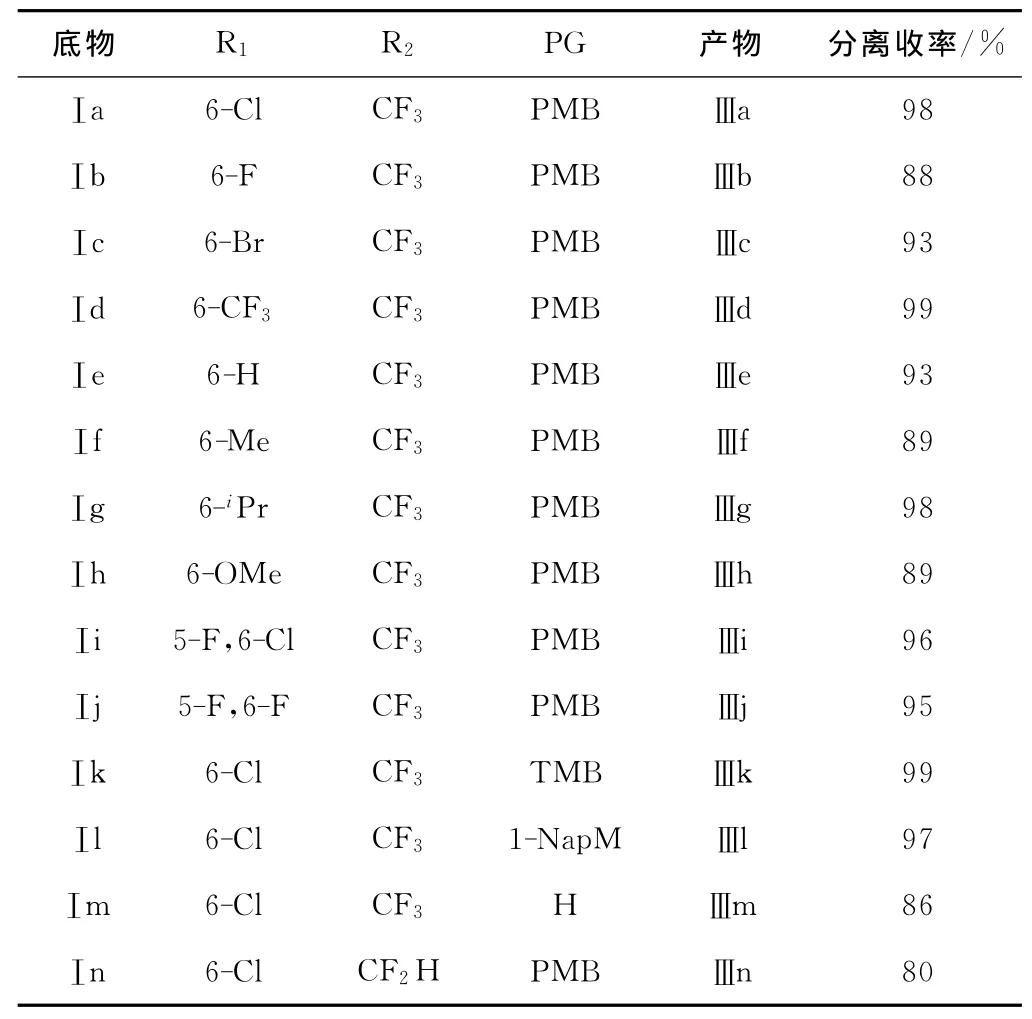
表2 亲核加成反应结果Tab.2 Results of nucleophilic addition
2.3 产物表征
Ⅲa:白色固体,收率98%,m.p.47~50 ℃。1HNMR (400MHz,CDCl3),δ:7.25 (s,1H),7.20~7.13(m,3H),6.84(d,J=8.6Hz,2H),6.82~6.75(m,2H),5.11(dd,J=45.3Hz,16.4Hz,2H),4.37~4.27(m,2H),3.76(s,3H),1.15(s,9H)。19FNMR(376MHz,CDCl3),δ:-79.06(s,3F)。13CNMR(100 MHz,CDCl3),δ:159.0,152.5,137.3,130.4,128.3,127.8,127.7,127.2,124.9(q,1JF-C=286.9Hz),117.6,116.4,114.4,70.8,68.9,61.5 (q,2JF-C=28.7 Hz),55.4,45.7,26.7。HRMS(ESI),m/z:found 491.1439[M+Na]+;calcd.for C22H24ClF3N4O2+Na:491.1438。IR (KBr),ν,cm-1:3218,3094,2971,1687,1514,1433,1396,1250,1174,809。
Ⅲb:白色固体,收率88%,m.p.48~51℃。1HNMR(400MHz,CDCl3),δ:7.17(d,J=8.5Hz,2H),7.02(d,J=8.7Hz,1H),6.96~6.91(m,1H),6.87~6.79(m,3H),6.72~6.56(m,1H),5.11(dd,J=39.6 Hz,16.4Hz,2H),4.30(s,2H),3.76(s,3H),1.15(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.94(s,3F),-120.53~ -120.56(m,1F)。13CNMR(100 MHz,CDCl3),δ:159.0,158.0(d,1JF-C=241.1Hz),152.6,134.9,128.5,127.7,125.0(q,1JF-C=286.4Hz),117.5(d,3JF-C=6.6Hz),117.3(d,2JF-C=22.2Hz),116.4 (d,3JF-C=7.6Hz),114.4,114.1 (d,2JF-C=24.7Hz),70.9,69.0,61.5(q,2JF-C=28.9Hz),55.4,45.9,26.7。HRMS(ESI),m/z:found 475.1735 [M+Na]+;calcd.for C22H24F4N4O2+ Na:475.1733。IR(KBr),ν,cm-1:3222,3092,2972,1686,1515,1447,1400,1250,1178。
Ⅲc:白色固体,收率93%,m.p.54~57℃。1HNMR(400MHz,CDCl3),δ:7.37(s,1H),7.31(d,J=8.8 Hz,1H),7.16(d,J=8.4Hz,2H),6.85~6.83(m,3H),6.73(d,J=8.9Hz,1H),5.10(dd,J=44.9Hz,16.4Hz,2H),4.36~4.27(m,2H),3.76(s,3H),1.15(s,9H)。19FNMR(376MHz,CDCl3),δ:-79.06(s,3F)。13CNMR(100MHz,CDCl3),δ:159.0,152.5,137.7,133.3,130.0,128.3,127.7,124.9(q,1JF-C=286.8Hz),118.0,116.7,115.0,114.4,70.8,68.9,61.4(q,2JF-C=28.2Hz),55.4,45.7,26.7。HRMS(ESI),m/z:found 535.0925 [M+Na]+;calcd.for C22H24BrF3N4O2+Na:535.0932。IR(KBr),ν,cm-1:3219,3092,2971,1688,1595,1512,1429,1393,1252,1173,1104,1034,810,523。
