Radial Compressive Property of Nerve Regeneration Conduit with PGLA Biodegradable Fibers
SONG Wei-ping(宋伟平),ZHANG Pei-hua(张佩华) ,WANG Wen-zu(王文祖)
1 Key Laboratory of Textile Science and Technology,Ministry of Education,Donghua University,Shanghai 201620,China
2 College of Textiles,Donghua University,Shanghai 201620,China
Introduction
Bioabsorbable nerve regeneration conduit as an alternative to autologous nerve grafting is mainly applied in peripheral nerve repair.It functions as a scaffold for peripheral nerve regeneration.As we all know,the axon of peripheral nerve naturally has the ability to extend outwards from the cut end to the distal stumps.The scaffold is required to create a favorable environment for the nerve regeneration[1].Since 1880,the development and adaptation of different nerve regeneration conduits has been optimized in order to generate better properties[2].The development of microsurgical techniques and repairing technology of peripheral nerve injury is closely associated to nerve conduit materials.Researchers usually achieve better performance by changing the materials of nerve conduits[3].Natural and synthetic materials have been used.Although natural materials have inherent bioactivity that may aid in nerve regeneration, synthetic materials offer several advantages:the physical properties can be more easily altered to produce materials with specific,desirable mechanical,and biochemical properties;it is easier to scale up production during manufacture;it may be more biomimetic and lead to enhanced regeneration[4].As the study progressed,there have been a variety of different bioabsorbable nerve conduits that could maintain their tubular structure in vivo and are flexible enough to be fixed to the nerve stump with a suture during operation.Most of these tubes are hollow.There are also porous and high permeability tubes with inner bracket structure which can be more favorable to nerve repair.At present,some nerve regeneration conduits are commercially available in the United States and Europe,and the clinical outcomes of these tubes have been reported[5–8].However,there have been problems in the using process.Soft tubular structures will appear collapse and prevent the growth of regenerated nerve.Conversely,the scaffold is too stiff to handle without difficulty during operation[9].Therefore, nerve regeneration conduits must maintain a certain level of strength until nerve regeneration is completed.In the study,we aim to investigate the mechanical properties of nerve regeneration conduits made from poly(glycolide-co-L-lactide)(PGLA)and to discuss how their radial compressive property changes with the inner diameter and length of tubes.
1 Experimental
1.1 Materials and preparation
Multifilament of PGLA (5.33tex/12F)was spun by Shanghai Tianqing Biomaterials Co., Ltd.The braided absorbable nerve regeneration conduits prepared by PGLA were fabricated on a conventional 2-D tubular braiding machine with 32 spindles.Figure 1 shows an optical microscope photograph of a PGLA cylinder.The mesh was interwoven with bundles.In order to achieve the required inner diameter of hollow tube,different diameters within the scope of 2-5 mm polytetrafluoroethylene (PTFE)cylindrical mandrels were used in the braiding process.The braiding process parameters are illustrated in Table 1.

Fig.1 Appearance of nerve regeneration conduit
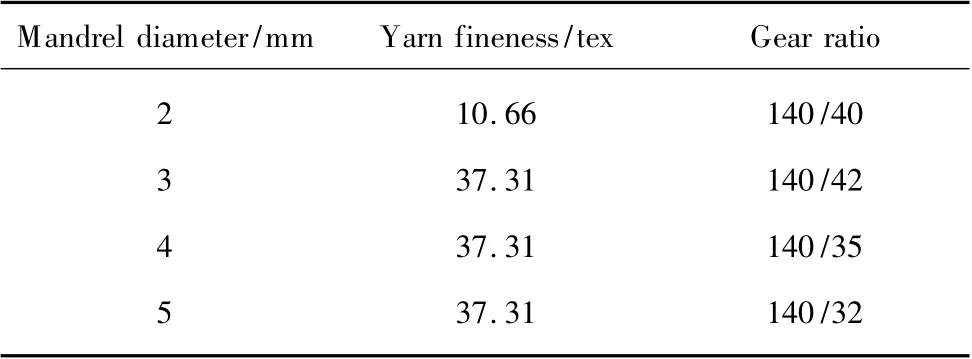
Table 1 The braiding process parameters
Heat setting was adopted at 100℃for 5 min to enhance the stability of the structure.The outer surfaces of the nerve regeneration conduits were coated manually by dipping into chitosan with an inherent viscosity of 3.5 for 30 s and then dried in air under room temperature.2-mm and 3-mm tubes were dried for 15 min and the other two tubes were 30 min.After drying,the next coating was made.The coating process was repeated 3 times.
1.2 Test index
(1)Outer diameter
Outer diameter was tested by the CH-12.7-BTSX electronic thickness gauge without load.Fifteen samples were extracted from nerve regeneration conduits for each type randomly and 3 tests per sample were performed.The measured average dimensions were recorded.
(2)Wall thickness
Nerve regeneration conduits were cut along the wall and eventually flattened.Wall thickness test was conducted on planar sample by using CH-12.7-BTSX electronic thickness gauge with a load of 44 g.The 5 test pieces were used independently for each type and each specimen was measured 5 times.Test data were expressed as means.
(3)Density
Density was evaluated by Y511B fabric density meter without pulling force.Each sample was measured in 3 different places and 5 specimens for each type were carried out.Test results were indicated as average values.
(4)Mechanical properties
The LLY-06D artificial biological pipeline compression tester was applied in radial compression mode for the mechanical tests (Fig.2).All tubular specimens with different lengths (2-5 cm)were put on the test platform respectively and compressed into half in the radial direction for 3 times with the rise of test platform.Tubes with mandrel diameter of approximately 2 and 3 mm were carried out with the compression velocity of 2 mm/min and the rest two was 6 mm/min.The initial gauge length was determined by outer diameter of nerve conduits.Compression time was 10 s and presser foot stayed at initial position for 10 s before the following compression.The contact tension was 0.5 cN.The 10 specimens were performed for each type and every sample was measured for only one time in the middle position.

