Effect of Aluminum on FOX-7 Structure
Abstract: The effect of aluminum on FOX-7 structure was considered. Density functional approach at the level of unrestricted B3LYP/6-311++G(d,p) level was adopted. The model considered 1-4 aluminum atoms per FOX-7 molecule. The results show that the molecular geometries as well as many molecular orbital characteristics of FOX-7 molecule are subjected multiplicity of the composites, FOX-7 +nAl (n=1-4). In the case of n=1-2, some conformational changes occur. In the presence of three aluminum atoms, the quartet state of the composite is stable but in the doublet state, one of the N—O bonds ruptures. As for the n=4 case the singlet and triplet states are stable but in the quintet state one of the N—O bonds cleaves. As the number of aluminum atoms increases, the HOMO-LUMO energy gap decreases generally.
Keywords: FOX-7;1,1-diamino-2,2-dinitroethylene;DADNE; explosives; aluminum; DFT
Introduction
Quite recently FOX-7 has been becoming popular. It is structurally 1,1-diamino-2,2-dinitroethylene (DADE, DADNE)[1]and was synthesized in 1998 by members of the Swedish Defense Research Agency (FOI)[2-3]. Many researchers have investigated its explosive potential thoroughly[4-18]. As an alternative route, it was also synthesized by the nitration of 4,6-dihydroxy-2-methylpyrimidine and then hydrolysis[19].
In spite of the fact that FOX-7 is simple in molecular composition and structure, it exhibits abundant chemical reactivity including coordination reactions, nucleophilic substitutions, acetylate reactions, oxidation and reduction reactions, electrophilic addition reactions etc.[20-21]. It is a novel high-energy insensitive material possessing good thermal stability and low sensitivity. Moreover, it exhibits excellent application performance among the insensitive ammunitions and solid propellants.
Interestingly enough FOX-7 has the same C/H/N/O ratio as RDX or HMX possesses, although no structural resemblance exists between them. However, FOX-7 is much less sensitive than RDX (in terms of impact, friction and electrostatic discharge sensitivities)[22].
FOX-7 exhibits many polymorphic forms of which theα-form reversibly turns intoβ-form by heat treatment[23-24]. At higher temperature,β-polymorph undergoes an irreversible conversion toγ-phase which decomposes at 504K[23]and its decomposition has been extensively searched[25]. The effect of high pressure on the crystal structure of FOX-7 has been also studied[26].
Several FOX-7 based propellant formulations have been developed in order to obtain a minimum or reduced smoke producing propellant composites[27].
A plastic bound explosive based on FOX-7 and an energetic binder have been prepared[28]. Thermo chemical calculations show that PBXs based on FOX-7 and energetic binders could serve as a replacement of Comp-B even at rather low solid loadings.
Effects of epoxidation and nitration on ballistic properties of FOX-7 were investigated within the realm of density functional theory (DFT)[29]. Various ground state properties of FOX-7 were calculated based on B3LYP/aug-cc-pVDZ predictions[30].
Laser ignitibility of FOX-7 has been investigated in order to achieve the direct optical ignition of an insensitive explosive[31]. Note that such a process will add more safety features to insensitive munitions (IM) or explosive devices.
The last couple of decades have evidenced extensive usage of theoretical and computational methods in the field of energetic materials[29,32-39]. Nowadays, density functional methods have become popular.
Recently, some novel derivatives of FOX-7 and their properties as energetic materials have been reported[40-41]. Also some aluminized FOX-7 compositions were reported[42-43]. Also some molecular orbital calculations were reported on aluminized FOX-7[44-47]. In the present study, FOX-7 +nAl (n=1-4) have been investigated at the molecular level within the restriction of density functional theory (DFT).
1 Method of calculation
Geometry optimizations of all the presently considered structures leading to energy minima were initially achieved by using MM2 method followed by semi-empirical PM3 self-consistent fields molecular orbital (SCF MO) method[48-49]at the restricted level[50-51]. Subsequent optimizations were achieved at Hartree-Fock level using various basis sets. Then, the geometry optimizations were managed within the framework of density functional theory (DFT) using unrestricted B3LYP functional (UB3LYP)[52-53]at the level of 6-311++G(d,p). The exchange term of B3LYP consists of hybrid Hartree-Fock and local spin density (LSD) exchange functions with Becke’s gradient correlation to LSD exchange[53-54]. Note that the correlation term of B3LYP consists of the Vosko, Wilk, Nusair (VWN3) local correlation functional[55]and Lee, Yang, Parr (LYP) correlation correction functional[56]. Presently, the vibrational analyses have been also done at the same level of calculations which had been performed for the optimizations. The total electronic energies (E) are corrected for the zero point vibrational energy (ZPE) to yieldEcvalues. The normal mode analysis for each structure yields no imaginary frequencies for the 3N-6 vibrational degrees of freedom, whereNis the number of atoms in the system. This indicates that the structure of each molecule corresponds to at least a local minimum on the potential energy surface. All these calculations were done by using the Spartan 06 package program[57].
2 Results and discussion
FOX-7 is a push-pull type conjugated nitramine explosive. The geminal amino groups supply some electron population to level off the electron demand of the geminal nitro groups. Thereby, the whole system (FOX-7) becomes an insensitive explosive material. Figure 1 shows the optimized structures of FOX-7 as well as FOX-7 +nAl (n=1-4) composites at different multiplicity states within the constraints of UB3LYP/6-311++G(d,p) level of calculations. The aluminum atom has 1s22s22p63s23p1ground state electronic configuration. The presence of an unpaired electron in aluminum engenders various multiplicities as the number of it increases. The mass fraction of Al in the presently considered composites are 15.41%(n=1), 26.70%(n=2) , 35.34%(n=3), 42.17%(n=4).