Ⅲd:白色固体,收率99%,m.p.48~51℃。1HNMR(400MHz,CDCl3),δ:7.52~7.47(m,2H),7.18(d,J=8.4Hz,2H),7.10~7.02(m,1H),6.96(d,J=8.7 Hz,1H),6.85(d,J=8.5Hz,2H),5.16(dd,J=38.0 Hz,16.4Hz,2H),4.42~4.34(m,2H),3.77(s,3H),1.13(s,9H)。19FNMR(376MHz,CDCl3),δ:-62.17(s,3F),-79.41(s,3F)。13CNMR(100MHz,CDCl3),δ:159.1,152.6,141.5,129.0,128.0,127.7,124.9(q,1JF-C=286.6Hz),124.6,124.6(q,2JF-C=33.2Hz),123.9(q,1JF-C=269.9Hz),116.5,115.3,114.5,70.7,69.0,61.7(q,2JF-C=29.0Hz),55.4,45.8,26.7。HRMS(ESI),m/z:found 525.1694[M+Na]+;calcd.for C23H24F6N4O2+Na:525.1701。IR (KBr),ν,cm-1:3219,3092,2971,1688,1595,1512,1429,1393,1252,1173,1104,1034,810。
Ⅲe:白色固体,收率93%,m.p.43~46℃。1HNMR(400MHz,CDCl3),δ:7.30~7.28(m,1H),7.25~7.17(m,3H),7.02(t,J=7.6Hz,1H),6.87(t,J=9.5Hz,3H),6.60(s,1H),5.15(q,J=16.4Hz,2H),4.36(q,J=13.8Hz,2H),3.77(s,3H),1.18 (s,9H)。19FNMR(376MHz,CDCl3),δ:-78.99(s,3F)。13CNMR(100MHz,CDCl3),δ:158.9,152.7,138.5,130.4,128.8,127.7,126.8,125.2(q,1JF-C=286.8Hz),122.4,116.1,115.0,114.3,71.1,68.8,61.5(q,2JF-C=27.6Hz),55.3,45.6,26.7。HRMS(ESI),m/z:found 457.1827 [M+Na]+;calcd.for C22H25F3N4O2+Na:457.1827。IR (KBr),ν,cm-1:3220,3087,2971,1686,1513,1462,1407,1250,1173,1035,750。
Ⅲf:白色固体,收率89%,m.p.164~166℃。1HNMR(400MHz,CDCl3),δ:7.18(d,J=8.4Hz,2H),7.08~6.99(m,2H),6.83(d,J=8.5Hz,2H),6.75(d,J=8.4Hz,1H),6.54~6.34(m,1H),5.11(dd,J=39.2Hz,16.3Hz,2H),4.33(dd,J=34.7 Hz,13.9Hz,2H),3.75(s,3H),2.25(s,3H),1.17(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.76(s,3F)。13CNMR(100MHz,CDCl3),δ:158.9,152.7,136.1,132.0,131.0,129.0,127.7,127.2,125.2(q,1JF-C=287.2Hz),116.1,115.0,114.3,71.3,68.8,61.4(q,2JF-C=28.2Hz),55.3,45.5,26.7,20.7。HRMS(ESI),m/z:found 471.1976[M + Na]+;calcd.for C23H27F3N4O2+Na:471.1984。IR (KBr),ν,cm-1:3221,3091,2970,1685,1515,1438,1250,1174,1034,810。
