高活性、可循环的Pt-Cu@3D石墨烯复合催化剂的制备和催化性能
王美淞 邹培培 黄艳丽 王媛媛 戴立益
(华东师范大学化学与分子工程学院,上海 200241)
高活性、可循环的Pt-Cu@3D石墨烯复合催化剂的制备和催化性能
王美淞 邹培培 黄艳丽 王媛媛*戴立益
(华东师范大学化学与分子工程学院,上海 200241)
以氧化石墨烯为前驱体,通过氧化还原法制备了具有三维大孔稳定结构的Pt-Cu@3D石墨烯催化剂。作为载体,三维石墨烯的宏观大孔结构具有大比表面积,可以更好地促进催化反应中的传质过程。以对硝基苯酚的还原为模型反应,通过实验确定Pt与Cu的最佳质量比为3 : 5,利用扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)、X射线衍射(XRD)和能量色散X射线光谱(EDX)等方法对最优催化剂的结构进行了表征。催化测试表明复合催化剂在对硝基苯酚还原反应中具有高效稳定、低成本、可循环的良好性能。
三维石墨烯;Pt-Cu纳米颗粒;催化反应;孔结构;复合材料
1 Introduction
Carbon-supported noble metal nanoparticles (NPs) have rapid development with wide applications in the field of catalysis, storage and energy conversion, and biomedicine1,2. As an important member of the carbon family, graphene have a variety of applications in catalysis and energy conversion andstorage3because of their unique 2D structures and excellent properties. It is generally believed that the graphene-based catalysts not only increase the nanosized catalyst surface area for electron transport but also provide better mass transport of reactants to catalysts4,5. However, irreversible agglomerates were easily driven by the strong van der Waals interaction consisting in graphene sheets, which greatly affect the superiority of the high specific surface area inherited from graphene. Self-assembling two dimensional graphene sheets into complex three dimensional macrostructures remains these superiority and further avoid a mass of aggregations6,7. Recently, three-dimensional graphene (3DG) has attracted a great deal of attention with its high specific surface area, strong mechanical strength, superior absorbability and thermal stability8-11. Such porous structures of 3DG could effectively avoid the stacking of the graphene sheets. 3DG materials keep the intrinsic physicochemical properties and endow the resulting materials with high specific surface area provided more anchoring sites for the catalyst12.
Pt-based nanomaterials have been widely used for chemical industry. Considering its expensive price and limited availability, there is an urgent need to develop substitutes for pure Pt catalysts. Pt-based bimetallic alloy NPs as a kind of important catalyst attracts researchers′ interests immensely. Pt-based bimetallic alloy NPs not only keep great catalytic activity but also reduce the content of Pt NPs to help lower costs. Bimetallic NPs such as Pt-Ni13, Pt-Sn14and Pt-Co15NPs exhibit good catalytic activity and stability. Moreover, the difficulty in determining the effect of alloying is that the activity of a supported catalyst can have a wide range of values depending on its microstructure and preparation method16. It is difficult to fine control the composition of bimetallic alloy NPs with different standard redox potential (SRP) between Pt and transition metals17. For avoiding the effect of different SRPs, a simple and common approach is to co-reduce two metal precursors to use a strong reducing agent. The precise control of the compositions of alloy nanocrystals with well-defined morphology remains a great challenge.
In this paper, we report the successful synthesis of the 3DG-based Pt-Cu NPs-containing (Pt-Cu@3DG) composite. Pt-based bimetallic alloy NPs not only keep good catalytic activity but also reduce the content of Pt NPs to lower the costs. The composite is prepared by introducing a stronger reductant. The accession of ascorbic acid achieves the chemical co-reduction of metal salts in the solution phase. The catalytic test indicates that the resulting composites can be used as an efficient, low costing, recyclable, and stability catalyst for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) under mild conditions.
2 Experimental
2.1 Materials and Reagents
Natural Graphite (AR), H2PtCl6·6H2O (AR), Cu(NO3)2·3H2O (AR) was purchased from Aladdin Company, NaBH4(AR) was purchased from Sinopharm Chemical Reagent Co., Ltd. Other materials were obtained from local suppliers.
2.2 Preparation of Pt-Cu@3DG composite hydrogel
The graphite oxide was prepared by a modified Hummers method18. Natural Graphite powder (2.5 g) and sodium nitrate (4.0 g) were mixed with 140mL concentrated sulfuric acid under vigorous agitation for 30 min at the room temperature. Then the mixture above was cooled in an ice bath. Afterwards, 7.5 g potassium permanganate was slowly added to make the sufficient oxidation of graphite with the permanent stirring. Subsequently, the ice bath should be removed and the composite was heated until 35 °C for 2 h. Then 200 mL distilled water was added to dilute the paste. Finally, 30% H2O2was added to react with the residual potassium permanganate. The prepared brown solution was washed with HCl (10%) three times. In the end, the brown solution was centrifuged to remove the aggregates followed by dialysis for seven days. Then chloroplatinic acid, copper nitrate and ascorbic acid (0.6 g) were added to the above GO dispersion (0.2 mg·g-1, 10 g). Afterwards, the mixture was stirred for 3 h, and then transferred into the Teflon-lined autoclave (120 °C, 2 h). Then it was cooled to the room temperature. The Pt-Cu@3DG composite hydrogel was formed with a height of 1.2 cm and the diameter of 1 cm. Then large amount of ethanol and water were used to wash the catalyst to remove the residual ascorbic acid.
