钙钛矿铁电纳米片诱导的P(VDF-TrFE)取向生长
刘 金 麦江泉 李 诗 任召辉,* 李 铭 武梦娇吴勇军 路新慧 李 翔 田 鹤 王宗荣 韩高荣,*
(1浙江大学材料科学与工程学院,硅材料国家重点实验室,唐仲英传感材料及应用研究中心,杭州 310027;2香港中文大学物理系,香港 新界 999077;3浙江大学电子显微镜中心,硅材料国家重点实验室,材料科学与工程学院,杭州 310027)
钙钛矿铁电纳米片诱导的P(VDF-TrFE)取向生长
刘 金1,†麦江泉2,†李 诗1任召辉1,*李 铭1武梦娇1吴勇军1路新慧2,*李 翔1田 鹤3王宗荣1韩高荣1,*
(1浙江大学材料科学与工程学院,硅材料国家重点实验室,唐仲英传感材料及应用研究中心,杭州 310027;2香港中文大学物理系,香港 新界 999077;3浙江大学电子显微镜中心,硅材料国家重点实验室,材料科学与工程学院,杭州 310027)
半结晶的铁电聚合物在柔性电子器件中极具应用前景,控制晶相生长对其性能优化至关重要。本文通过引入少量(0.2%)单晶单畴的PbTiO3纳米片对P(VDF-TrFE) (简称PVTF)铁电薄膜的生长进行有效调节,获得了高度取向的铁电薄膜且铁电性能得到了大幅提高。PbTiO3纳米片铁电极化对PVTF极性分子的诱导作用可能是薄膜取向生长与性能提高的原因。
P(VDF-TrFE);PbTiO3;纳米片;取向生长;铁电性
1 Introduction
As a classical ferroelectric polymer, PVDF and its copolymer PVTF have been extensively explored in terms of dielectric, piezoelectric and ferroelectric properties, affording wide potential applications in flexible electronics from energy harvesting, data storage, sensors and actuators1-5. The electric properties and device performance of PVTF films highly depend on the crystallographic orientation of β phase (ferroelectric phase)6-8. A prominent example is that a PVTF film with pressure-induced oriented crystalline exhibits an improved remnant polarization, 1.6 times higher than that of disordered counterpart9. To obtain such a preferential orientation, different physical templates, including anodic aluminium oxide (AAO)10, organosilicate (OS) lamellae11, SAM-modified Au substrates12, graphene layer13, etc. have been employed. Among them, a confinement effect originating from microstructure of the templates was demonstrated to effectively induce the polarization direction along the long axis of one dimensional PVTF nanotubes14. However, the preparation and removal of the templates have been found to be very complex and tedious, significantly limiting large scale material and device fabrication. In addition, the epitaxy growth method normally employed for inorganic crystalline films cannot be simply applied for organic ferroelectric polymers due to the semi-crystalline character. Hence, it remains a great challenge to develop a facile strategy to fabricate highly oriented PVTF film.
In this work, for the first time, we demonstrated a facile and effective approach to mediate the growth and thus the orientation of PVTF films by a strong electrostatic interaction between PVTF and perovskite ferroelectric nanoplates. A very low introduction (0.2% (w)) of single-crystal and single-domain PbTiO3(denoted by PTO) nanoplates can effectively mediate the nucleation and growth process of crystalline phase within PVTF, giving rise to highly (110)/(200) oriented films. We attributed this oriented crystallization behaviour to an alignment of polarization directions of the polymer and the nanoplates. As a result, the ferroelectric polarization of the films have been significantly improved by 53%.
Negative or positive polar surface of ferroelectric perovskite oxides has been explored to accelerate the crystal growth of inorganic crystals15and to influence the selective deposition of Ag particles on ferroelectric substrates by a photochemical method16. Moreover, the ordering of polar water molecules is observed within the ice films growing on the surface of PVTF17.
The electrostatic interaction between polar surface of ferroelectrics and different inorganic objects leads to fasnating phenomena. However, an effect of ferroelectric polarization on the crystal growth of semi-crystalline polymer films has not been achieved yet, and the difficulty arises from the semi-crystalline nature of polymer films with complex molecular configuration. To realize this, a strong electrostatic interaction is highly required. As a prototypical ferroelectric oxide, PTO is an ideal material to investigate the effect of electrostatic interaction on the growth of PVTF because of its simple perovskite structure and large spontaneous polarization (Ps)18.