Fig.2 Schematic representation for the compression tests
The radial compressive force and the elastic recovery ratio of the nerve regeneration conduit were used to represent the tube's radial compression property.The compressive force and displacement that occurred during compression were monitored on a chart recorder for data analysis.In this study,the radial compressive force was the mean value of the force that tubes were first compressed into half.The elastic recovery ratio (ε)is defined by

where L0is the displacement before presser foot first came into contact with the specimen;L1is the displacement when the tube was last compressed into half;L2is the displacement of contact tension.
2 Results and Discussion
2.1 Structural parameters of nerve regeneration conduits
The test results of outer diameter,wall thickness and density of nerve regeneration conduits are shown in Table 2.The data indicate that the difference in wall thickness and density of neural conduits is fairly small except the 2-mm tubes.2-mm tubes are braided with finer yarns.The wall thickness is thinner than others and density is obviously large.Because of larger difference of yarns,the density of 2-mm tubes is not enough to make their thickness increased significantly.It is important to point out that inner diameter of tubes becomes small after removing the cylindrical mandrel.As the inner diameter of tubes increases,shrinkage ratio of tubes decreases.The main reason is the tension in weaving process.The larger the diameter of tubes is, the smaller the tension is.Consequently,the contraction of tubes with larger diameter is not obvious.
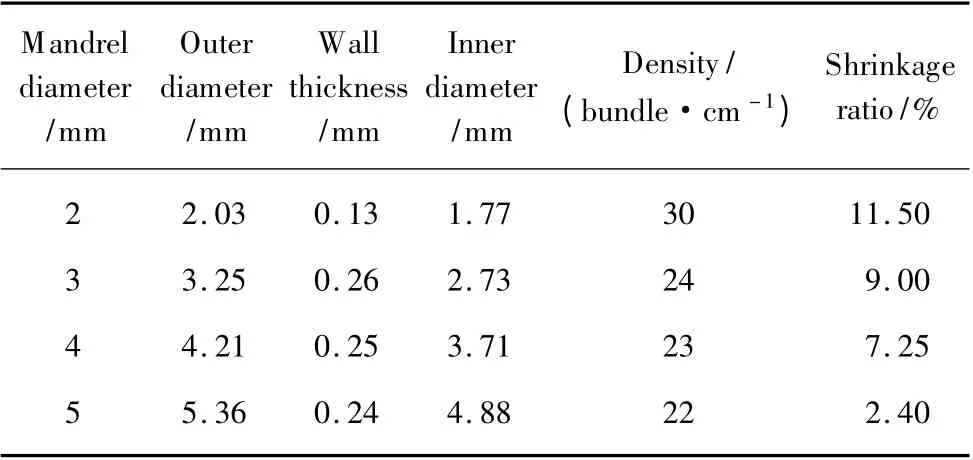
Table 2 Structural parameters of nerve regeneration conduits
2.2 Mechanical properties of nerver egeneration conduits
As depicted in Fig.3,radial compressive force of nerve regeneration conduits with different inner diameters changes greatly.Due to the thin thickness,radial compressive force of 2-mm tubes is the lowest.For other tubes with mandrel diameter of 3 mm whatever lengths obtain the biggest radial compressive force.Because its wall is relatively thick and space inside the tube is small.So tubes with 3-mm are not easy to collapse.The same nerve regeneration conduits of different lengths have obvious difference.Because the contact area between tube and presser foot is the same in the test,but space inside the tubes is different.The 4-cm specimens give better mechanical performance of high strength for the same specimen type.
In Fig.4,elastic recovery ratio is plotted against the inner diameter of nerve regeneration conduits.There is almost no difference among the same neural conduits with different length.Under the same condition,the smaller the diameter of tubes with thicker wall is,the poor the elastic recovery ratio is.So the elastic recovery ratio of 3-mm tubes is comparatively low.Because the wall of tubes is soft,the elastic recovery ratio of 2-mm tubes is significantly higher.

