Effect of Molecular Weight of PCL on the Structure and Mechanical Properties of PCL/PET Composite Vascular Scaffold Prototype
LI Chao-jing(李超婧),MOHAMMED Abedalwafa,WANG Fu-jun(王富军) ,GE Peng(葛 鹏),CHEN Pei-feng(陈培峰),WANG Lu(王 璐)
1 Key Laboratory of Textile Science and Technology,Ministry of Education,Donghua University,Shanghai 201620,China
2 College of Textiles,Donghua University,Shanghai 201620,China
Introduction
Atherosclerosis is a sickness of arteries that causes more deaths and disability than any other disease in the world[1].Vascular grafts are widely used to replace the damaged arteries,and commercial ones are mainly produced by knitting or weaving method[2-3],which usually have excellent mechanical properties.Knitting constructions are made from interloping yarns in horizontal rows and vertical columns of stitches.And these constructions account for more than 50% of the structures available,due to stretch more easily,more flexible and easily comfortable,and have better handling characteristics than woven graft designs[4].Weft-knit structure has extensibility and flexibility and shows good compliance,thus it has a promising application[5-6].Unfortunately knitting structure cannot be used directly due to high porosity,leakage of blood,and low biocompatibility property.Composite scaffolds consisting of polyester weft-knit tubular fabrics have been coated with polyurethane (PU),to improve the mechanical properties,elasticity,and blood-proof properties[5,7-8].The PU is not suitable for vascular graft due to its toxicity toward the end stages of degradation,which have been attributed to the accumulation of degradation products of the urethane segments[9].
Poly (ε-caprolactone)(PCL)is a promising material due to its characteristics such as biodegradability and biocompatibility[10-14].Many studies show that PCL has been successfully applied as scaffolds in tissue engineering,because it can be fabricated to porous structure with many different kinds of methods[15-17].The molecular weight and nature of the polymer has an influence on mechanical properties and physical properties of the film[18].It affects the ability of the polymer chains to crystallize or exhibit secondary interactions such as hydrogen bonding.The crystallinity and secondary interactions can give polymers additional strength[19].In addition,the molecular weight and nature of the polymer also has influences on micromechanical morphology and porous structure.The physical and chemical properties of PCL scaffolds with various porosities have significant differences,during the degradation process[20].
The main aim of this paper is to study the effect of the PCL molecular weight on the mechanical properties and the porous structure of PCL vascular scaffold reinforced with polyethylene terephthalate (PET)monofilament weft-knit tubular fabric.And we use scanning electron microscopy (SEM),universal mechanical tester, and radial compression apparatus to characterize the samples.
1 Materials and Methods
1.1 Materials
PET monofilament (30D)was used for preparing weft-knit tubular fabric.PCL (Mw=50 000,80 000,and 180 000)was purchased from Shenzhen Brightchina Industrial Co.,Ltd.(China).Acetic acid was purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China),which was used as solvent.
1.2 Sample preparation
PET was fabricated into a 6 mm diameter tubular fabrics by using small diameter single jersey weft knitting machine,which had 10 needles/inch.The density of the PET weft-knit tubular fabric was 12.61 g/m2within 29.21 loops in wale direction and 71.43 loops in course direction.Figure 1 (a)shows the structure of the weft-knit fabric,which is the optical microscope image.

Fig.1 Observation of the (a)tubular fabric layer and (b)final PCL scaffold prototype
The PCL with three different molecular weights (50 000,80 000,and 180 000)were dissolved separately in the acetic acid to form 10% (weight ratio)solutions.The mixtures were stirred continuously at room temperature for 5 h until a homogeneous solution was formed.Then PCL solution was coated on both sides of the tubular knitting structure prior to mounting it on a 6 mm diameter polytetrafluorothylene (PTFE)rod which was served as the mold to control the inside diameter of the tubular scaffold.After coating the samples were cooled and hold at -10 ℃for 12 h for drying preparation.Finally they were dried by freeze drying method at the temperature of-60 ℃and pressure below 15 Pa for 5 h.Figure 1 (b)shows PCL scaffold reinforced with PET weft-knit fabric.