Ⅲg:白 色 固 体,收 率 98%,m.p.49~54 ℃。1HNMR(400MHz,CDCl3),δ:7.20(d,J=8.5Hz,2H),7.09(d,J=8.8Hz,2H),6.85~6.79(m,3H),6.45(s,1H),5.20~5.04(m,2H),4.40(d,J=13.9 Hz,1H),4.30(d,J=13.9Hz,1H),3.76(s,3H),1.18(s,15H)。19FNMR(376MHz,CDCl3),δ:-78.78(s,3F)。13CNMR(100MHz,CDCl3),δ:158.9,152.7,143.0,136.2,129.0,128.4,127.8,125.3(q,1JF-C=287.1Hz),124.7,116.0,115.0,114.3,71.3,68.8,61.4(q,2JF-C=28.3Hz),55.3,45.6,33.4,26.8,24.0。HRMS(ESI),m/z:found 499.2286[M +Na]+;calcd.for C25H31F3N4O2+Na:499.2297。IR(KBr),ν,cm-1:3222,3095,2964,1686,1615,1513,1442,1404,1245,1172,1028,901,812,620。
Ⅲh:白色固体,收率89%,m.p.52~55℃。1HNMR(400MHz,CDCl3),δ:7.18(d,J=8.4Hz,2H),6.84~6.82(m,3H),6.78(s,2H),6.38 (s,1H),5.10(dd,J=39.3Hz,16.4Hz,2H),4.32(q,J=13.9 Hz,2H),3.75(s,3H),3.72(s,3H),1.18(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.52 (s,3F)。13CNMR(100MHz,CDCl3),δ:158.9,155.0,152.6,132.1,129.0,127.7,125.2(q,1JF-C=287.1Hz),117.3,116.1,115.6,114.3,112.8,71.3,68.9,61.4(q,2JF-C=28.4Hz),55.7,55.4,45.7,26.7。HRMS(ESI),m/z:found 487.1932 [M+Na]+;calcd.for C23H27F3N4O3+Na:487.1933。IR (KBr),ν,cm-1:3219,3087,2970,1683,1516,1455,1178,1029,810,670,613。
Ⅲi:白色固体,收率96%,m.p.63~66 ℃。1HNMR(400MHz,CDCl3),δ:7.28(t,J=8.4Hz,1H),7.18~7.08(m,3H),6.86(d,J=8.5Hz,2H),6.65(d,J=9.0Hz,1H),5.14(dd,J=35.1Hz,16.4 Hz,2H),4.58(d,J=14.5Hz,1H),4.33~4.29(m,1H),3.79(s,3H),1.16(s,9H)。19FNMR(376MHz,CDCl3),δ:-79.91~-79.95(m,3F),-109.19(s,F)。13CNMR(100MHz,CDCl3),δ:159.1,155.4(d,1JF-C=250.3Hz),152.6,139.0(d,3JF-C=4.9Hz),132.0,128.1,127.7,125.1(q,1JF-C=287.4Hz),115.5 (d,2JF-C=20Hz),114.5,111.4 (d,3JF-C=3.5Hz),106.3(d,2JF-C=16.2Hz),68.8,68.7,61.4(q,2JF-C=30.2Hz),55.4,46.1,26.7。HRMS(ESI),m/z:found 509.1340[M +Na]+;calcd.for C22H23ClF4N4O2+Na:509.1343。IR(KBr),ν,cm-1:3220,3092,2972,1691,1613,1586,1515,1455,1397,1250,1178,1036,801,615。