Fig.1(a, b) present the schematic view of the crystal structures for tetragonal PTO (JCPDS 70-0746) and the orthorhombic β-phase PVTF (JCPDS 42-1649), where the Psis along the c axis and b axis, respectively. The relative displacement of Ti ions from oxygen plane of octahedron in Fig.1(a) gives rise to a Psof PTO. In our previous work, single-crystal and single-domain PTO nanoplates were synthesized hydrothermally via a self-templated growth19. A cross-section STEM image of an individual PTO nanoplate is shown in Fig.1(c), and the nanoplate adopts faceted planes with a smooth surface. Fig.1(d) depicts the corresponding atomic-level HAADF-STEM of the nanoplate (red area in Fig.1(c)), where the displacement of titanium atoms deviation from the body-center downwards can be observed, opposite to the ferroelectric polarization direction20. Thus, the polarization direction (pointing by an orange arrow) of the nanoplate is perpendicular to the surface in Fig.1(d). In particular, the Curie temperature of the nanoplates was determined to be 487 °C (Fig.S2), very close to 490 °C of bulk PTO, implying a large Ps of the nanoplates with an order of 60~70 μC·cm-221. This value is much larger than those of polar organic molecules and ferroelectric polymer17,22,23. When unscreened, an electrostatic field derived from the polar surface of PTO nanoplates could be high as 108V·cm-123.
2 Experiment section
2.1 Material preparation

Fig.1 Schematic view of the crystal structures for (a) tetragonal PbTiO3and (b) orthorhombic β-phase PVTF, (c) Cross-section TEM image of individual PTO nanoplate and (d) corresponding atomic level HAADF-STEM (SEM image and XRD pattern see Fig.S1(a, b)
Single-crystal and single-domain ferroelectric PTO nanoplates were synthesized by hydrothermal synthesismethod19. TiO2nanosheets were prepared according to the method reported in the reference24. 25 mL of Ti(OBu)4and 4 mL of hydrofluoric acid solution were mixed in a dried Teflon autoclave with a capacity of 50 mL, and then kept at 200 °C for 24 h. After being cooled to room temperature, the white powder was washed with ethanol and distilled water for several times and then dried at 80 °C for 6 h.
PVTF (the mole ratio of VDF to TrFE is 70/30) powder was dissolved in tetrahydrofuran (THF) and then a certain PTO (or TiO2) powder was introduced into the solution. The mixed hybrid was then processed with stirring for 15 min and then sonicating 30 min in order to disperse the nanoplates completely. Here we designed the weight concentration of PTO (or TiO2) nanoplates as 0.2%, 0.5%, and 1.0% within PVTF. The pure PVTF and PVTF films with 0.2% PTO (TiO2) nanoplates were prepared by a spin coating technique on the ITO glass substrates under a speed of 1500 rpm for 30 s. In particular, the substrates were cleaned by sonicating in the deionized water, acetone and ethanol for 15 min, respectively. Finally, the as-prepared films were heated under vacuum condition for different time and then cooled down to room temperature. In order to measure the electric properties, Ag film was sputtered onto the surface of films as top electrode while ITO was used as the bottom electrode.