1.3 Geometrical characteristics
The geometrical characteristic was determined according to Standard ISO 7198:1998[21].Calculate and record the porosity(P,%)of each sample from Eq.(1).

where M is mass per unit area of the composite scaffold;h is thickness;and d is weighted average density of PCL and PET.
1.4 Microporous structure
The surface and cross section morphology of PCL scaffold reinforced with weft-knit tubular fabric were assessed using a JSM-5600LV SEM Japan JEOL.Before the examination,samples were gold covered under nitrogen at an excitation voltage of 15 kV.
1.5 Mechanical properties
The longitudinal and circumferential direction tensile strengths of the samples were tested using a universal mechanical tester (YG-B026H),Wenzhou Darong Textile Instrument Co.,Ltd.,China,according to Standard ISO 7198:1998[21].The strain rate was 50 mm/min,and the gauge length for the longitudinal and circumferential test was 60 mm and 20 mm respectively.Particularly,radial tensile fracture strain value was calculated according to Eq.(2).The results were average of 5 times testing.
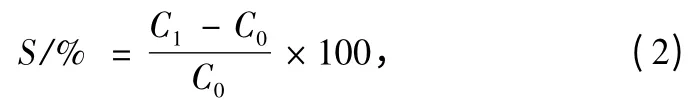
where C1is perimeter when the sample breaks;C0is the original perimeter;and S is the breaking strain of circumferential direction.
The radial compressive strength was tested by the radical compression apparatus (YG061),Laizhou Electronic Instrument Co.,Ltd.,China.The testing method for the elastic recovery according to the principle in Ref.[22].The strain rate is 10 mm/min,and the samples are compressed to 50% of the original diameter in the radial direction.The results were average of 3 times testing.The compressive strength is shown in Fig.2,which means a force when pressure stops loading on the sample.The elastic recovery (E)of each sample calculates from Eq.(3),according to the curve in Fig.2.


Fig.2 Calculation of the elastic recovery
2 Results and Discussion
2.1 Geometrical analysis
The geometrical characteristic for composite scaffold with different molecular weight is shown in Table 1.The PCL content in each sample is kept as constant,so the changes of porosity and thickness due to different molecular weight can be clearly observed in Fig.3.In general,an increasing molecular weight seems to result in the formation of bigger pores,especially when the concentration of the solution is kept constant,due to the molar concentration decreasing with increasing molecular weight.The micro-porous structures in the PCL have been fabricated using the freeze drying method.The number of moles of PCL in per unit volume of solution reduced and the acetic acid could form lager size of crystal when increasing molecular weight.After the drying processes,the acetic acid crystal can be extracted from the mixture,and the larger size porous structure will be formed within the PCL membranes.So when the content of PCL and the total weight of the samples are constant,the thickness and the porosity are increased slightly with increasing the molecular weight.
