Study on catalytic redox of potassium ferrocyanide at self assembly membrane of surfactant
, , ,
XIN Shigang2, ZHANG Hongbo2
(1. Institute of Catalysis for Engery and Environment, Shenyang Normal University, Shenyang 110034, China; 2. College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, China)
Study on catalytic redox of potassium ferrocyanide at self assembly membrane of surfactant
ZHUYongchun1,2,MENGXiangyu2,KUANGYawen2,
XINShigang2,ZHANGHongbo2
(1. Institute of Catalysis for Engery and Environment, Shenyang Normal University, Shenyang 110034, China; 2. College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, China)
The self assembly membrane of cetyl trimethylammonium brominide at castor oil coated graphite composite electrode was investigated by cyclic voltammetry and pm7 quantum chemical method with ferrocyanide redox couple as a probe. The self assemblying process shows a two-step exponential association function with the apparent first order rate constants of 0.002 377 s-1and 0.000 721 5 s-1, respectively. The self assembly membrane modified castor oil coated graphite composite electrode shows a catalytic behavior to ferrocyanide a more than 100 mV redox peak potential neganitive shift, 5 times increase in peak current, and about 30 mV decrease in peak-peak potential difference indicating the increase of rate constant of the electrode reactions. The concentration of cetyltrimethylammonium bramide has a great influence on the formation of the self-assembly membrane and also the rate constant of the electrode reaction. The pm7 quantum chemical calculation results reveal the stability of the self-assembly membrane and the association complexes between ferrocyanide and ferricyanide with triple cetyl trimethylammonium bromide. The exchangable electrons from the energy of the frontier orbital show the redox tendency of the ferrocianide and ferricyanide as well as their association complexes with triple cetyl trimethylammonium brominide, which is qualitatively accord well with the experimental results.
self assembly membrane of cetyl trimethylammonium brominide surfactant; cyclic voltammetry; graphite composite electrode; caster oil coating
0 Introduction
Self-assembly[1-4]and self organization[5]are natural and spontaneous processes that are of increasing importance in chemistry, physics, biology and material science. The former commonly indicates one ordered molecular layer on a two-dimensional surface, the latter are more complex structure in one, two and three dimensions depending its environmental conditions, concentration, substrate and molecular property. These processes depend on the interactions between molecules and molecule/substrate[6]. A self-organized molecular pattern with control of structure and derived concomitant function could provide promising materials for the construction of nano devices depends on the properties of the molecule and substrate and their composition and concentration.
Amphiphilic molecules, such as surfactants, have two parts, a water-soluble, hydrophilic part and a water-insoluble, hydrophobic part. They can self-assemble into layers in an aqueous solution with its heat faces air. This “bilayer” structure represents the universal building blocks of all membranes found in biological systems. On the micrometer scale they can be described as smooth surfaces, and their self-organization can be understood in terms of a few parameters such as the enclosed volume, the preferred of spontaneous’ curvature and the bending rigidity. In general, these parameters depend on the colloidal self-organization on smaller scales. In this way, the flexible soft membranes provide a direct connection between the nano- and the micro-world. Self organization of surfactants at solid/liquid interfaces[7]or oil/water interfaces[8]and their kinetics[9-10]have been studied by STM[7], AFM[11], electrochemistry[12-16], coherent anti-Stokes Raman scattering microscopy[10],surface tension measurement[11], fluorescent techniques[17], stoped flow techniques[18]. Some theoretical considerations and simulations have been published such as electrostatic analogy[19], molecular dynamics simulations[20], spontaneous formation of lipid structures at oil/water/lipid interfaces[10],off-lattice Monte Carlo approach[21]modeling surfactant adsorption on hydrophobic surfaces and interactions[22-23]and quantum chemical calculation of charge distribution in the molecules[9,24]. The ordered membrane offers some capability in chemical catalysis[25], electrochemical catalysis, and used for biosensors and electron transferred in bio-molecules[11-15].
In this paper, the self assembly membrane (SAM) of CTAB from an aqueous solution at the castor oil coated graphite composite electrode surface was monitored by cyclic voltammetry with a electrochemical probe of ferrocyanide. The SAM modified electrode shows a special electrochemical catalytic behavior to ferrocyanide. The formation and the catalytic behavior were also studied by PM7 quantum chemical method which offers some qualitative evidences from thermodynamic and molecular electronic structural senses. Some interesting results were reported here.
1 Experimental and calculation method
Instrument and reagents
The electrochemical experiments were carried out on CHI620b electrochemical equipment (made in China). A conventional three-electrode cell was used with a piece of spiral platinum wire as the counter electrode, a homemade graphite composite electrode(GCE) in a glass tube as the working electrode(geometry area is about 0.12 cm2) and a KCl saturated calomel electrode(SCE) as the reference electrode. All potentials were reported here with respect to this reference electrode.
The 20.0 mM CTAB aqueous solutions were prepared with analytical pure CTAB (purchased from Beijing Chemical Co.). 1.0 M KCl was prepared with analytical pure KCl (purchased from Beijing Chemical Co.) as electrolyte solution through the all experiments. 2.00 mM K4Fe(CN)6aqueous solution was prepared with analytical pure K4Fe(CN)6.3H2O(purchasing from Shanghai Chemical Co.). All solutions prepared with ultrapurified water (Resistance≥18.2 MΩ/cm) from a Millipore synthesis A-10 water purification system (Billerica, MA). All the solutions were pre-deaerated with high-pure nitrogen for 20 min prior to the electrochemical experiments.