2.2 Characterization
The XRD patterns were collected on a Thermo ARLXTRA powder diffractometer with Cu Kαradiation (λ = 1.54056 nm-1). SEM images were obtained from a Hitachi field emission SEM MODEL S-4800 at 5 kV. TEM specimens were examined by using an FEI Titan G2 80-200 Chemi 280 STEM with an accelerating voltage of 200 kV. Non-contact mode AFM (XE-100E, Park, Korea) was conducted to analyze topographic of films and Fourier transform infrared (FTIR) (Tensor 27, Bruker, Germany) was used to identify the molecule structures of films. The thickness of pure PVTF and PVTF (0.2%) films were detected by a step profiler (DEKTAK-XT, Bruker, America). The enthalpy values for the two samples were derived from TG curves recorded on a PE DSC 7. The Curie temperature of PTO nanoplates was detected by a technique of TG-DTA, combining TG ignition measurement and DTA analysis, equipped with the analyzer TGA7 (Perkin Elmer, San Jose, CA, USA) and DTA7 (Perkin Elmer, San Jose, CA, USA), respectively. The GIWAXS measurements were conducted at 23A SWAXS beamline at the National Synchrotron Radiation Research Center, Hsinchu, Taiwan, using a 10 KeV primary beam, 2.0° incident angle and C9728DK area detector. Dielectric properties of the samples were measured by Agilent 4292A precision impedance analyzer (HP4294ALRC) between 1 KHz and 1 MHz. Ferroelectric properties of the samples were measured at 100 Hz using a RT66A ferroelectric tester (Radiant Technologies Inc., Albuquerque, NM, USA).
3 Results and Discucssion
For such nanoplates, the exposed (001) is positive or negative polar surface, and the direction of Psis uniformed within the nanoplate along c axis. Different concentration of PTO nanoplates was introduced into PVTF films with similar thicknesses about 800 nm, prepared by spin-coating on ITO substrates (see Experiment section). According to the XRD patterns in Fig.S3(a), the peak intensity of (001) is the highest among those of PTO in the film, implying that most PTO nanoplates are lying in PVTF films. When PTO nanoplates were introduced, the intensity of (110)/(200) diffraction peaks in PVTF films with 0.2%, 0.5% and 1.0% PTO nanoplates is higher than that of PVTF (0%) films (Fig.S3(a)), implying that an orientation growth could occur. In particular, PVTF films with 0.2% (w) PTO nanoplates (denoted by PVTF (0.2%) films) present the highest relative intensity ratio of (110)/(200) (~19.8°). Furthermore, grazing incidence wide-angle X-ray scattering (GIWAXS) was used to investigate molecular orientation. Fig.2(a, b) show GIWAXS patterns of pure PVTF and PVTF (0.2%) films, respectively. It can be clearly observed that at q ≈ 14 nm-1, PVTF (0.2%) film has two distinct reflections at meridian and 60° away from the meridian indicating a highly preferential orientation, while the pure PVTF film shows a ring-like pattern representing a randomly distributed orientation. The two reflections are corresponding to (200) and (110). These reflections arise from either (110) or (200) of PVTF crystals, based on the fact that the PVTF crystal phase has a pseudo hexagonal orthorhombic lattice which is characterized by aratio of its a and b axes and in turn leads to nearly equal (200) and (110) spacings25. Two possible orientations of the PVTF films are illustrated in Fig.3(a, b). The case one is that (200) of PVTF parallel to (001) of PTO, where the Ps direction of PVTF is perpendicular to that of PTO, as shown in Fig.3(a). This configuration gives rise to the reflection of (200) on the meridian. In the case two, (110) of PVTF is paralleled to (001) of PTO, where the reflection on the meridian is thus indexed as (110) in Fig.3(b). Although the two reflections are hard to be distinguished due to the same lattice constant, we presume that the case in Fig.3(b) is more possible since the polarization direction of PVFT and PTO should align together to induce this preferential orientation. And the aligned dipoles of PVTF would further affect the other dipoles around. In addition, 0.5%, and 1.0% samples also show the similar two reflections but their intensity is relatively weak (Fig.S4). This result indicates an orientation growth also occurred in these films as well, consistent with the XRD results of Fig.S3(a). In the emerged work, it has been discussed that an oriented growth of PVTF films could be thickness-dependent due to molecular confinement effect. However, such effect is limited to ultrathin films (<100 nm), resulting in a decreased ferroelectric property26,27. Therefore, the molecular confinement effect cannot give rise to the highly preferential orientation of PVTF films in our work.