2.3 Exploring of the best loading of bimetallic NPs
Keeping the total mass of metal as 8% of graphene oxide and changing the mass ratio of Pt and Cu, a series of catalysts were synthesized, and used to catalyze the reduction of 4-NP. The absorbance of resultant was recorded by UV-Vis absorption spectra when the reaction was at 6 min. According to the formula: A = kc, (A: absorbance, c: concentration, k: absorption coefficient) conversion was calculated to obtain the change of the concentration. As shown in Table 1, the loading amount of Pt increased from 3% to 7%, while the amount of Cu gradually decreased from 5% to 1%. The conversion of 4-NP decreased from 98.6% to 76%, measured as the reaction proceeds at 6 min by UV-Vis absorption spectra. So, the best catalyst should be 3% Pt-5% Cu@3DG composite.
2.4 General procedure for reduction of 4-NP
In order to investigate the catalytic activity of Pt-Cu@3DGcatalyst, 6 mL 4-NP (0.033 mmol·L-1) and the freshly prepared 14 mL NaBH4(0.4 mmol·L-1) was mixed homogeneously at room temperature. Then the catalyst (Pt-Cu@3DG) was added into the mixture. The reduction progress was monitored with UV-Vis spectroscopy.

Table 1 Loaded amount of Pt-Cu in different catalysts and the conversion
2.5 Characterization
X-ray diffraction (XRD) patterns of the Pt-Cu@3DG were performed on a Bruker D8 diffractometer with Cu Kαradiation with a scan speed 10 (°)·min-1. Transmitting electron microscopy (TEM) was performed on a JEOL 2100F instrument operating at 30 kV. Inductively coupled plasma emission spectroscopy (ICP-AES) measurements were obtained with the Thermo Scientific iCAP 6300 instrument. Scanning electron microscopy (SEM) was performed on a Philips XL 30 microscope operating at 30 kV.
X-ray photoelectron spectroscopy (XPS) measurements were carried out on a Versaprobe Phi 5000 ULVAC-PHI spectrometer with an Al Kα(1486.6 eV) radiator, and the vacuum in the analysis chamber was maintained at about 1000-900 Pa. The binding energy was calibrated by means of the C 1s peak energy of 284.6 eV.
3 Results and discussion
3.1 Stucture of Pt-Cu@3DG composite
After the hydrothermal treatment of GO, Fig.1a shows a peak at 23.92°, meaning that the GO (10.92°)19has been reduced and the carbon sp2bonding restored. The Pt-Cu alloy NPs exhibits three characteristic diffraction peaks, corresponding to the (111), (200) and (220) planes around 41.9°, 48.0° and 71.2°. The diffraction peaks of the Pt-Cu@3DG shift to higher angles as compared to the Pt@3DG, indicating that a lattice contraction arises from the substitution of the smaller Cu atoms for the larger Pt atoms. The XRD pattern of Pt-Cu@3DG sample indicates that the material is polyphasic, with the strongest signal correlated with the (111) reflection of pure Pt, and no significant signal of a Pt-Cu alloy of any composition19,20.
XPS is used to analyze the elemental composition and heterocarbon components of Pt-Cu@3DG (Fig.1(b-d)). In brief, the asymmetric C 1s spectrum of Pt-Cu@3DG hydrogel in Fig.1b shows four peaks at 284.6, 286.6, 287.7 and 289.0 eV, which are assigned to the sp2-hybridized C, the C in C―O bonds, the carbonyl C and the carboxylate C (O=C―O), respectively. The C―C functional group has the most content (57.95%), other oxidation species such as the carbonyl C (14.11%) and carboxylate C (4.28%) are presented with lower quantity. These results confirm that 3DG was mainly reduced from GO through the removal of most epoxide and hydroxyl functional groups. The XPS spectrum of Pt 4f of the Pt-Cu@3DG composite exhibits the presence of three pairs of doublets, as shown in Fig.1c. The most intense doublet with binding energies of 71.0 (Pt 4f7/2) and 74.5 eV (Pt 4f5/2) is attributed to Pt0(66.3%). Peaks at 72.2 (Pt 4f7/2), 75.6 eV (Pt 4f5/2) and 74.2 (Pt 4f7/2), 78.3 eV (Pt 4f5/2) could be assigned to Pt(II) and Pt(IV), that both of them anchored with the C―O groups on 3DG21. The Cu 2p spectrum exhibits peaks at 932.4 (Cu 2p3/2) and 952.4 eV (Cu 2p1/2), indicative of elemental Cu0(Fig.1d). Thus, XPS measurements demonstrate that Pt(IV) and Cu(II) precursors are reduced successfully. For the Pt-Cu@3DG composite hydrogel, the binding energies of Pt 4f shift to lower binding energies because of the presence of Cu, further confirming the formation of Pt―Cu alloy22. Theequilibrium potentials (E0) of the electrochemical reactions [PtCl6]2+ 2e → [PtCl4]2+ 2Cl , 0.68 V, and [PtCl4]2+ 2e → Pt + 4Cl , 0.755 V, are substantially higher than that of the equilibrium potential of the electrochemical reaction Cu2++ 2e → Cu, 0.34 V. The higher equilibrium potential for platinum indicates that platinum ions would be preferentially reduced when a reducing agent is added to an aqueous solution containing a mixture of platinum and copper ions. Hence, the formation of the Pt-Cu alloy NPs can be least favorable23,24.