The preparation of working electrode
Graphite power (200#), epoxy resin and polyamide resin (purchased from Beijing Chemical Co.) in the weight ratio of 8∶2∶2 were mixed and made into a paste, pressed into a clean glass tube (inner diameter is about 4 mm) with a copper wire from the other end of the tube as electrode lead, and solidified in air for 72 h. The top end of thebar(The resistance about 40 Ω/cm) serves as working electrode called graphite composite electrode (GCE)[ 26]. The newly prepared GESE was polished with 200#~1 000#sandpapers and glassy paper sequentially, and the used electrode was polished again to renew the surface for reuse.
Experimental procedure for dynamic measurement
The newly polished GECE was coated a thin layer of castor oil with a glass tube by hand, slightly polished on a piece of glassy paper to keep the coated layer thin and uniform. The electrode were set into 0.50 M KCl aqueous solution including 0.50 mM K4Fe(CN)6and given concentration of CTAB, performed cyclic voltametry experiments at 80 mV/s scan rate every other 3~5 min until the CV curve obtained was identical to the prior one.
Models of molecules and molecular clusters
CTAB is a cationic surfactant with a long cetyl group as the hydrophobic chain tail and an ammonium as the cationic head. When a CTAB aqueous solution contacts with a organic substance such as the castor oil, the long tail of CTAB inserts into the organic phase and leaves its hydrophilic heat faces the water phase, in this manner, it forms a dense molecular membrane between the two phases, and called it as a self assembling membrane or self organization membrane, and transfers the hydrophobic property of the organic phase surface into a hydrophilic one. The nearest distance between the heats and tails depending on the molecular attraction forces of the tails and rejection forces of the heats. The simplest interaction model and smallest unit selected from the between molecules in the SAM can be described as CTAB, CTAB2and CTAB3in Fig.1. The suitable simplest unit is CTAB3.
Ferrocyanide(FeCN6) is a bianionic complex composed of a ferrous ion and six cyanide, its potassium salt is soluble inaqueous solution. When it contacts with SAM of CTAB, it will replaces the bromide from the SAM and forms an ionic association as described CTAB3-FeCN6K4and CTAB3-FeCN6K3Br in Fig.1.
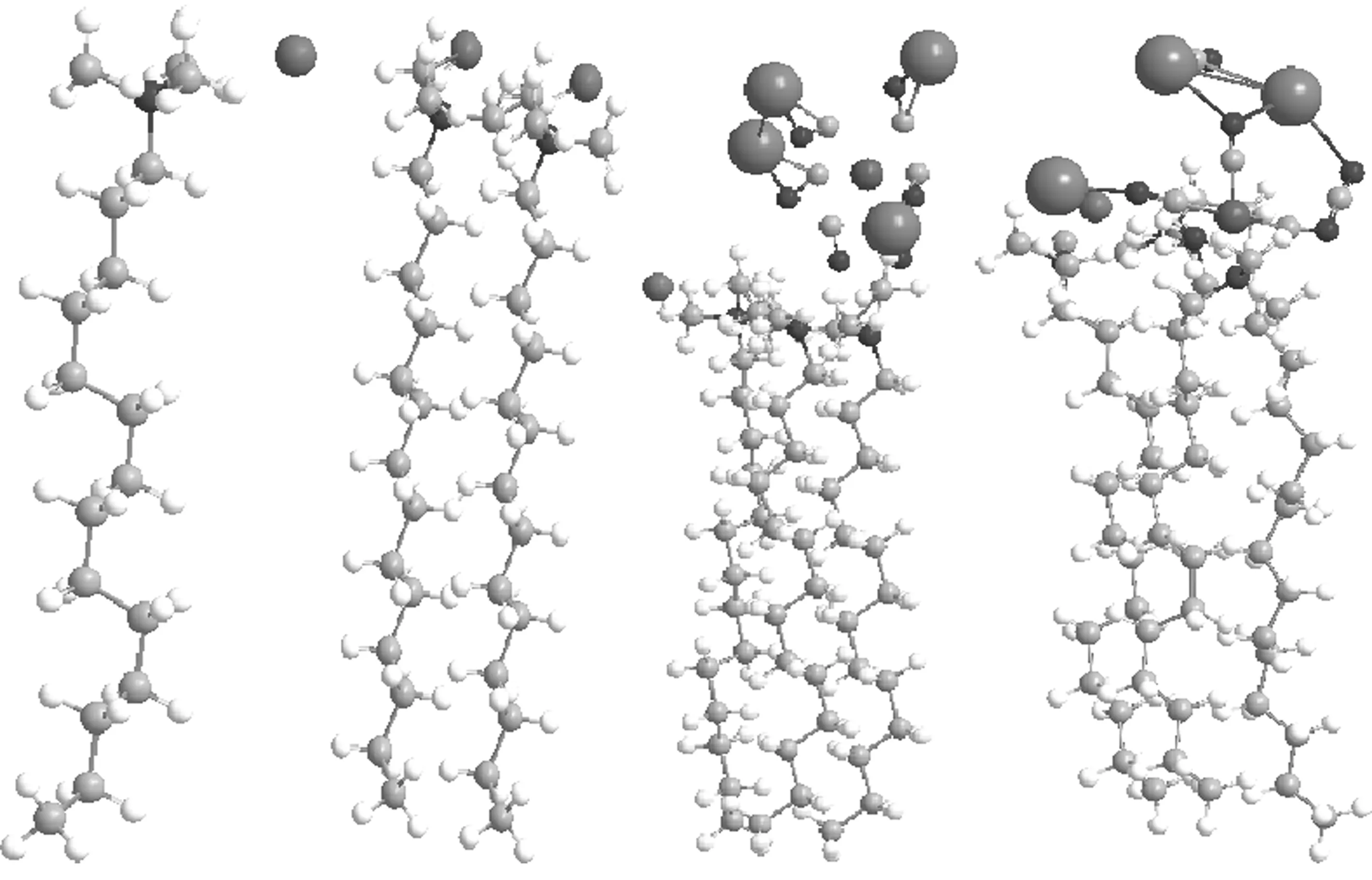
Fig.1 Models of molecule and molecular clusters
Calculation details
All quantum chemical calculation in this work was performed with MP6 semi-empirical quantum chemical method in MOPAC2012 software[27]on personal computer. All molecules and molecular clusters were designed by PCMOD 7.0 and further optimized with MOPAC2012 software[28]before the energy and molecular orbital calculations. The heat and entropy of formation and frontier orbitals were calculated by the semi-empirical calculations in vacuum condition. There were not considered about the influence of solvent effects in the calculation, partly due to the calculation complexity and the methyl silicone oil of organic phase mixed with the graphite powder and greatly reduced the influences of water molecules on the molecule and molecular clusters. All of the obtained data were analyzed and evaluated for the stability and the electron transfer properties of each molecular cluster with thermodynamic and quantum chemical concepts.