Ⅲj:白 色 固 体,收 率95%,m.p.48~51 ℃。1HNMR (400MHz,CDCl3),δ:7.15(d,J=8.5Hz,2H),7.06(dd,J=17.8Hz,9.0Hz,1H),6.99(s,1H),6.84(d,J=8.6Hz,2H),6.61~6.58(m,1H),5.11(q,J=16.4Hz,2H),4.57(d,J=14.5Hz,1H),4.33~4.26(m,1H),3.76(s,3H),1.14(s,9H)。19FNMR(376MHz,CDCl3),δ:-79.94(d,J=12.8Hz,3F),-132.80~-132.85(m,1F),-144.42~-144.51(m,1F)。13CNMR(100MHz,CDCl3),δ:159.1,152.7,148.5(dd,1JF-C=250.8Hz,2JF-C=14.7Hz),146.5(dd,1JF-C=243.3Hz,2JF-C= 14.2 Hz),135.7,128.2,127.7,125.1(q,1JF-C= 287.6 Hz),118.6(d,2JF-C= 17.8Hz),114.5,110.4,106.8(d,2JF-C= 12.5Hz),68.9,68.6,61.3(q,2JF-C=29.7 Hz),55.4,46.1,26.7。HRMS(ESI),m/z:found 493.1632[M+Na]+;calcd.for C22H23F5N4O2+Na:493.1639。IR (KBr),ν,cm-1:3222,3094,2972,1690,1612,1514,1477,1400,1250,1176,1036,803,720,643。
Ⅲk:白色固体,收率99%,m.p.190~195℃。1HNMR(400MHz,CDCl3),δ:7.23(s,1H),7.13(d,J=7.1Hz,1H),6.82(s,3H),6.68(d,J=8.9Hz,1H),5.38(d,J=16.3Hz,1H),5.04(d,J=16.3 Hz,1H),4.34~4.28(m,2H),2.31(s,6H),2.24(s,3H),1.14(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.80(s,3F)。13CNMR(100MHz,CDCl3),δ:152.4,137.7,136.8,136.3,130.4,130.4,129.7,129.0,127.7,127.4,124.7(q,1JF-C=286.7Hz),120.4,117.2,115.8,71.4,68.9,61.8(q,2JF-C=28.6Hz),43.2,26.7,20.9,20.2,19.3。HRMS(ESI),m/z:found 503.1800[M+Na]+;calcd.for C24H28ClF3N4O+Na:503.1801。IR(KBr),ν,cm-1:3216,3092,2968,1689,1608,1431,1399,1259,1174,1042,805,697。
Ⅲl:白色固体,收率97%,m.p.194~197 ℃。1HNMR(400MHz,CDCl3),δ:8.06(d,J=8.2Hz,1H),7.92(d,J=7.9Hz,1H),7.77(d,J=8.2Hz,1H),7.63~7.55(m,2H),7.36~7.31(m,2H),7.13~7.08(m,2H),6.84(s,1H),6.58(d,J=8.9Hz,1H),5.69~5.60(m,2H),4.41~4.35(m,2H),1.19(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.91(s,3F)。13CNMR(100MHz,CDCl3),δ:152.4,137.4,134.0,130.6,130.5,130.4,129.2,127.9,127.9,127.2,126.6,126.1,125.7,125.0(q,1JF-C=286.6Hz),122.6,122.4,117.5,116.6,70.9,69.0,61.6(q,2JF-C=28.1Hz),44.3,26.7。HRMS(ESI),m/z:found 511.1484[M+Na]+;calcd.for C25H24ClF3N4O+Na:511.1488。IR(KBr),ν,cm-1:3218,3095,2969,1690,1600,1507,1429,1400,1256,1173,792,560。