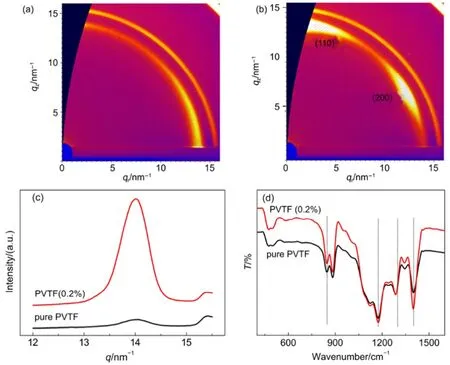
Fig.2 (a, b) GIWAXS patterns, (c) corresponding intensity profiles along qzaxis and (d) FTIR spectrum of pure PVTF film and PVTF (0.2%) film
We also argued that an improved crystallization cannot be the origin of such phenomenon because it can only lead to a general increase of all peaks instead of a single peak. Furthermore, the crystallinity degree Xcfor the two samples has been determined by DSC curves (Fig.S5) to be 59.6% and 61.5%, respectively. Despite of similar crystallinity, the morphology by AFM imaging is obviously different, from particle shape in pure PVTF film to long-rod shape in PVTF (0.2%) film, as shown in Fig.S6(a, b). On the basis of the corresponding 3D view image, an average diameter of the particles in pure PVTF (Fig.S6(c)) is statically about 0.38 μm, and the rods in PVTF (0.2%) film in Fig.S6(d) was determined to have an average diameter of 0.45 μm and length of 1.54 μm. The anisotropic morphological shape is most likely to result from the highly oriented growth of the film induced by the introduction of PTO nanoplates.
On the basis of the model in Fig.3(b), the polarization axis (b axis) of PVTF film tend to be aligned by that of PTO nanoplate and thus form an angle of 60° with the normal direction of the nanoplates. Such configuration would lead to a strong electrostatic interaction between molecular chain of PVTF and PTO. The interaction can be manifested by the band of 1400 cm-1of PVTF in FTIR spectrum, corresponding to the ωCH2coupled with νasC-C28. From the FTIR spectra (Fig.2(d)), it can be observed that the peak intensity of the band of 1400 cm-1in PVTF (0.2%) film becomes much higher than that of pure PVTF, supporting our model of Fig.3(b). The other bands at 846 cm-1, 1174 cm-1and 1286 cm-1in Fig.2(d) are indexed as the CF2symmetric stretching, the CF2asymmetric stretching and the long trans sequence29-31.
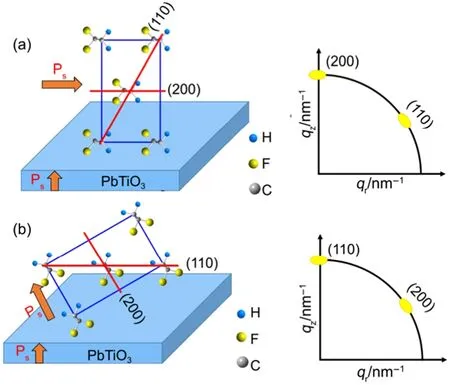
Fig.3 A schematic diagram of two possible cases that the molecular preferentially oriented
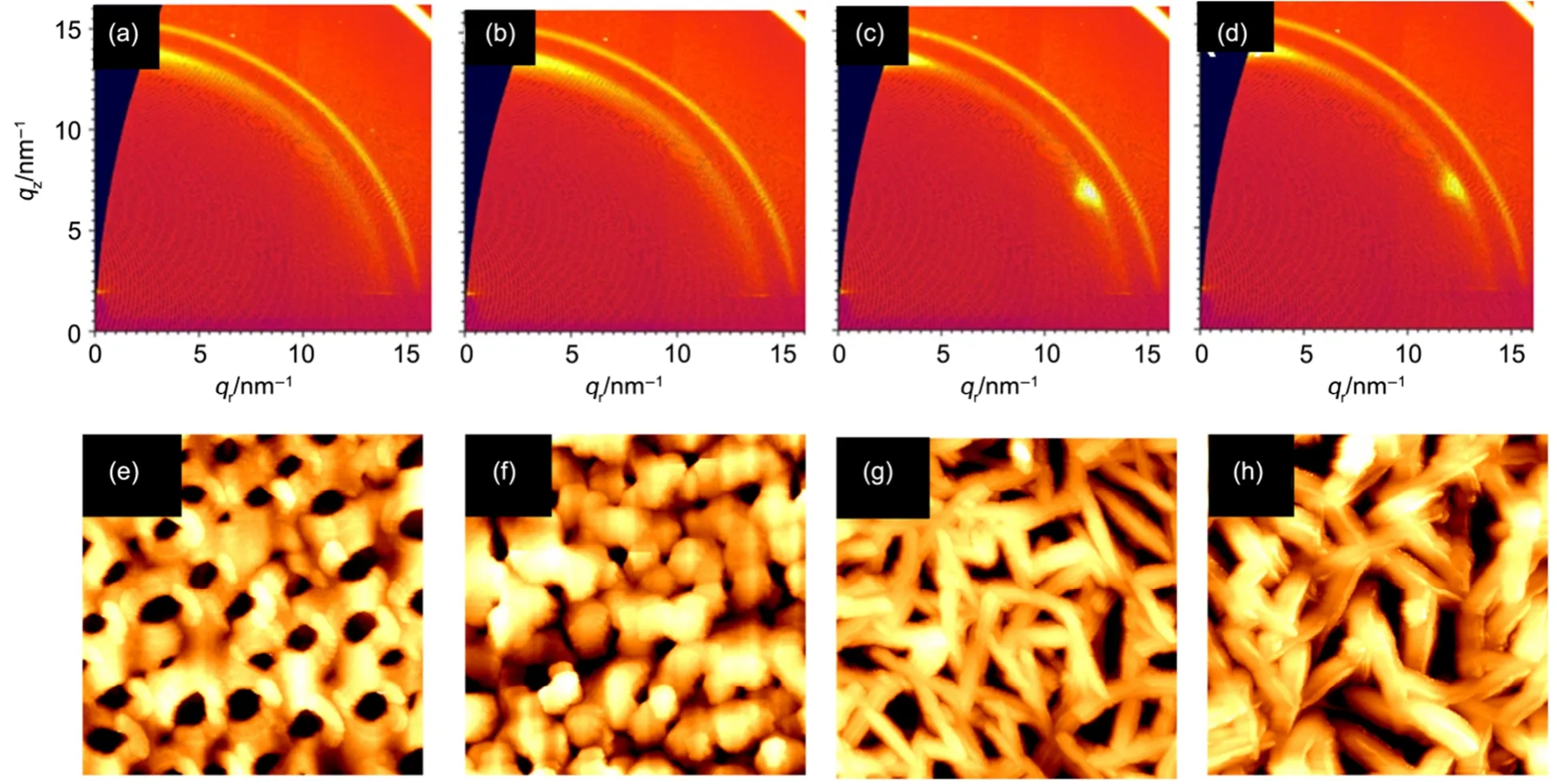
Fig.4 GIWAXS patterns of PVTF (0.2%) films heat treated at 180 °C for (a) 0.5 h, (b) 1.0 h, (c) 1.5 h, (d) 2.0 h and (e-h) corresponding AFM topographic images (5 μm × 5 μm)
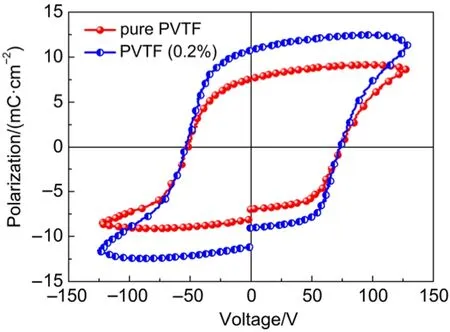