Heat of formation of molecule or molecular clusters can be easily obtained from the PM6 calculation in MOPAC2012, and used to evaluate the stability of molecule or molecular clusters. According to thermodynamic principle, the Gibbs free energy(ΔGf) of formation of a molecule or molecular cluster can be calculated with corresponding formation enthalpy(Hf) and formation entropy(Sf), and described as[29],
The Gibbs free energy of formation can be calculated from heat and entropy of formations for a molecule or molecular cluster and used for evaluation of the molecular stability and the spontaneity of the formation reaction.
The electrochemical catalytic property can be estimated by exchangeable electrons[26]in the molecule or molecular cluster, which can be obtained from the frontier orbitals from the Pm7 calculation in mopac2016. The frontier orbitals include the highest occupied molecular orbital(HOMO) and the lowest unoccupied molecular orbital(LUMO). The exchangeable electrons can be calculated by means of the frontier orbital gap(ΔE), chemical potential(), molecular hardness(η) and charge exchange(ΔN) from A to B molecules. All of these terms are defined as,
Here ΔNwas originally used to describe the electron transfer quantity from molecule A to molecule B, but in our case, it is used to describe the electron transfer from molecule A to another electrode or output electric circuit withμB,ηB=0, here molecule B serves as environment or CTAB modified on GCE surface.
2 Results and discussions
2.1 cyclic votammetry behavior of ferrocyanide
Cyclic voltammetry of ferrocyanide at bared GCE in 0.5 M KCl electrolyte solution including 0.5 mMk4FE(CN)6shows a paired redox peaks (courve 3 in Fig.1) located at 231 mV and 140 mV with currents of 13.16 and 13.03 μA, respectively. It is a typical reversible processes with a larger peak-peak separation of 99 mV. At the castor oil coasted SGE, It shows a smaller redox peaks (curve 1 in Fig.1) located at 252 mV(6.57 μA) and 149 mV(8.03 μA). All the redox peaks slightly shift to positive direction with peak-peak separation of 103 mV and small charging current due to the hydrophobic property of castor oil. At self assembly of hexadecyltrimethylammonium bromide modified castor oil coasted GCE, it shows a redox peaks with larger peak potential negatively shifts and a larger peak current increases as shown in Fig.1 curve 4. The oxidation peak locates at 96 mV with peak current of 36.31 μA, which is 1.76 times to bare SGE and 4.5 times to castor oil coasted SGE. The reduction peak locates at 34 mV with peak current of 81.28, which is 5.2 times to bare SGE and 9.1 times to castor oil coasted SGE. The peak current ratio is far away from 1 such as curve1 in Fig.2, about 2.2 time of the cathodic to the anodic. The small peak potential separation and the larger negative shifts of the peak potentials attribute to the transformation of the electrode surface property from hydrophobic one at the caster oil coasting to the hydrophilic at the self assembly of hexadecyltrimethylammonium bromide modification on the castor oil coasted GCE surface and the catalytic effect on the redox reaction of ferrocyanide with the SAM of CTAB.
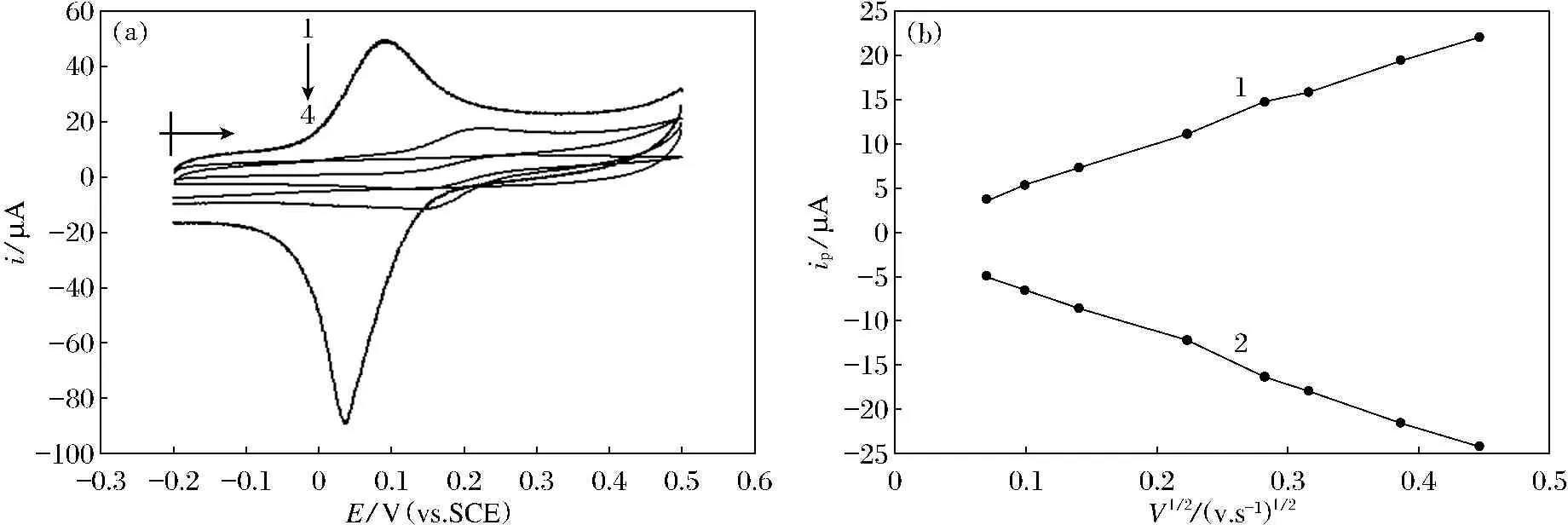
Fig.2TheCVsofferrocyanidein0.5MKClelectrolytesolutionincluding0.5mMK4FE(CN)6atScanrateof80mV/s(A)andrelationsofoxidationpeakcurrent(1)andreductionpeakcurrent(2)withscanrate(B).TheelectrodeinAare:theselfassemblyofCTABmodifiedcastoroilcoastedGCE(1);thebareSGE(2);thebaredSGEinelectrolytewithoutK4FE(CN)6c(3)andthecastoroilcoastedGCE(4).