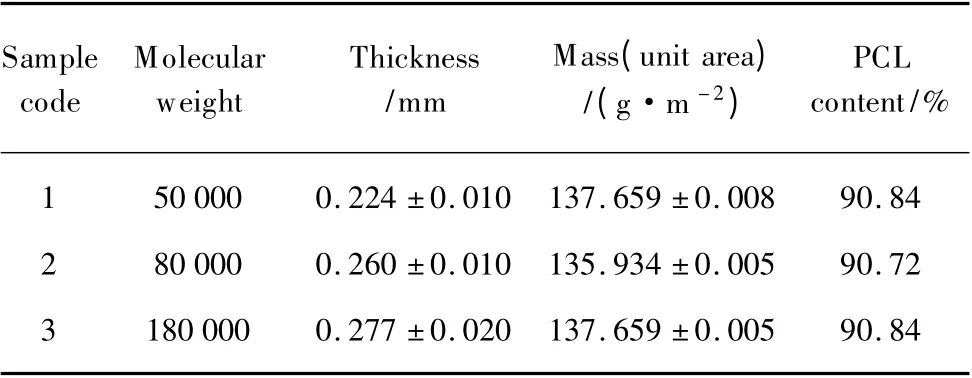
Table 1 Geometrical characteristics
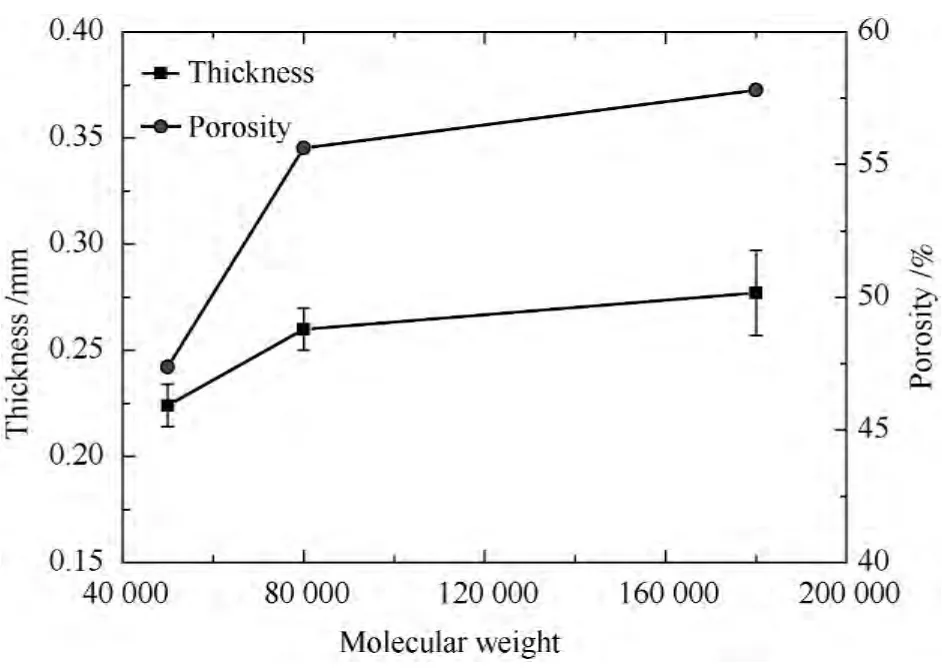
Fig.3 Effect of the molecular weight on the porosity and the thickness
2.2 Morphology of micro-porous structure
Freeze drying method has been used here to fabricate micro-porous structures in the PCL.Figure 4 shows the SEM images for the surface and cross section of PCL with three different molecular weights.The PCL membrane has a pore sizes ranging around 10 μm and above (especially the sample produced with molecular weight 180 000).As seen in Fig.4,the porosity of PCL increases with increasing molecular weight of PCL.From the image of the surface structure,it can be found that with the increasing of the molecular weight,the size and the distribution of the pore become much more uniform.Reasons for this phenomenon are the phase separation mechanism.It means when the temperature is low enough to allow the freeze of the solution,it would result in solid-liquid demixing,which forms frozen solvent and concentrated polymer phases.After the removal of the frozen solvent,the remained space would become pores.With the molecular weight increasing the chain of the molecular would be longer,which leads to greater extent of phase separation,so the morphology of the pores is better.

Fig.4 SEM images for the microstructure:(a),(b),and (c)are the surface sections of the PCL composite vascular graft;(d),(e),and (f)are the cross sections of the PCL films,fabrication with different molecular weight of PCL
2.3 Tensile strength
The composite scaffol d combines the good elasticity and blood-proof properties of PCL with the strength and stability of knitting structure.As shown in Table 2 the molecular weight has not immense effect on the breaking stress and strain at break,and this simply effect is due to the composite structure of the samples,where the tubular fabric part provides most of the strength support rather than PCL membrane.This means the samples,which have the same textile structure but with different PCL composite membranes,keep consistent tensile strength.

Table 2 Effect of the molecular weight on the breaking stress and strain at break
2.4 Radial compressive strength
General analysis of the Table 3 has shown that all of the samples have acceptable compressive strength and elastic recovery.