Fig.5 Ferroelectric hysteresis loops of pure PVTF and PVTF (0.2%) films
The growth process of PVTF film was further investigated by using PVTF (0.2%) films after a heat treatment of 180 °C for different time. The intensified GIWAXS reflection in Fig.4(a) suggests that the film became crystalline after 0.5 h heat treatment, and the crystallization gradually improved up to 1 h in Fig.4(b). A longer time of 1.5 h could also induce the (110)/(200) orientation growth of the film, confirmed by the two intensified reflections in Fig.4(c). The orientation of the film remained when the heat treatment was prolonged to 2.0 h in Fig.4(d). Corresponding topographic morphology of the samples were characterized by AFM in Fig.4(e-h). PVTF (0.2%) film was initially crystallized into particle, as shown in Fig.4(e, f), and then particle crystals gradually grew to a rod-like shape (Fig.4(g, h)). The above results provide the solid evidence to support that the films experience a nucleation-growth process, accompanied with the orientation and the distinct morphology change. One should note that a high temperature of 180 °C used for preparation is above melting temperature (Tm) (~150 °C) of PVTF (Fig.S5), at which the molecular chains of PVTF can freely reorganize. This could be particularly important for ferroelectric polarization inducing the array of the chains and subsequent preferential orientation of the films, as shown in Fig.1(d). When the thickness is below 100 nm, the annealing temperature could play an important role in modifying confinement effect and thus the orientation of P(VDF-TrFE) films26,27,32. However, no such orientation growth has been observed when our samples, such as PVTF (0.2%) films, were prepared at below Tm(120 °C) (see Fig.S7) Furthermore, non-ferroelectric TiO2nanoplates (SEM image see Fig.S8) were introduced to investigate their influence on the growth of PVTF films. And such PVTF films did not show such orientation like Fig.2(b) by analysing GIWAXS patterns (Fig.S9). It is thus reasonable to conclude that the temperature above Tmand ferroelectric polarization are crucial for the oriented growth of PVTF films. Accompanying with the oriented growth, the peak intensity at 1400 cm-1was significantly enhanced in FTIR spectra of PVTF (0.2%) films (Fig.S10) as the heat time was prolonged from 0.5 h to 2 h at 180 °C. These results strongly support our model in Fig.3(b). In particular, a significant improvement in ferroelectric property has been achieved in such PVTF (0.2%) films.