Ⅲm:白色固体,收率86%,m.p.162~165℃。1HNMR(400MHz,CDCl3),δ:9.90(s,1H),7.27(s,1H),7.22(d,J=8.2Hz,1H),6.82(d,J=8.3Hz,1H),6.73(s,1H),4.32(s,2H),1.14(s,9H)。19FNMR(376MHz,CDCl3),δ:-78.84(s,3F)。13CNMR(100 MHz,CDCl3),δ:153.6,135.9,130.7,127.9,127.0,124.8(q,1JF-C=286.2Hz),116.6,115.4,71.0,69.0,62.7(q,2JF-C=28.6Hz),26.7。HRMS(ESI),m/z:found 347.0887 [M-H]+;calcd.for C14H16ClF3N4O -H:347.0886。IR (KBr),ν,cm-1:3318,3210,3124,3067,2978,1700,1502,1434,1254,1195,1173,865,827,739。
Ⅲn:白色固体,收率80%,m.p.47~51℃。1HNMR(400MHz,CDCl3),δ:7.21(s,1H),7.17~7.13(m,3H),6.84(d,J=8.5Hz,2H),6.75(d,J=8.9Hz,1H),6.30~6.23(m,1H),5.92(t,J=55.6Hz,1H),5.16(d,J=16.4Hz,1H),4.99(d,J=16.4Hz,1H),4.26 (d,J=13.8Hz,1H),4.14 (d,J=13.8 Hz,1H),3.76(s,3H),1.17(s,9H)。19FNMR(376 MHz,CDCl3),δ:-128.95(dd,J=278.24Hz,1F),-131.40(dd,J=278.62Hz,1F)。13CNMR(100 MHz,CDCl3),δ:159.0,153.0,137.2,129.9,128.5,127.7,127.7,126.6,119.0,116.2,115.5(t,1JF-C=250.8Hz),114.4,71.8,69.0,60.7(t,2JF-C=21.2 Hz),55.4,45.7,26.8。HRMS(ESI),m/z:found 473.1526 [M+Na]+;calcd.for C22H25ClF2N4O2+Na:473.1532。IR (KBr),ν,cm-1:3224,3096,2970,1685,1604,1513,1396,1249,1176,1083,809,549。
Ⅳa:白色固体,收率 94%,m.p.67~72℃。1HNMR(400MHz,CDCl3),δ:7.30(s,1H),7.23~7.11(m,4H),6.84(d,J=8.6Hz,2H),6.78(d,J=8.9Hz,1H),6.33(s,1H),5.18(d,J=16.4Hz,1H),5.00(d,J=16.5Hz,1H),3.76(s,3H),1.21(s,9H)。19FNMR(376MHz,CDCl3),δ:-79.31(s,3F)。13CNMR(100MHz,CDCl3),δ:159.0,152.3,137.1,130.3,129.1,128.4,127.8,127.7,127.2,124.5(q,1JC-F=286.8Hz),118.1,116.5,114.4,61.7(q,2JC-F=28.6Hz),55.4,54.5,45.7,28.5。HRMS(ESI),m/z:found 491.1426 [M +Na]+;calcd.for C22H24ClF3N4O2+Na:491.1438。IR(KBr),ν,cm-1:3416,3222,3096,2970,1684,1510,1427,1247,1180,1031,812。
3 结论
在碱的促进下,通过甲醛N-叔丁基腙与环状N-酰基三氟酮亚胺的亲核加成反应,首次合成了新型三氟甲基取代偶氮化合物。以Na2CO3为碱、以二氯甲烷为溶剂,在室温下,得到了收率80%~99%的系列加成产物;进一步研究表明该加成产物在酸性条件下容易发生异构化反应形成腙,它是一类非常重要的中间体,易转化为醛和腈类化合物。
[1]Kosmrlj J,Kocevar M,Polanc S.Diazenes as powerful and versatile tools in organic synthesis[J].Synlett,2009,(14):2217-2235.