The peak currents both anodic and cathodic were plot against the squared root of scan rate, and shows two straight lines as shown in Fig.1B with regression equations ofipa=1.634v1/2+1.318,(R2=0.997 8,SD=0.359 5) andipc=1.550v1/2+0.345 6(R2=0.998 8,SD=0.246 1), respectively. these results indicate that at SAM of HTAB modified GCE, ferrocyanide still shows the diffusion controlled processes.
Ferric-ferous couple are a reversible redox system in aqueous solution, in the experimental conditions, it becomes a quisi reversible one due to its environmental changes. The theoretical study shows the peak current (ip) can be described with a current functionψ[30],
Fis the Faladi chanstant, A, electrode surface,D0, diffusion constant of the oxidation form,f=F/RT,v, scan rate.The current function is a characterization parameter directly ralated to the electrochemical reaction rate constant,
The characterization values of functionΨ(E) has been listed in liturature, and can be regressed into a reciprocal lograthm function as,
Taking the values ofDO=DR=5.0×10-6cm2·s-1,f=38.95,π=3.142, scan rate=0.08 V·s-1in this work, then theψandk0can be calculated listed in Table 1.
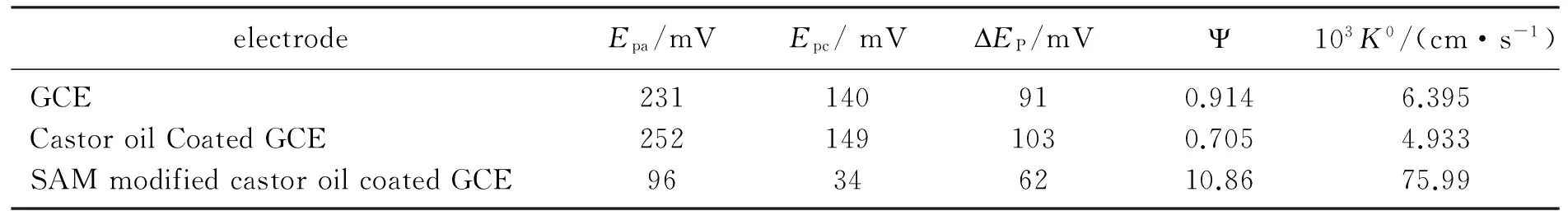
Tab.1 The peak potentials and corresponding rate constants of ferrocyanide at different electrodes
The estimated electrode reaction rate constant can be seen that castor oil coating slowdowns the reaction rate due to its hydrophobic property, while the modification of SAM of CTAB extremely increases the rate constant for about 11 times compared with the GCE due to its surface hydrophilic transformation. These results are very similar to the catalytic behavior of SAMs to the electrochemical redox couple[13-16, 31]. The electrochemical property of the surface of GCE is more near to that of the SAM coated GCE.
2.2 The Kinetics of self assembly membrane of CTAB Dynamics of self assembly of CTAB
GCE was polished with glassy paper and dried in air for one day, then coated one thin layer of caster oil carefully. Put the electrodes in 0.5 M KCl aqueous solution including 0.5 mM K4Fe(CN)6and 5.0 mM cetyltrimwthylammonium bromide, and performed cyclic voltammetry at 80 mV/s scan rate in potential range of -0.20~0.50 V every 3~5 minutes, the resulting CV curves and the curves of peak currents against self-assembly time as shown in Fig.1. At the first scan, ferrocyanide redox couple shows a anodic peak at 0.130 V, and a cathodic peak at 0.043 V. With the increase of scans or assemble time, the peak currents increase greatly, anodic current increases for about 88 times and cathodic current increases for about 12 times. The anodic peak potential moves to negative direction from 0.130 V to 0.091 V, the cathodic potential slightly moves to negative direction from 0.043 V to 0.038 V for self-assembly time of 4036 second. This means the SAM further self-organized into a two layers of membrane. Both of the two layer membrane has different electrochemical catalytic behaviors.
The kinetic curve monitored with the reduction peak current is more sensitive than that with the oxidation peak current, so the one monitored with reduction peak current curve (1) is used for the regression of the kinetic equation. The curve (1) id also a bifunction with the first part upto 1860s shown in Fig.3. The first part regressed into a componential association equation as,

(9)
From this equation the parant first order formation rate constant can be obtained as 0.002 377 s-1.