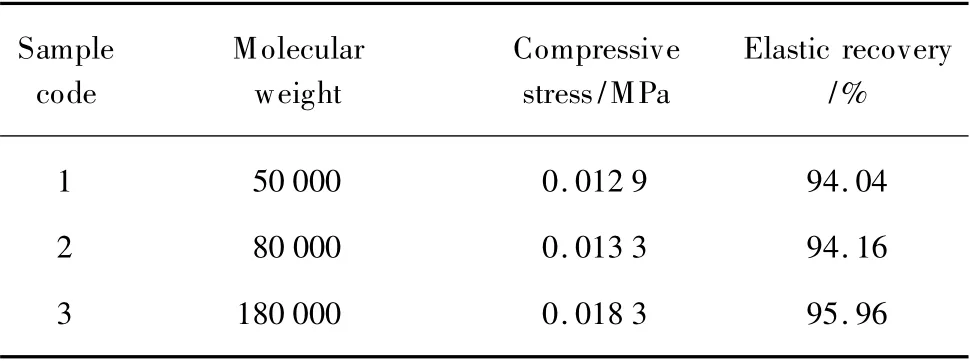
Table 3 Effect of the molecular weight on the radial compressive strength and elastic recovery
It can be seen that the influences of molecular weight on the compressive stress and elastic recovery are evident.Among the three molecular weights analyzed,the molecular weight 180 000 has a higher ability to resist the mechanical compression and higher elastic recovery than the molecular weight 50 000 when the PCL content (%)is kept constant.This is due to the changing of porous structure, which influences physical properties of the sample.
3 Conclusions
PCL vascular prototype scaffolds reinforced with PET weftknit tubular fabrics had been made successfully.The influence of the molecular weight was mainly on porous structure and mechanical properties,especially on the elastic recovery rate,which provides theoretical support for clinical applications.With increasing the molecular weight of PCL,the porosity of the composite scaffold improved significantly.The SEM image also illustrated this point,the pore size became larger and the distribution of the pore was more uniform.However,the breaking stress and strain at break in both longitudinal and circumferential directions.Another phenomenon was the compressive stress increasing with molecular weight increasing.In short,considering the preparation of PCL scaffold reinforced with weft-knit fabric, which possesses good mechanical properties as well as good porous structure,PCL raw material with higher molecular weight has preferable potential in application.
[1]Faxon D P,Fuster V,Libby P,et al.Atherosclerotic Vascular Disease Conference:Writing Group III:Pathophysiology [J].Circulation,2004,109(21):2617-2625.
[2]Manju S,Muraleedharan C V,Rajeev A,et al.Evaluation of Alginate Dialdehyde Cross-Linked Gelatin Hydrogel as a Biodegradable Sealant for Polyester Vascular Graft[J].Journal of Biomedical Materials Research Part B,2011,98(1):139-149.
[3]Kowalewski R, Zimnoch L, Wojtukiewicz M Z, et al.Expression of Fibrinolysis Activators and Their Inhibitor in Neointima of Polyester Vascular Grafts[J].Biomaterials,2004,25(28):5987-5993.
[4]Pourdeyhimi B,Text C.A Review of Structural and Material Properties of Vascular Grafts [J].Journal of Biomaterials Applications,1987,2(2):163-204.
[5]Xu W L,Zhou F,Ouyang C X,et al.Mechanical Properties of Small-Diameter Polyurethane Vascular Grafts Reinforced by Weft-Knitted Tubular Fabric [J].Journal of Biomedical Materials Research Part A,2010,92(1):1-8.
[6]Ge Z,Goh J C,Wang L,et al.Characterization of Knitted Polymeric Scaffolds for Potential Use in Ligament Tissue Engineering [J].Journal of Biomaterials Science - Polymer Edition,2005,16(9):1179-1192.
[7]Xu W L,Zhou F,Ouyang C X,et al.Small Diameter Polyurethane Vascular Graft Reinforced by Elastic Weft-Knitted Tubular Fabric of Polyester/Spandex [J].Fibers and Polymers,2008,9(1):71-75.
[8]Yang H J,Xu W L,Ouyang C X,et al.Circumferential Compliance of Small Diameter Polyurethane Vascular Grafts Reinforced with Elastic Tubular Fabric[J].Fibres &Textiles in Eastern Europe,2009,17(6):89-92.
[9]van Minnen B,Stegenga B,van Leeuwen M B,et al.A Long-Term in vitro Biocompatibility Study of a Biodegradable Polyurethane and Its Degradation Products [J].Journal of Biomedical Materials Research Part A,2006,76(2):377-385.