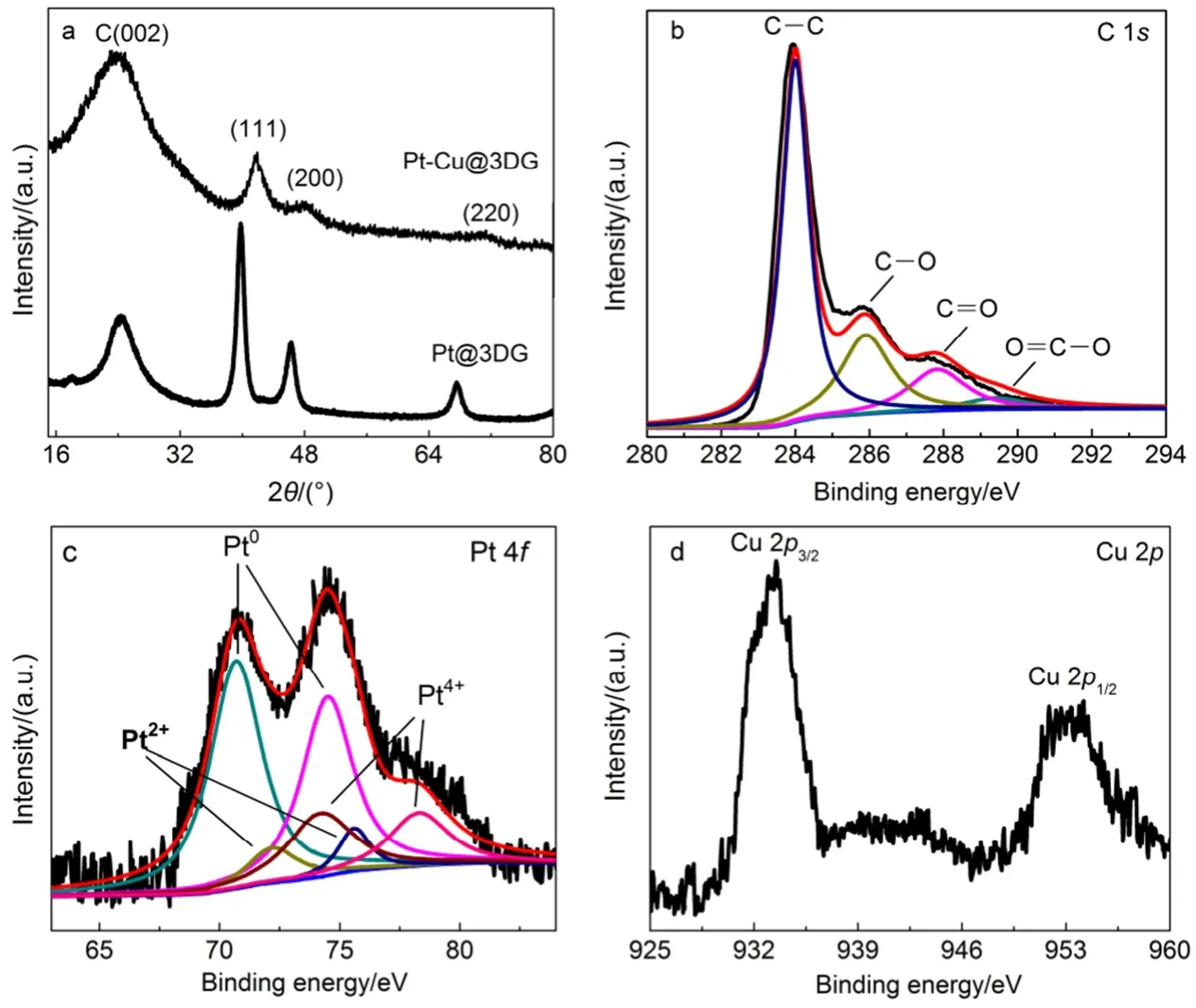
Fig.1 (a) XRD patterns of Pt-Cu@3DG and Pt@3DG, (b-d) XPS spectra of Pt-Cu@3DG in (b) C 1s, (c) Pt 4f and (d) Cu 2p regions
A TEM image of Pt-Cu@3DG is shown in Fig.2a before the catalytic reaction. The homogenous and uniform distribution of Pt-Cu alloy NPs on the 3DG may be due to in situ reduction of metal salts with an average size of around 130 nm (Fig.2b). The lattice fringes are highlighted on a particle (Fig.2c), indicating the crystalline nature of the materials. In addition, the regular d-spacing (0.238 nm)of Pt-Cu alloy NPs is well matched with the (111) planes of XRD. The EDX spectrum in Fig.2d confirms the presence of Cu and Pt from the particles.