On the basis of ferroelectric hysteresis loops in Fig.5, the remnant polarization (Pr) in Fig.5 increased from 7.0 μC·cm-2to 10.7 μC·cm-2. We believe that the improved properties should be attributed to the oriented growth and dipole arrangement in the films. One may argue that the introduction of PTO nanoplates might also contribute to the results in Fig.5. To investigate this aspect, the ferroelectric property of PVTF films with different concentrations of PTO that nanoplates were measured. The results indicate the properties were not improved upon the increased concentrations of PTO nanoplates (Fig.S11 and Fig.S12). When the concentration of PTO nanoplates is 0.2%, the properties of the films could be optimized.
4 Summary
In summary, a highly oriented PVTF film was obtained by the introduction of a very small amount of single-crystal and single-domain PTO nanoplates. The corresponding structural analysis revealed that with the optimum concentration of 0.2% (w), the electrostatic interaction between PTO polar surface and dipoles of PVTF molecular chains can effectively mediate the nucleation and growth of PVTF, giving rise to a strong orientation. By this means, the ferroelectric polarization of the samples have been improved by 53%, which is of great significance for device applications. The findings suggest that the introduction of ferroelectric nanoplates may be a facile approach to tailor the crystalline growth of polar organic materials and their fundamental physical properties.Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Soin, N.; Boyer, D.; Prashanthi, K.; Sharma, S.; Narasimulu, A. A; Luo, J.; Shah, T. H.; Siores, E.; Thundat, T. Chem. Commun. 2015, 51, 8257. doi: 10.1039/c5cc01688f
(2) Hu, Z.; Tian, M.; Nysten, B.; Jonas, A. M. Nat. Mater. 2009, 8, 62. doi: 10.1038/NMAT2339
(3) Chen, X. Z.; Li, Q.; Chen, X.; Guo, X.; Ge, H. X.; Liu, Y.; Shen, Q. D. Adv. Funct. Mater. 2013, 23, 3124. doi: 10.1002/adfm.201203042
(4) Wang, X.; Wang, P.; Wang, J.; Hu, W.; Zhou, X.; Guo, N.; Huang, H.; Sun, S.; Shen, H.; Lin, T.; Tang, M. Adv. Mater. 2015, 27, 6575. doi: 10.1002/adma.201503340