[2]Takahashi H,Ueda A,Nagai S.Synthesis of new macro-azo-initiators having two kinds of azo linkages in repeating unit and block copolymerization by two-step radical polymerization initiated with them[J].Journal of Polymer Science Part A:Polymer Chemistry,1997,35(1):69-76.
[3]Kennedy D A,Vembu N,Fronzek F R,et al.Synthesis of mutual azo prodrugs of anti-inflammatory agents and peptides facilitated byα-aminoisobutyric acid[J].The Journal of Organic Chemistry,2011,76(23):9641-9647.
[4]Engel P S.Mechanism of the thermal and photochemical decomposition of azoalkanes[J].Chen Rev,1980,80(2):99-150.
[5]Blank O,Raschke N,Heinrich M R.Hydroperoxides and aryl diazonium salts as reagents for the functionalization of non-activated olefins[J].Tetrahedron Letters,2010,51(13):1758-1760.
[6]Heinrich M R,Blank O,Wetzel A.Oxidative and reductive carbodiazenylation of nonactivated olefins[J].The Journal of Organic Chemistry,2007,72(2):476-484.
[7]Heinrich M R,Blank O,W lfel S.Reductive carbodiazenylation of nonactivated olefins via aryl diazonium salts[J].Organic Letters,2006,8(15):3323-3325.
[8]Prechter A,Gr ger H,Heinrich M R.Synthesis of(S)-(+)-cericlamine through lipase-catalyzed aminolysis of azo acetates[J].Organic & Biomolecular Chemistry,2012,10(17):3384-3387.
[9]Fernandez M,Uria U,Vicario J L,et al.Enantioselective conjugate addition of donor-acceptor hydrazones toα,β-unsaturated aldehydes through formal diaza-ene reaction:Access to 1,4-dicarbonyl compounds[J].Journal of the American Chemical Society,2012,134(29):11872-11875.
[10]Crespo-Pena A,Monge D,Martin-Zamora E,et al.Asymmetric formal carbonyl-ene reactions of formaldehyde tert-butyl hydrazone withα-keto esters:Dual activation by bis-urea catalysts[J].Journal of the American Chemical Society,2012,134(31):12912-12915.
[11]Monge D,Daza S,Bernal P,et al.Synthesis of enantioenriched azo compounds:Organocatalytic Michael addition of formaldehyde N-tert-butyl hydrazone to nitroalkenes[J].Organic & Biomolecular Chemistry,2013,11(2):326-335.
[12]Rueping M,Sugiono E,Theissmann T,et al.An enantioselective chiral Br nsted acid catalyzed imino-azaenamine reaction[J].Organic Letters,2007,9(6):1065-1068.
[13]Kirsch P.当代有机氟化学 [M].上海:华东理工大学出版社,2006:8-15.
[14]Corbett J W,Ko S S,Rodgers J D,et al.Inhibition of clinically relevant mutant variants of HIV-1by quinazolinone non-nucleoside reverse transcriptase inhibitors[J].Journal of Medinical Chemistry,2000,43(10):2019-2030.
[15]Lehn J S,Javed S,Hoffman D M.Synthesis of zirconium,hafnium,and tantalum complexes with sterically demanding hydrazide ligands[J].Inorganic Chemistry,2007,46(3):993-1000.
[16]Fernández R,Martín-Zamora E,Pareja C,et al.Synthesis of enantiopureα-alkoxy-α-trifluoromethyl aldehydes and carboxylic acids from trifluoromethyl ketones[J].Angewandte Chemie International Edition,1998,37(24):3428-3430.
[17]Gómez-Guillén M,Lassaletta J M.1-Methyl(or phenyl)-5-(penta-O-acetyl-D-galacto-pentitol-1-yl)pyrazoles from the reactions of 3,4,5,6,7-penta-O-acetyl-1,2-dideoxy-1-nitro-D-galactohept-1-enitol with aldehyde methyl(or phenyl)hydrazones[J].Carbohydrate Research,1991,210:175-189.