3.2 Catalytic activity of Pt-Cu@3DG composite
For exploring the influence of concentration of GO solution on the composite, a series of concentrations of GO solution: 1, 2, 4 mg·g-1were prepared for the synthesis of Pt-Cu@3DG. The SEM of resulting composites exhibited pore structure, as shown in Fig.3(a-c). The reduced graphene sheets assembled easily with the increasing concentration of GO. The aggregation of sheets made the 3D structure lose its advantage of porosity when the concentration of GO solution increased to 4 mg·g-1. Therefore, the number of effective oxygen-containing groups is decreasing and the size of metal NPs increases withthe growing concentration of GO. Three kinds of Pt-Cu alloy NPs distribution are investigated by TEM dispersing on the graphene uniformly, as shown in Fig.3(d-f).
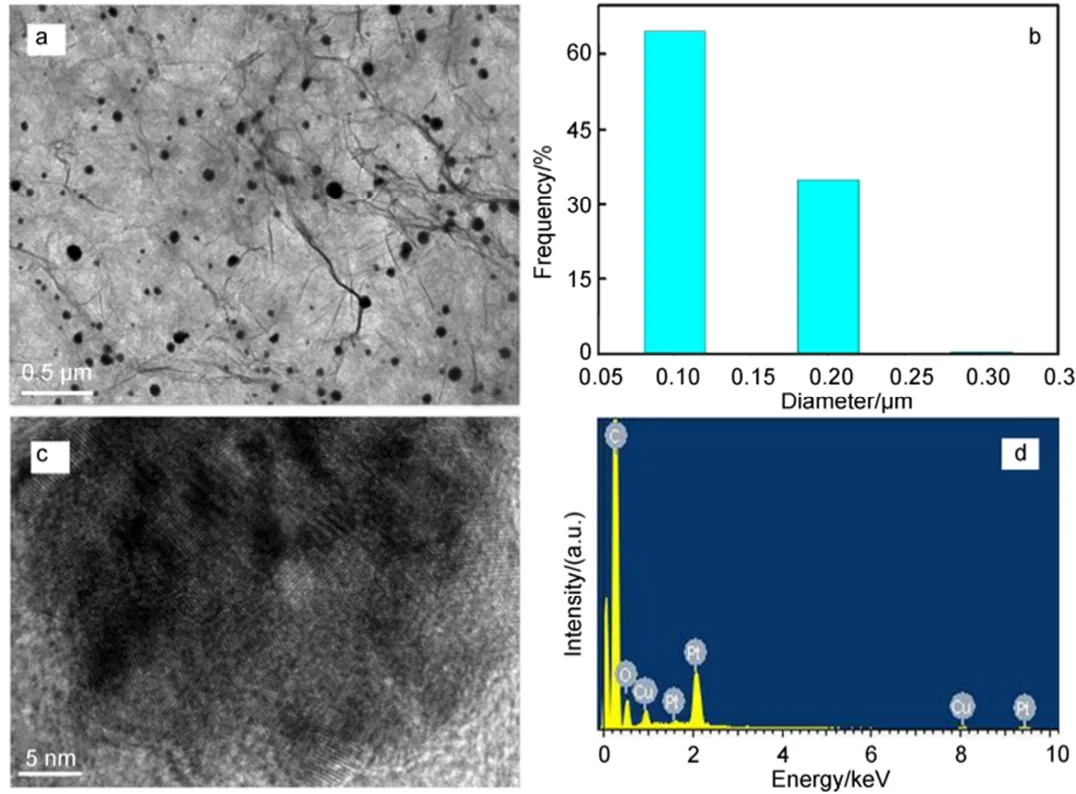
Fig.2 (a) TEM and (c) HR-TEM images of Pt-Cu@3DG catalyst, (b) diameter of particle size distribution of Pt-Cu alloy nanoparticles before the reaction, (d) EDX spectrum of Pt-Cu@3DG catalyst
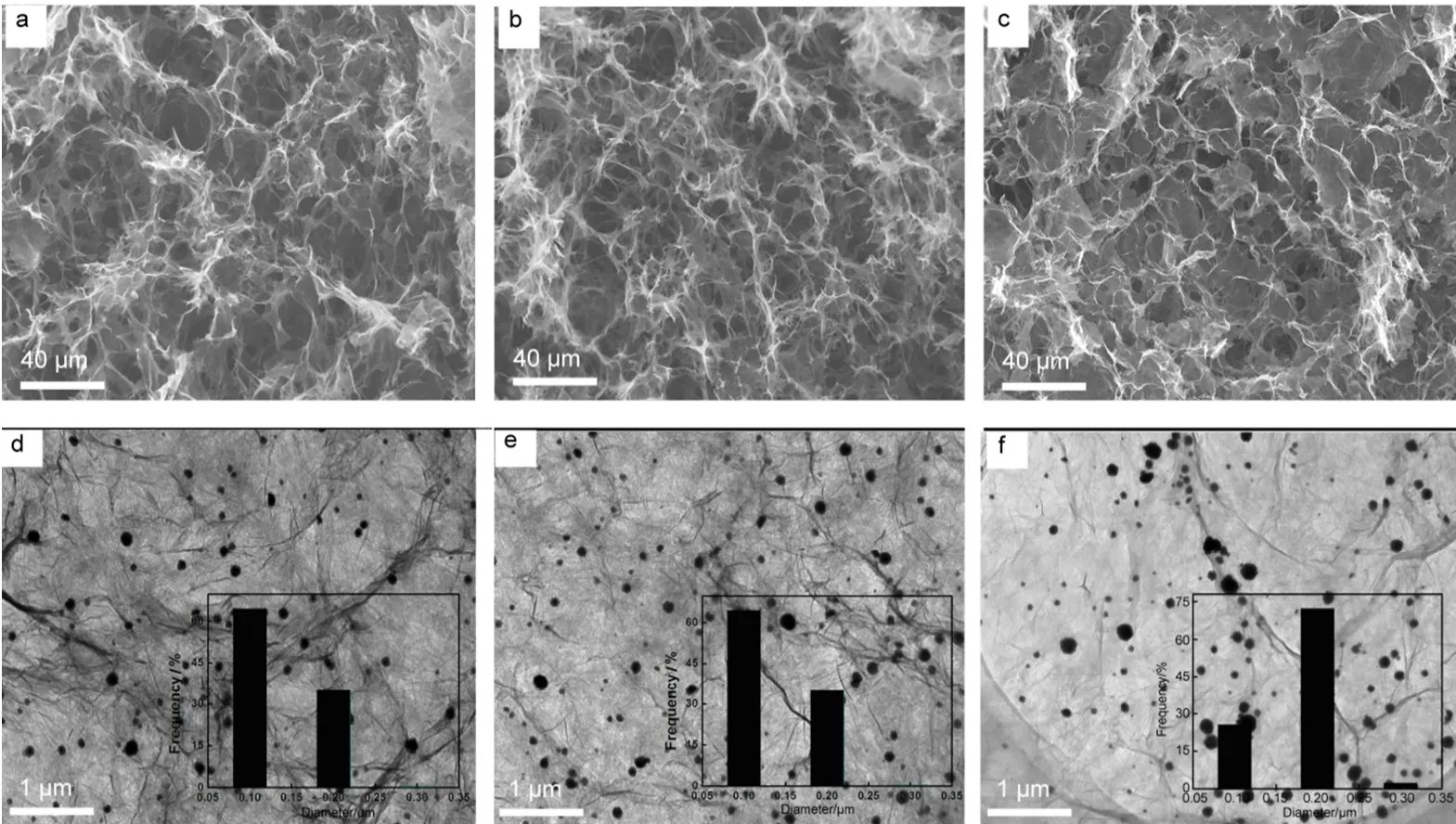
Fig.3 (a-c) SEM and (d-f) TEM images of Pt-Cu@3DG GO: (a, d) 1 mg·g-1; (b, e) 2 mg·g-1; (c, f) 4 mg·g-1. Inset shows the corresponding size distribution of Pt-Cu alloy NPs.