(5) Lee, J. S.; Shin, K. Y.; Kim, C.; Jang, J. Chem. Commun. 2013, 49, 11047. doi: 10.1039/c3cc46807k
(6) Ohigashi, H.; Omote, K.; Gomyo, M. C. Appl. Phys. Lett. 1995, 66, 3281. doi: 10.1063/1.113730
(7) García-Gutiérrez, M. C.; Linares, A.; Hernández, J. J.; Rueda, D. R.; Ezquerra, T. A.; Poza, P.; Davies, R. J. Nano Lett. 2010, 10, 1472. doi: 10.1021/nl100429u
(8) Wu, Y.; Li, X.; Jonas, A. M.; Hu, Z. Phys. Rev. Lett. 2015, 115, 267601. doi: 10.1103/PhysRevLett.115.267601
(9) Shin, Y. J.; Kim, R. H.; Jung, H. J.; Kang, S. J.; Park, Y. J.; Bae, I. Park, C. ACS Appl. Mater. Interfaces 2011, 3, 4736. doi: 10.1021/am201202w
(10) Cauda, V.; Torre, B.; Falqui, A.; Canavese, G.; Stassi, S.; Bein, T.; Pizzi, M. Chem. Mater. 2012, 24, 4215. doi: 10.1021/cm302594s
(11) Kang, S. J.; Bae, I.; Shin, Y. J.; Park, Y. J.; Huh, J.; Park, S. M.; Kim, H. C.; Park, C. Nano Lett. 2010, 11, 138. doi: 10.1021/nl103094e
(12) Park, Y. J.; Kang, S. J.; Park, C.; Lotz, B.; Thierry, A.; Kim, K. J.; Huh, J. Macromolecules 2008, 41, 109. doi: 10.1021/ma0718705
(13) Kim, K. L.; Lee, W.; Hwang, S. K.; Joo, S. H.; Cho, S. M.; Song, G.; Cho, S. H.; Jeong, B.; Hwang, I.; Ahn, J. H.; Yu, Y. J. Nano Lett. 2015, 16, 334. doi: 10.1021/acs.nanolett.5b03882
(14) Bhavanasi, V.; Kusuma, D. Y.; Lee, P. S. Adv. Energy Mater.
(15) Sun, X.; Ma, C.; Wang, Y.; Li, H. J. Cryst. Growth 2002, 234,
404. doi: 10.1016/S0022-0248(01)01695-5
(16) Burbure, N. V.; Salvador, P. A.; Rohrer, G. S. Chem. Mater. 2010, 22, 5823. doi:10.1021/cm1018025
(17) Rosa, L. G.; Xiao, J.; Losovyj, Y. B.; Gao, Y.; Yakovkin, I. N.; Zeng, X. C.; Dowben, P. A. J. Am. Chem. Soc. 2005, 127, 17261. doi: 10.1021/ja054159t
(18) Fong, D. D.; Kolpak, A. M.; Eastman, J. A.; Streiffer, S. K.; Fuoss, P. H.; Stephenson, G. B.; Thompson, C.; Kim, D. M.; Choi, K. J.; Eom, C. B.; Grinberg, I. Phys. Rev. Lett. 2006, 96, 127601. doi: 10.1103/PhysRevLett.96.127601
(19) Chao, C.; Ren, Z.; Zhu, Y.; Xiao, Z.; Liu, Z.; Xu, G.; Mai, J.; Li, X.; Shen, G.; Han, G. Angew. Chem. Int. Ed. 2012, 51, 9283. doi: 10.1002/anie.201204792
(20) Jia, C. L.; Nagarajan, V.; He, J. Q.; Houben, L.; Zhao, T.; Ramesh, R.; Urban, K.; Waser, R. Nat. Mater. 2007, 6, 64. doi: 10.1038/nmat1808
(21) Fridkin, V. M. Ferroelectric Semiconductors, Consultants Bureau: New York, NY, USA 1980.