Fig.3TheCVsofferrocyanideatcastoroilcoastedGCEduringtheselfassembleingofCTAB(A),andoxidationpeakcurrent(1)andreductionpeakcurrentcurvesofpeakcurrentsaganistthewithselfassemblingtime(B)atscanrateof80mV/sin0.5MKCl, 0.5mMK4FE(CN)6aqueoussolution.Time(s)inA: 1, 0, 2, 154; 3, 332; 4, 658; 5, 1 395; 6, 2 378; 7, 3 105; 8, 4 036.
The second part is also regressed into a exponential association equation as,

(10)
This equation gives out the apparent first order formation rate constant for the second layer of SAM as 0.000 721 5 s-1. The first layer formation is more faster, about 3 times higher than that of the second layer, that means the second layer is more difficult to form with more complex structure than the first layer[32-33]. The oxidation peak current in curve (2) can also used to extracts process rate constant, and regress into a exponential association equation with the apparent first order formation rate constant of 0.002 359 s-1, but it is not sensitiver compared with redcution peak current. For the first layer formation, both of them have only about 2.7% of the deviation.
2.3 The effect of concentration of CTAB on the self assembling
For a given surfactant, the formation of SAM is a function of concentration of surfactant. The kinetic experiments were performed with different concentration of CTAB. The relationships between peak currents and self-assembly time are typical S form of curves with two steps corresponding to the monolayer formation and second layer formation in the concentration of 5 mMol/L of CTAB, below this concentration only one step appears, much more over this concentration may be more than two steps appears as shown in Fig.4.

Fig.4 The final CVs of ferrocyanide at self organization membrane of CTAB at different concentrations of CTAB in 0.5 M KCl including 0.5 mM K4[Fe(CN)6]. Scan rate: 80 mV/s,Concentration of CTAB(mM): 1, 2.0; 2,15; 3,5.0;4,0.50
In the first CV curve (t=0), the reduction peak locates at 0.040 V which is 82 mV shift to negative direction compared with that at bared electrode (0.122 V). The oxidation peak locates at 0.130 V, which is also 91 mV negatively shift. With the self organization progressing, the peak potential future negatively shifts, and peak current increases. At the self organization time of 3 105 s, anodic peak potential future shifted to 0.103 V from 0.130 V, the cathodic potential slightly shifts to 0.037 V from 0.40 V. The anodic current increases from 3.06A to 33.43A, which is about 11 times increase. The cathodic current increases from 0.927A to 80.5A, which is about 87 times increase. The larger increase of the cathodic current results in the great change in the ratio ofipa/ipc. The plot of peak currents in CV curves against self organization time are two-step sigmoid curve as shown in Fig.3B. The first part in the time range of 0~1 378 s is a exponential curve with a nonlinear regression equation with the first order rate constant ofk1=0.009 26 s-1.

(11)
The second part follows a Gaussian model with a regression equationas,
ip2=58.76+23.76exp[1.076×10-7(t-t0)2](2=0.385 7)
(12)
with the apperent rate constant of 0.000 103 3 s-1.
The relationship between peak current and self organization time indicates that there are two stages of the membrane formation with the accumulation of CTAB molecules at the castor oil/water interface, and can be described with two-cumulant expansion equation[ 2].
thereA,B,k1andk2are adjustable parameters,is the characterized time. The equation(3) is just the combination of the experimental regression equations ofip1andip2.
Concentration effects on self organization process
CTAB is a cationic surfactant with critical micelle concentration (CMC) of 0.96 mM[34]. At 5.0 mM concentration, a two-step sigmoid dynamic curve was obtained, lower or higher than this concentration may obtain a single step sigmoid curve. The experiments were performed at CTAB concentrations of 0.5 mM, 2.0 mM, and 15.0 mM repeatedly, another three dynamic curves with single sigmoid and three cyclic voltammograms at final of the self organization processes were obtained as shown in Fig.4. The anodic peak potential are almost the same at around 103 mV, compared with that at bared electrode(231 mV), it negatively shift for about 127 mV. While the cathodic peak potential slightly changed around 32.5 mV, which is negatively shift from the bared electrode for about 107.5 mV. According to the electron transfer rate constant[30]estimation method, the electrochemical rate constant was calculated as listed in table 2. The self organization membrane at 5.0 mM of CTAB shows the largest electrochemical rate constant,75.99×10-3cm·s-1, and the next one at 0.50 mM of CTAB (23.75×10-3cm·s-1), the smallest one is at 15.0 mM of CTAB(9.236×10-3cm·s-1). These result indicate the higher concentration of CTAB may forms a multilayer of self organization membrane, which is not favorable for electron transfer. One or two perfect self organization membrane may be the suitable membrane for electron transfer, and unperfect membrane is unfavorable one.

Tab.2 The corresponding parameters of self organization membranes at different concentrations
2.4 The theoretical calculations of self assembly membrane of CTAB and its interaction with ferrocyanide couples
Calculation of the formations of SAM of CTAB and association complexes with ferrocyanide.