To explore the influence of distribution of metal NPs, a series of concentrations of GO solution: 1, 1.5, 2, 3, 4, 5 mg·g-1were prepared for the synthesis of Pt-Cu@3DG and the catalysts were used in the reduction of the 4-NP. The effect of the catalyst was tested by the conversion of reactants when the reaction was at 6 min, as shown in Fig.4. When the concentration of GO solution is 1 mg·g-1, the conversion of reaction is 87.3% with a smaller NPs distribution. The concentration increases to 2 mg·g-1, the higher conversion 98.6% is achieved. It is found that the conversion is higher, although the size of NPs distribution is larger than the composite with 1 mg·g-1. Raising the concentration of GO solution to 4 mg·g-1, the conversion has no obvious change. When the concentration of GO solution is 5 mg·g-1, the conversion of reaction was 96%. The assembly of reduced graphene sheets influences the catalytic performance. We infer that the determining factor of reaction rate is not only the particle size, but also the appropriate concentration of catalyst carrier precursor and the interaction between the bimetallic alloy NPs24-26. The conversion of reactants using with Pt@3DG, Pt-Cu@3DG and Pt-Cu catalyst is showed in Fig.4b. The catalytic effect of Pt-Cu alloy NPs is better than Pt NPs with the same content of Pt. The conversion of reactants with Pt-Cu@3DG is larger than Pt-Cu catalyst and the Pt-Cu@3DG composite shows greater cyclicity.