(22) Wang, F.; Lack, A.; Xie, Z.; Frübing, P.; Taubert, A.; Gerhard, R. Appl. Phys. Lett. 2012, 100, 062903. doi: 10.1063/1.3683526
(23) Salimi, A.; Yousefi, A. A. J. Polym. Sci. B: Polym. Phys. 2004, 42, 3487. doi: 10.1002/polb.20223
(24) Han, X.; Kuang, Q.; Jin, M.; Xie Z.; Zheng, L. J. Am. Chem. Soc. 2009, 131, 3152. doi: 10.1021/ja8092373
(25) Park, Y. J.; Kang, S. J.; Lotz, B.; Brinkmann, M.; Thierry, A.; Kim, K. J.; Park, C. Macromolecules 2008, 41, 8648. doi: 10.1021/ma801495k
(26) Guo, D.; Setter, N. Macromolecules 2013, 46, 1883. doi: 10.1021/ma302377q
(27) Urayama, K.; Tsuji, M.; Neher, D. Macromolecules 2000, 33, 8269. doi: 10.1021/ma000855w
(28) Prabu, A. A.; Lee, J. S.; Kim, K. J.; Lee, H. S. Vibrational Spectroscopy 2006, 41, 1-13. doi: 10.1016/j.vibspec.2005.11.005
(29) Li, W.; Guo, S.; Tang, Y.; Zhao, X. J. Appl. Polym. Sci. 2004, 91, 2903. doi: 10.1002/app.13503
(30) Shin, Y. J.; Kang, S. J.; Jung, H. J.; Park, Y. J.; Bae, I.; Choi, D. H. Park, C. ACS Appl. Mater. Interfaces 2011, 3, 582. doi: 10.1021/am1011657
(31) Zhu, H.; Mitsuishi, M.; Miyashita, T. Macromolecules 2012, 45, 9076. doi: 10.1021/ma301711g
(32) Guo, D.; Stolichnov, I.; Setter, N. J. Phys. Chem. B 2011, 115, 13455. doi: 10.1021/jp2061442
Perovskite Ferroelectric Nanoplates Induced a Highly Oriented Growth of P(VDF-TrFE) Films
LIU Jin1,†MAI Jiang-Quan2,†LI Shi1REN Zhao-Hui1,*LI Ming1WU Meng-Jiao1WU Yong-Jun1LU Xin-Hui2,*LI Xiang1TIAN He3WANG Zong-Rong1HAN Gao-Rong1,*
(1State Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Cyrus Tang Center for Sensor Materials and Application, Zhejiang University, Hangzhou 310027, P. R. China;2Department of Physics, Chinese University of Hong Kong, New Territories, Hong Kong 999077, P. R. China;3State Key Laboratory of Silicon Material, School of Material Science & Engineering, Center of Electron Microscope, Zhejiang University, Hangzhou 310027, P. R. China)
Ferroelectric polymers are particularly attractive for applications in flexible electronic devices, and controlling its crystalline phase growth is crucial for obtaining optimized ferroelectric properties. Herein we report that a very low introduction (0.2% (w)) of single-domain ferroelectric PbTiO3nanoplates can effectively mediate the nucleation and subsequent growth of a crystalline phase within P(VDF-TrFE) (denoted by PVTF), forming highly oriented films and significantly improving the ferroelectric properties due to an alignment of the polarization directions of the polymer and the nanoplates.
P(VDF-TrFE); PbTiO3; Nanoplate; Orientation growth; Ferroelectricity
February 15, 2017; Revised: February 27, 2017; Published online: March 6, 2017.
O641
, 4, 1400723.
10.1002/aenm.201400723
doi: 10.3866/PKU.WHXB201702281
*Corresponding authors. REN Zhao-Hui, Email: renzh@zju.edu.cn. LU Xin-Hui, Email: xhlu@phy.cuhk.edu.hk. HAN Gao-Rong, Email: hgr@zju.edu.cn.
†These authors contributed equally to this work.
The project was supported by the National Natural Science Foundation of China (51232006, 51472218), the National Key Basic Research Special Foundation, China (973) (2015CB654901), Fundamental Research Funds for the Central Universities, China (2016FZA4005) and RGC of Hong Kong GRF (14303314) and CUHK Direct Grant, China (4053128).
国家自然科学基金(51232006, 51472218),国家重点基础研究发展项目(973)(2015CB654901),中央高校基本科研业务费专项资金(2016FZA4005),香港研究资助局科研基金(14303314),香港中文大学基金(4053128)资助项目
© Editorial office of Acta Physico-Chimica Sinica
——材料科学与工程