CTAB is a cation surfactant with a long cetyl carbon chain tail. Itself likely to forms a double layers or micell with the long tail inside and the cation head outside in aqueous solution, but when its aqueous solution contacts with a organic phase such as caster oil, its long tail inserts into the organic phase and lets the cation heat outside the organic phase. In this way,the small organic phase will be well distributed in to the aqueous solution. In our graphite composite electrode surface, the long carbon chain inserts into the castor oil layer and leaves the cation head outside the castor oil surface. The orientation pattern of the CTAB molecules and the distance between the nearest cation heads depend on the head size, charge, repulsion force between heads and attraction force between the long carbon chain tails and concentration of CTAB in the solution, such as one CTAB, double CTAB, and triple CTAB. The simplest, smallest and stable unit of the pattern is triple CTAB. This can be seen from the pm7 thermodynamic calculation results listed in table 3. The formation heats of all molecule and molecular clusters and negative values indicating, all of them are stable in enthalpy sense, but after considered the entropy contribute, the Gibbs free energy is not always the case. The free energy of one CTAB is positive, that means the formation of CTAB is not a spontaneous process, while the double CTAB and triple CTAB are negative ones. The decrease of the formation free energy of the CTAB cluster with number of CTAB molecule from one to the double is 186 kJ/mol, further to the triple is 150 kJ/mol. The negative of the free energy is obtained, but the increase rate is decreased compared with the last step. So the triple CTAB is the suitable unit for all the pattern formation and also for the formation of association complex with ferrocyanide. Potassium Ferricyanide is not stable one compared with potassium ferocyanide. But after formation of an association complex with triple CTAB cluster, it becomes more stable than the association complex of triple CTAB with ferrocyanide due to the interactions of one more anion group with the cation heads in the triple CTAB and the smaller entropy contribution. Because of the stronger interaction of fericyanide with triple CTAB, the oxidation peak potential at the modified GCE has a larger potential shift of 135 mV, compared with 106 mV of the reduction peak potential shift of ferrocyanide with triple CTAB.
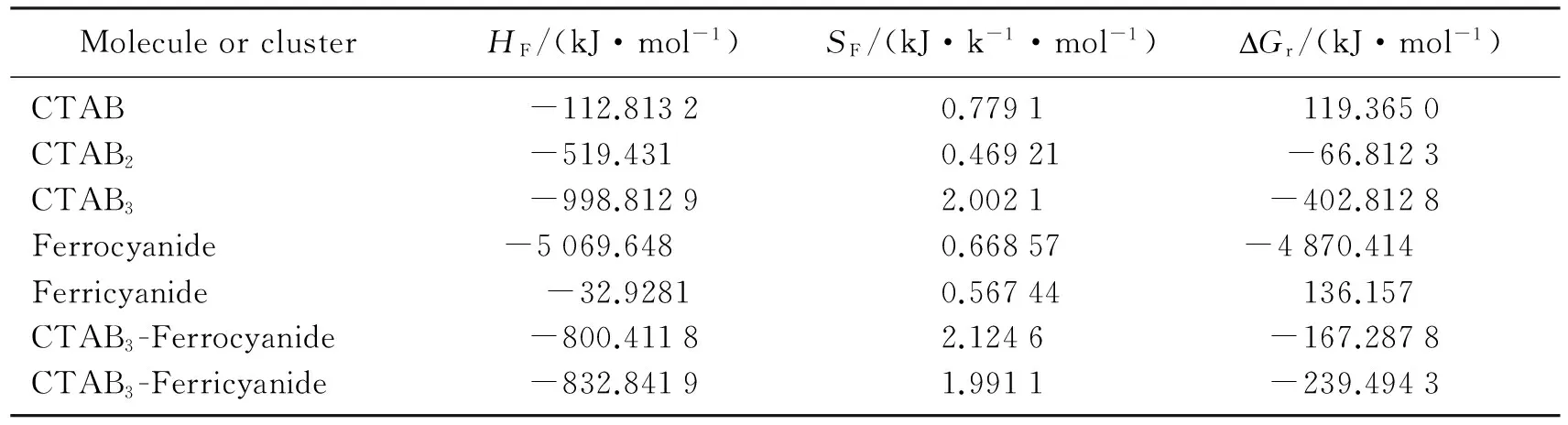
Tab.3 The corresponding parameters of self organization membranes at different concentrations
The pm7 calculation can also gives out the electronic structure property of the molecule and the molecular clusters as listed in table 4. With the increase of number of CTAB, the energy of the HOMO increases from -7.664 eV to -7.065 eV, while the energy of LUMO decreases from 0.057 eV to -0.992 eV, and the exchangable electron increases from 0.395 7 to 0.663 2, that means that the increase of the number of CTAB, the formed molecular clusters increase redox property and electron transfer property, and favorable the redox reaction occurs on this surface. TheHOMO of ferrocyanide and its association complex and the LUMO of ferricyanide and its association complexes with triple CTAB are mainly composed of the atom orbitals of the central iron atom, these are accord well with the common sense that ferrocyanide lose an electron from central iron atom to becomes ferricyanide. Ferrocyanide and ferricyanide have different energy of frontier orbitals, but they have the same exchangable electron, 0.589 9, so they have similar oxidation and reduction peak currents in cyclic voltamogram at graphite composite electrode. After the formation of assiciation complex with triple CTAB, the exchangable electron decrease to a different levels, 0.514 9 for ferrocyanide and 0.486 1 for fericyanide. Compared with the ferricyanide or ferrocyanide, all the association complexes are favorable for receiving nor for giving out electron, and the triple CTAB is also favorable for transfer electron from electrode to the ferricyanide group in the association complexes. This may be the reason for the current ratio of 1∶2.2 for the oxidation peak current and reduction peak current at the SAM modified GCE, the reduction peak current is greatly increase than the oxidation peak current.