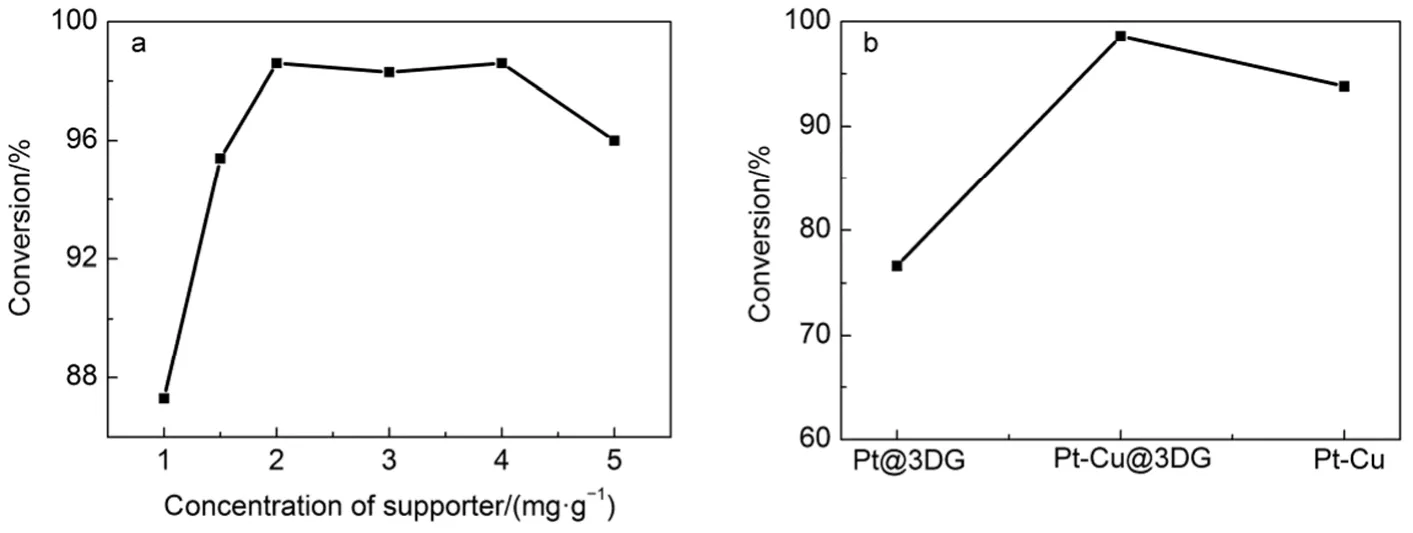
Fig.4 (a) Effects of the concentration of catalyst carrier precursor (GO) versus the conversion; (b) conversion of reactants using with Pt@3DG, Pt-Cu@3DG and Pt-Cu catalyst
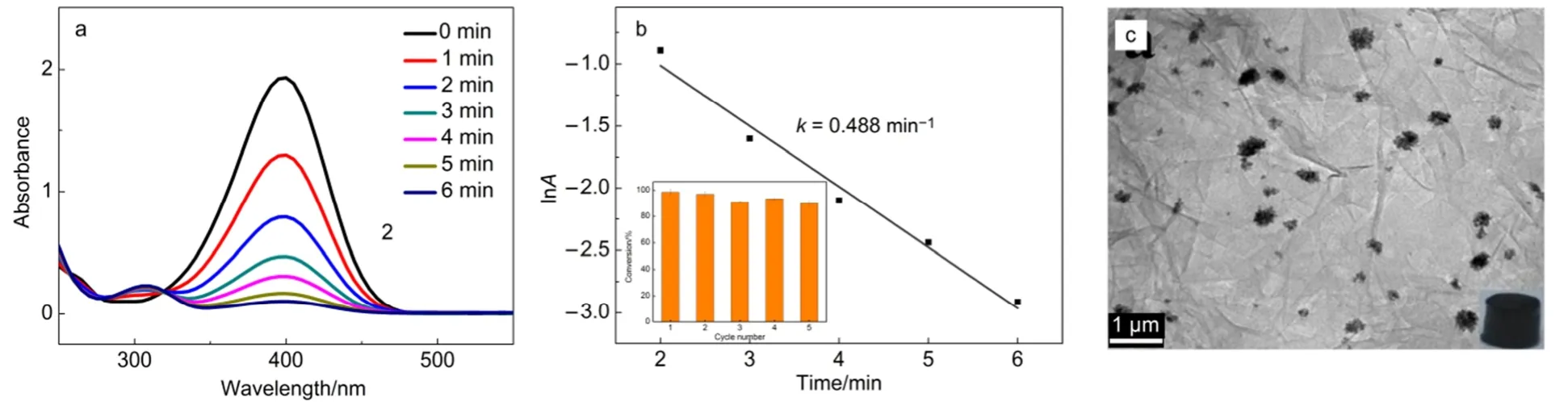
Fig.5 (a) Successive UV-Vis absorption spectra of the 4-NP by NaBH4in the presence of Pt-Cu@3DG; (b) shows the speed constant k (inset shows the recyclability of the Pt-Cu@3DG); (c) TEM image of the Pt-Cu@3DG catalyst after 5 cycles
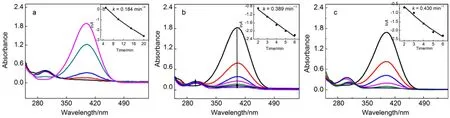
Fig.6 Successive UV-Vis absorption spectra of the 4-NP by NaBH4in the presence of 3%Pt@3DG (a), 8%Pt@3DG (b), 3% Pt-5% Cu (c) Inset shows the speed constant k.