[10]Edlund U,Danmark S,Albertsson A C.A Strategy for the Covalent Functionalization of Resorbable Polymers with Heparin and Osteoinductive Growth Factor [J].Biomacromolecules,2008,9(3):901-905.
[11]Wan Y,Feng G,Shen F H,et al.Biphasic Scaffold for Annulus Fibrosus Tissue Regeneration[J].Biomaterials,2008,29(6):643-652.
[12]Ng K W,Achuth H N,Moochhala S,et al.In vivo Evaluation of an Ultra-Thin Polycaprolactone Film as a Wound Dressing [J].Journal of Biomaterials Science - Polymer Edition,2007,18(7):925-938.
[13]Mo X,Weber H J,Ramakrishna S,et al.PCL-PGLA Composite Tubular Scaffold Preparation and Biocompatibility Investigation[J].International Journal of Artificial Organs,2006,29(8):790-799.
[14]Wong D Y,Hollister S J,Krebsbach P H,et al.Poly(epsiloncaprolactone)and Poly (L-lactic-co-glycolic acid)Degradable Polymer Sponges Attenuate Astrocyte Response and Lesion Growth in Acute Traumatic Brain Injury[J].Tissue Engineering,2007,13(10):2515-2523.
[15]Chakoli A N,Wei C,Sui J H,et al.Efficient Load Transfer to Functionalized Carbon Nanotubes as Reinforcement in Polymer Nanocomposites[J].International Journal of Modern Physics B,2009,23(6/7):1401-1406.
[16]Ho M H,Kuo P Y,Hsieh H J,et al.Preparation of Porous Scaffolds by Using Freeze-Extraction and Freeze-Gelation Methods[J].Biomaterials,2004,25(1):129-138.
[17]Sasmazel H T,Manolache S,Gümüʂ dereliog∨lu M,et al.Water/O2-Plasma-Assisted Treatment of PCL Membranes for Biosignal Immobilization[J].Journal of Biomaterials Science - Polymer Edition,2009,20(7/8):1137-1162.
[18]Stenzel-Rosenbaum M H,Davis T P,Fane A G,et al.Porous Polymer Films and Honeycomb Structures Made by the Selforganization of Well-Defined Macromolecular Structures Created by Living Radical Polymerization Techniques[J].Angewandte Chemie -International Edition,2001,40(18):3428-3432.
[19]Ratner B D,Hoffman A S,Schoen F J,et al.Biomaterials Science:an Introduction to Materials in Medicine [M].Amsterdam,Boston:Elsevier Academic Press,2004:58-60.
[20]Zhang Q C,Jiang Y,Zhang Y,et al.Effect of Porosity on Long-Term Degradation of Poly (ε-caprolactone)Scaffolds and Their Cellular Response[J].Polymer Degradation and Stability,2013,98(1):209-218.
[21]ANSI/AAMI/ISO7198.Cardiovascular Implants-Tubular Vascular Prostheses[S].British:British Standards Institution,1998
[22]Liu G H,Hu H,Zhang P H,et al.Radial Compressive Properties of the Biodegradable Braided Regeneration Tubes for Peripheral Nerve Repair [J].Journal of Industrial Textiles,2006,36(1):35-46.
 Journal of Donghua University(English Edition)2013年5期
Journal of Donghua University(English Edition)2013年5期
- Journal of Donghua University(English Edition)的其它文章
- Electrospun Small Diameter Tubes to Mimic Mechanical Properties of Native Blood Vessels Using Poly(L-lactide-co-ε-caprolactone)and Silk Fibroin:a Preliminary Study
- Properties of Scaffold Reinforcement for Tendon Tissue Engineering in vitro Degradation
- Mineralized Composite Nanofibrous Mats for Bone Tissue Engineering
- Promoted Cytocompatibility of Silk Fibroin Fiber Vascular Graft through Chemical Grafting with Bioactive Molecules
- Fatigue Performance of Fabrics of Stent-Grafts Supported with Z-Stents vs.Ringed Stents
- Effect of Media on the in vitro Degradation of Biodegradable Ureteral Stent