Fig.3 Radial compressive force of nerve conduit

Fig.4 Elastic recovery ratio of nerve conduit
3 Conclusions
In this work, the results demonstrate that radial compressive force of nerve regeneration conduits is closely related to the inner diameter and length of tubes,but the effect of these two factors on elastic recovery ratio is not obvious.This preliminary experiment is expected to provide a useful alternative for peripheral nerve repair.It must be pointed out that coating method will affect the test result and correct operation is rather significant.So far,it is still a difficult problem and need to be adequately resolved.
[1]Tanaka S,Takigawa T,Ichihara S,et al.Mechanical Properties of the Bioabsorbable Polyglycolic Acid—Collagen Nerve Guide Tube[J].Polymer Engineering and Science,2006,46(10):1461-1467.
[2]Ijpma F F,van De Graaf R C,Meek M F.The Early History of Tubulation in Nerve Repair[J].The Journal of Hand Surgery,2008,33(5):581-586.
[3]Chen L J,Yu J C,Fu Q,et al.Clinical Application of Nerve Conduits in Repairing Peripheral Nerve Injury [J].Chinese Journal of Tissue Engineering Research,2012,16(34):6413-6420.(in Chinese)
[4]Gunn J W,Turner S D,Mann B K.Adhesive and Mechanical Properties of Hydrogels Influence Neurite Extension[J].Journal of Biomedical Materials Research:Part A,2005,72(1):91-97.
[5]Koshimune M,Takamatsu K,Nakatsuka H,et al.Creating Bioabsorbable Schwann Cell Coated Conduits through Tissue Engineering[J].Bio-Medical Materials and Engineering,2003,13(3):223-229.
[6]Guenard V,Kleitman N,Morrissey T K,et al.Syngeneic Schwann Cells Derived from Adult Nerves Seeded in Semipermeable Guidance Channels Enhance Peripheral Nerve Regeneration[J].The Journal of Neuroscience,1992,12(9):3310-3320.
[7]Hadlock T,Sundback C,Hunter D,et al.A Polymer Foam Conduit Seeded with Schwann Cells Promotes Guided Peripheral Nerve Regeneration[J].Tissue Engineering,2000,6(2):119-127.
[8]Takahashi K,Yamanaka S.Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors[J].Cell,2006,126(4):663-676.
[9]Uemura T,Takamatsu K,Ikeda M,et al.A Tissue-Engineered Bioabsorbable Nerve Conduit Created by Three-Dimensional Culture of Induced Pluripotent Stem Cell-Derived Neurospheres[J].Bio-medical Materials and Engineering,2011,21(5/6):333-339.
 Journal of Donghua University(English Edition)2013年5期
Journal of Donghua University(English Edition)2013年5期
- Journal of Donghua University(English Edition)的其它文章
- Electrospun Small Diameter Tubes to Mimic Mechanical Properties of Native Blood Vessels Using Poly(L-lactide-co-ε-caprolactone)and Silk Fibroin:a Preliminary Study
- Properties of Scaffold Reinforcement for Tendon Tissue Engineering in vitro Degradation
- Mineralized Composite Nanofibrous Mats for Bone Tissue Engineering
- Promoted Cytocompatibility of Silk Fibroin Fiber Vascular Graft through Chemical Grafting with Bioactive Molecules
- Fatigue Performance of Fabrics of Stent-Grafts Supported with Z-Stents vs.Ringed Stents
- Effect of Media on the in vitro Degradation of Biodegradable Ureteral Stent