The catalytic performance of the Pt-Cu@3DG composite is studied by the reduction of 4-NP to 4-AP with NaBH4, a representative reaction to evaluate the catalytic activity of metal NPs. The reduction process could be easily monitored byUV-Vis spectra because of the feature absorption at 400 and 300 nm for 4-NP and 4-AP, respectively. The peak at 400 nm vanishes gradually when the catalysts have been added, and concomitantly the absorption peak at around 300 nm is emerged. It takes 6 min to complete the catalytic reduction as shown in Fig.5(a). To investigate the catalytic stability of the Pt-Cu@3DG, the catalyst is recycled up to 5 times and the TEM of Pt-Cu@3DG catalyst is showed in Fig.5(c) after 5 cycles. The obvious aggregation of metal NPs is the main reason for decrease of catalyst activity. It could be seen that Pt-Gu@3DG catalyst only displays a slight decrease after five cycles, indicating the excellent recyclability.
Fig.5b indicates a linear correlation between lnA and reduction time, showing that the reaction is a pseudo-first-order. We calculated the speed constant k is 0.488 min-1. When the reduction is catalyzed by 4% Pt@3DG composite with the same content of Pt, the speed constant k is 0.184 min-1. When the reduction is catalyzed by 8% Pt@3DG composite with the same content of metal, the speed constant k is 0.389 min-1. When the reduction is catalyzed by Pt-Cu composite, the speed constant k is 0.430 min-1(Fig.6). These experimental data exhibited that Pt-Cu@3DG composite have better catalytic activity.
4 Conclusions
In a word, the Pt-Gu@3DG composite hydrogel, an efficient, low costing, recyclable, and stability catalyst is prepared successfully by a mild chemical reduction with ascorbic acid. The Pt-based bimetallic alloy NPs not only keep good catalytic activity but also lower the costs. The composite possesses interconnected macroporous structure, large specific surface area and thermal stability, resulting in excellent catalytic activity for reduction of 4-NP. Besides, the catalyst is recovered and reused easily, which could be recycled for 5 times. On the basis of the results, the Pt-Cu@3DG composite hydrogel has huge potential in industrial catalysis.
(1) Bai, X. J.; Hou, M.; Liu, C.; Wang, B.; Cao, H.; Wang, D. Acta Phys. -Chim. Sin. 2017, 33, 377. [白雪君, 侯 敏, 刘 婵, 王 彪,曹 辉, 王 东. 物理化学学报, 2017, 33, 377.] doi: 10.3866/PKU.WHXB201610272
(2) Liang, Y. Y.; Li, Y. G.; Wang, H. L.; Zhou, J. G.; Wang, J.; Regier, T.; Dai, H. J. Nat. Mater. 2011, 10, 780. doi: 10.1038/nmat3087
(3) Wu, Z. S.; Sun, Y.; Tan, Y. Z.; Yang, S. B.; Feng, X. L.; Müllen, K. J. Am. Chem. Soc. 2012, 134, 19532. doi: 10.1021/ja308676h
(4) Scheuermann, C. M.; Rumi, L.; Steurer, P.; Mvlhaupt, R. J. Am. Chem. Soc. 2009, 131, 8262. doi: 10.1021/ja901105a
(5) Subrahmanyam, K. S.; Manna, A. K.; Pati, S. K.; Rao, C. R. Chem. Phys. Lett. 2010, 497, 70. doi:10.1016/j.cplett.2010. 07.091
(6) Qiu, L.; Liu, J. Z.; Chang, S. L. Y.; Wu, Y. Z.; Li, D. Nat. Commun. 2012, 3, 187. doi: 10.1038/ncomms2251
(7) Xu, Y. X.; Sheng, K. X.; Li, C.; Shi, G. Q. ACS Nano 2010, 4, 4324. doi: 10.1021/nn101187z
(8) Ji, X. L.; Lee, K. T.; Zhang, L.; Zhang, J. J.; Botton, G. A.; Couillard, M.; Nazar, L. F. Nat. Chem. 2010, 2, 286. doi: 10.1038/nchem.553
(9) Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Katsnelson, M. I.; Grigorieva, I. V.; Dubonos, S. V.; Firsov, A. A. Nature 2005, 438, 197. doi: 10.1038/nature04233
(10) Li, Y. R; Chen, J.; Huang, L.; Li, C.; Shi, G. Q. Adv. Electron. Mater. 2015, 1, 1. doi: 10.1002/aelm.201500004
(13) Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Science 2007, 315, 493. doi:10.1126/science.1135941