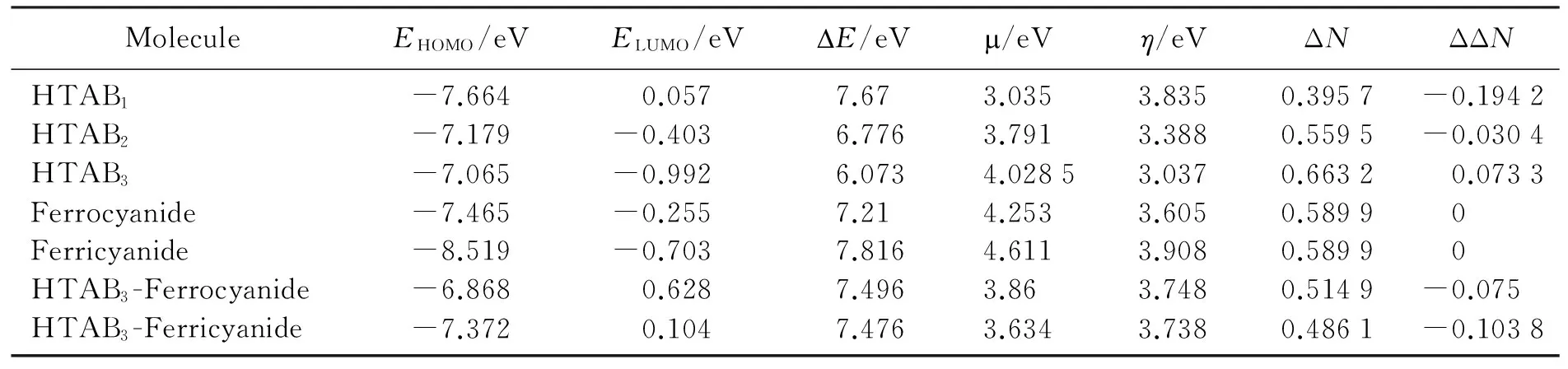
Tab.4 The corresponding parameters of self organization membranes at different concentration
3 Conclusions
As a summary of thispaper, the self organization membrane of CTAB cationic surfactant was formed at a castor oil coated GCE from aqueous solution, and monitored by cyclic votammetry. the self organiztion process follows a two-step exponential association function at 5 mmol/L of the CTAB concentration. The SOM shows a catalytic behavior to the redox of ferrocyanide by formation of the assiciation complexes with triple CTAB with increase of rate constants of the redox reaction of ferrocyanide. The concentration of CTAB shows a great influence on the rate constant. The quantum chemical calculation with pm7 in mopac 2016 offers the thermodynamic and electronic structure evidences to support the experimental result quanlitatively.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No.20875063), The project Foundation of Education Department of Liaoning Province (2004-c022) and The project Foundation of Technology Bureau of Shenyang (2007GX-32).
[ 1 ]LANGMUIR I. The mechanism of the surface phenomena of flotation[J]. Trans Faraday Soc, 1920,15(6):62-74.
[ 2 ]ISRAELACHVILI J N,MITCHELL D J,NINHAM B W. Theory of self-assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers[J]. J Chem Soc-Farad Trans Ⅱ, 1976,72(24):1525-1568.
[ 3 ]HOUGH D B,RENDALL H M. Adsorption of ionic surfactants in adsorption from solution at the solid/liquid interface[M]. London: Academic Press, 1983:247-319.
[ 4 ]ATKIN R,CRAIG V S J,WANLESS E J,et al. Mechanism of cationic surfactant adsorption at the solid-aqueous interface[J]. Adv Colloi Interf Sci, 2003,103(3):219-304.
[ 5 ]HOLMBERG K,JONSSON B,KRONBERG B,et al. Surfactants and polymers in aqueous solution[M]. 2nd ed. Chichester: John Wiley & Sons, 2003.
[ 6 ]LOVE C J,ESTROFF A L,KRIEBEL K J,et al. Self-assebbled monolayers of thiolates on metals as a form of nanotechnology[J]. Chem Rev, 2005,105(4):1103-1169.
[ 7 ]BHATTACHARYA A,MAHANTI S D. Self-assembly of neutral and ionic surfactants: An off-lattice Monte Carlo approach[J]. J CHEM PHYS, 1998,108(24):10281-10293.
[ 8 ]GIASSON S,KUHL L T,ISRAELACHVILI N J. Adsorption and interaction forces of Micellar and Microemulsion Solutions in Ultrathin Films[J]. Langmuir, 1998,14(4):891-898.
[ 9 ]HUIBERS D T P. Quantum-chemical calculations of the charge distribution in Ionic Surfactants[J]. Langmuir, 1999,15(22):7546-7550.
[10]RICHMOND G L. Molecular bonding and interactions at aqueo lus surfaces as probed by vibrational sum frequency spectroscopy[J]. Chem Rev, 2002,102(8):2693-2724.
[11]AHMAD S I,FRIBERG S. Catalysis in Micellar and Liquid-Crystalline Phases.1. System Water-Hexadecyltrimethylammonium Bromide-Hexanol[J]. J Am Chem Soc, 1972,94(15):5196-5199.
[12]KANG J W,ZHUO L,LU X Q,et al. Electrochemical behavior of dopamine at a quercetin-SAM-modified gold electrode and analytical application[J]. J Sol Stat Electrochem, 2005,9(2):114-120.
[13]LOVRIC M,KOMORSKY-LOVRIC E,COMPTON R G. Electrodes covered with random arrays of microdroplets: heterogeneous electron transfer coupled to catalytic reaction at the liquid/liquid interface in the prism film geometry approximation[J]. Electrochim Acta 2005,50(6):1377-1381.
[14]LIU B,Bard A J,MIRKIN M V,et al. Electron transfer at self-assembled monolayers measured by scanning electrochemical microscopy[J]. J Am Chem Soc. 2004,126(5):1485-1492.
[15]TATSUMI H,KATANO H. Voltammetric study of electron transfer across the 1,6-dichlorohexane|water interface with the bis(pentamethylcyclopentadienyl)iron(Ⅱ/Ⅲ) redox couple[J]. J Electroanal Chem, 2004,563(2):269-275.
[16]DAVIES T J,GAMER A C,DAVIES S G,et al. Cyclic voltammetry at microdroplet modified electrodes. A comparison of the reaction of vicinal dibromides with vitamin B-12s at the liquid/liquid interface with the corresponding homogeneous process: evidence for polar-solvent effects at the liquid/liquid interface[J]. J Electroanal Chem, 2004,570(2):171-185.