(14) Liu, Y.; Li, D. C.; Stamenkovic, V. R.; Soled, S.; Henao, J. D.; Sun, S. H. ACS Catal. 2011, 1, 1719. doi: 10.1021/cs200430r
(15) Yang, H. Z.; Zhang, J.; Sun, K.; Zou, S. Z.; Fang. J. Y. Angew. Chem. Int. Edit. 2010, 49, 6848. doi: 10.1002/anie.201002888
(16) Stamenkovic, V. R.; Mun, B.; Arenz, S. M.; Mayrhofer, K. J. J.; Lucas, C. A.; Wang, G. F.; Ross, P. N.; Markovic, N. M. Nat. Mater. 2007, 6, 241. doi: 10.1038/nmat1840
(17) Jia, Y.; Su. J.; Chen, Z.; Tan, B.; Chen, Q.; Cao, Z.; Jiang, Y.; Xie, Z.; Zheng, L. RSC Adv. 2015, 5, 18153. doi: 10.1039/c4ra15673k
(18) Nie, R.; Shi, J.; Du, W.; Hou, Z. Appl. Catal. A: Gen. 2014, 473, 1. doi: 10.1016/j.apcata.2013.12.029
(19) Gümeci, C.; Cearnaigh, D. U.; Casadonte, D. J.; Korzeniewski, C. J. Mater. Chem. A 2013, 1, 2322. doi: 10.1039/c2ta00957a
(20) Dai, L.; Mo, S. G.; Qin, Q.; Zhao, X. J.; Zheng, N. F. Small 2016, 12, 1572. doi: 10.1002/smll.201502741
(21) Liu, Z.; Lee, J. Y.; Chen, W.; Han, M.; Can, L. M. Langmuir 2004, 20, 181. doi: 10.1021/la035204i
(22) Yu, X.; Wang, D.; Peng, Q.; Li, Y. Chem. Eur. J. 2013, 19, 233. doi: 10.1002/chem.201203332
(23) Hwang, B. J.; Tsai, Y. W.; Sarma, L. S.; Tseng, Y. L.; Liu, D. C.; Lee, J. F. J Phys. Chem. B 2004, 108, 20427. doi: 10.1021/jp0495592
(24) Gong, M. X.; Fu, C. T.; Chen, Y.; Tang, Y. W.; Lu, T. H. ACS Appl. Mater. Inter. 2014, 6, 7301. doi: 10.1021/am500656j
(25) Mott, D.; Luo, J.; Smith, A. Nano. Res. Lett. 2007, 2, 12. doi: 10.1007/s11671-006-9022-8
(26) Zhao, W. G.; Su, L.; Zhou, Z. N.; Zhang, H. J.; Lu, L. L.; Zhang, S. W. Acta Phys. -Chim. Sin. 2015, 31, 145. [赵万国, 苏 丽, 周振宁,张海军, 鲁礼林, 张少伟. 物理化学学报, 2015, 31, 145.] doi: 10.3866/PKU.WHXB2014102
Three-Dimensional Graphene-Based Pt-Cu Nanoparticles-Containing Composite as Highly Active and Recyclable Catalyst
WANG Mei-Song ZOU Pei-Pei HUANG Yan-Li WANG Yuan-Yuan*DAI Li-Yi
(College of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200241, P. R. China)
A composite hydrogel consisting of well-dispersed Pt-Cu nanoparticles (NPs) supported on three-dimensional (3D) graphene (Pt-Cu@3DG) was successfully prepared by mild chemical reduction. The 3D interconnected macroporous structure of the graphene framework not only possesses large specific surface area that allows high metal loadings, but also facilitates mass transfer during the catalytic reaction. The Pt-Cu bimetallic alloy NPs show good catalytic activity compared with Pt NPs and reduce the content of Pt NPs used, thereby lowering costs. The morphology and composition of the Pt-Cu@3DG composite were investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and energy-dispersive X-ray spectroscopy (EDX). The catalysis studies indicate that the resulting composites can be used as an efficient, inexpensive, recyclable, and stable catalyst for the reduction of 4-nitrophenol to 4-aminophenol under mild conditions.
Three dimensional graphene; Pt-Cu nanoparticle; Catalytic reaction; Pore structure; Composite
December 12, 2016; Revised: March 8, 2017; Published online: March 31, 2017.
O643
Geim, A. K. Science 2009, 324, 1530.
10.1126/science.1158877 (12) Zhang, M.; Lu, X.; Wang, H. Y.; Liu, X.; Qin, Y. J.; Zhang, P.; Guo, Z. X. RSC Adv. 2016, 6, 35945. doi: 10.1039/c6ra01772j
doi: 10.3866/PKU.WHXB201703311
*Corresponding author: Email: ecnu_yywang@163.com; Tel/Fax: +86-21-54340133.
The project was supported by the Key Project of Shanghai Science and Technology Committee (14231200300) and Shanghai Key Laboratory of Green Chemistry and Chemical Processes.
上海市重点工程科学和技术委员会(14231200300)及上海市绿色化学与化工过程绿色化重点实验室
© Editorial office of Acta Physico-Chimica Sinica