[17]KUSUMI A,IKE H,NAKADA C,et al. Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules[J]. Semin Immunol, 2005,17(1):3-21.
[18]GEORGANOPOULOU D G,CARUANA D J,STRUTWOLF J,et al. Electron transfer mediated by glucose oxidase at the liquid/liquid interface[J]. Farad Disc, 2000,116:109-118.
[19]GYEPI-GARBRAH S H,ILEROVA R. The first direct comparison of self-assembly and Langmuir-Blodgett deposition techniques: Two routes to highly organized[J].Phys Chem Chem Phys, 2002,4(14):3436-3442.
[20]LAWRENCE S N,THOMPSON M,DAVIS J,et al. Carbon-epoxy electrodes: unambiguous identification of authentic triple-phase (insulator/solution/electrode) processes[J]. Chem Comm, 2002,10:1028-1029.
[21]COLONNA B,ECHEGOYEN L. Templated SAMs for metal ion recognition[J].Chem Comm, 2001,12:1104-1105.
[22]GORDILLO J G,SCHIFFRIN J D. The electrochemistry of ubiquinone-10 in a phospholipid model membrane[J]. Farad Disc, 2000,116(1):89-107.
[23]FLOATE S L J,CORDEMANS E,COMPTON G R. A sonotrode for electroanalysis: the determination of copper in passivating media[J]. Analyst, 2002,127(8):1094-1099.
[24]GAUA J J,LANB H E,DUNNB B,et al. A MEMS based amperometric detector for E. Coli bacteria using self-assembled monolayers[J].Biosens Bioelectron, 2001,16(2):745-755.
[25]NAGATANI H,SAMEC Z,BREVET P,et al. Adsorption and aggregation of meso-Tetrakis(4-carboxyphenyl)porphyrinato Zinc(II) at the Polarized Water 1,2-Dichloroethane Interface[J]. J Phys Chem B, 2003,107(3):786-790.
[26]LAWRENCE S N,THOMPSON M,DAVIS J,et al. Carbon-epoxy electrodes: unambiguous identification of authentic triple-phase (insulator/solution/electrode) processes[J]. Chem Comm, 2002,10:1028-1029.
[27]STEWART J J P. Optimization of parameters for semiemprical methods V: modification of NDDO approximation and applications to 70 elements[J] J Mol model, 2007,46(3):1173-1213.
[28]STEWART J J P. Application of the pm6 method to modeling the solid state[J]. J Mol Model, 2008,14(6):499-535.
[29]ZHU Y,JIANG Q,SU G,et al. Electrochemical catalytic oxidation of p-aminophenol at p-phenol modified graphite paste electrode by cyclic voltammetry and PM6 semi-empirical molecular orbital theory[J]. J Shenyang norm Uni (natural Sci Ed), 2016,34(3):343-353.
[30]BARD A J,FAULKNER L R. Electrochemical methods fundations and applications[M]. 2d ed. New York: JOHNWILEY & SONS, INC, 2003:240-243.
[31]MOSER J,PUNCHIHEW S,INFELTA P P,et al. Surface complexation of colloidal semiconductors strongly enhances interfacial electron-transfer rates[J]. Langmuir, 1991,7(12):3012-3018.
[32]MCDERMOTT D C,KANELLEAS D,THOMAS R K,et al. Study of the adsorption from aqueous solution of mixtures of nonionic and cationic surfactants on crystalline quartz using the technique of neutron reflection[J]. Langmuir, 1993,9(9):2404-2407.
[33]PATIST A,KANICKY R J,SHUKLA K P,et al. Importance of Micellar Kinetics in relation to technological processes[J]. J Collo Interf Sci, 2002,245(1):1-15.
[34]KARABORNI S,ESSELIN K K,HILBERS P A J,et al. Simulating surfactant self-assembly[J]. J Phys Condens Matt, 1994,23A(6):A351-A356.
1673-5862(2017)04-0475-10
表面活性剂自组装膜上亚铁氰化钾电催化研究
朱永春1,2, 孟祥玉2, 匡雅文2, 辛士刚2, 张洪波2
(1. 沈阳师范大学 能源与环境催化研究所, 沈阳 110034; 2. 沈阳师范大学 化学化工学院, 沈阳 110034)
以亚铁氰化钾为探针,循环伏安和Pm7量子化学方法研究了十六烷基三甲基溴化铵在蓖麻油涂层石墨复合电极上形成的自组装膜,该自组装过程遵从两步指数解离方程,其表观一级动力学速率常数分别为0.002 377 s-1和0.000 721 5 s-1。该自组装膜表现出了对亚铁氰化钾氧化还原的电化学催化作用, 其相应的氧化还原峰电位负移,峰电流增加及峰-峰电位差减小,并给出了氧化还原速率常数的增加。十六烷基三甲基溴化铵在水溶液中的浓度对自组装过程和速率常数有很大影响。Pm7量子化学计算揭示了自组装膜和亚铁氰化钾和铁青化钾与三十六烷基三甲基溴化铵形成的缔合络合物的稳定性, 由前线轨道能量计算的可移动电子数定性地解释了铁青化钾和亚铁氰化钾及其与三十六烷基三甲基溴化铵缔合物的氧化还原趋势。
十六烷基三甲基溴化铵自组装膜; 循环伏安; Pm7量子化学方法; 石墨复合电极; 蓖麻油涂层
date: 2017-09-08.
Supported: Project supported by National Natural Science Fundation of China(20807563).
Biography: ZHU Yongchun(1955-), male, was born in Huaide city of Jilin province,professor of Shenyang Normal University,doctor.
O646DocumentcodeA
10.3969/ j.issn.1673-5862.2017.04.019