As seen in Figures 1 and 2, whenn=1 , the composite remains sound with some small bond length changes (Figure 2). Whenn=2 the multiplicity states arise. In the singlet case, one of the N—O bond lengths elongates (0.152nm) accompanied by some conformational changes. Whereas in the triplet state, the bond lengths are not very different than the respective ones in FOX-7 structure, however some conformational variations occur. As for then=3 case, the doublet and quartet states may emerge. In the doublet case, one of the N—O bonds highly elongates (0.404nm) which is indicative of bond cleavage. Ye et al., investigated decomposition of FOX-7 for Al13 cluster and the results indicated that the cleavage of the N—O bond[44]. However, as presently indicated, even a much smaller number of aluminum atoms may cause decomposition of FOX-7. In the case ofn=4, the singlet and triplet states seem to be stable but the quintet state undergoes N—O bond cleave (see Figures 1 and 2). It is to be noted that in all the composites studied, except the triplet ofn=4 case, aluminum atoms reside somewhere around the nitro groups, but in the exceptional case mentioned above they prefer to be around the amino groups.
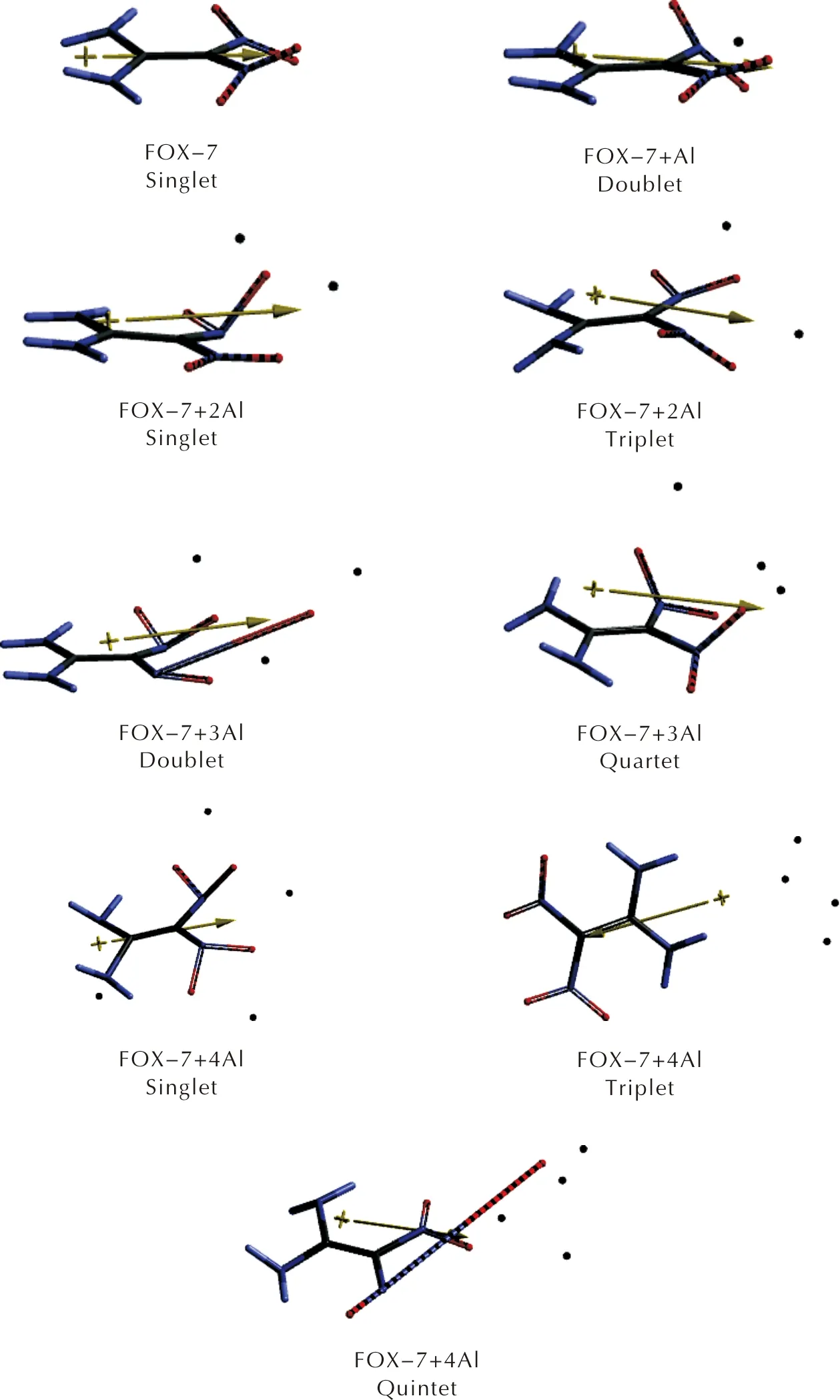
Fig.1 Optimized structures of FOX-7 and some of its aluminized composites(n=1-4)
Figures 3 and 4 respectively stand for Mulliken and natural electrostatic charges on the atoms of the composites considered. In each case, the aluminum atom(s) possesses some positive partial charge development which is indicative of transfer of some electron population to FOX-7 moiety. Note that the natural charges are similar to Mulliken charges but include more advanced mathematics so that the results behave better for large basis sets[57].
As seen in Figures 3 and 4, aluminum atoms acquire some positive partial charges in the composites. As the local assembly of three Al atoms form a doublet spin-state or four of them constitute a quintet state, a danger of decomposition of FOX-7 arises. Note that the Al percentage for this situation to be likely, is 35.34%(n=3) and 42.17%(n=4).
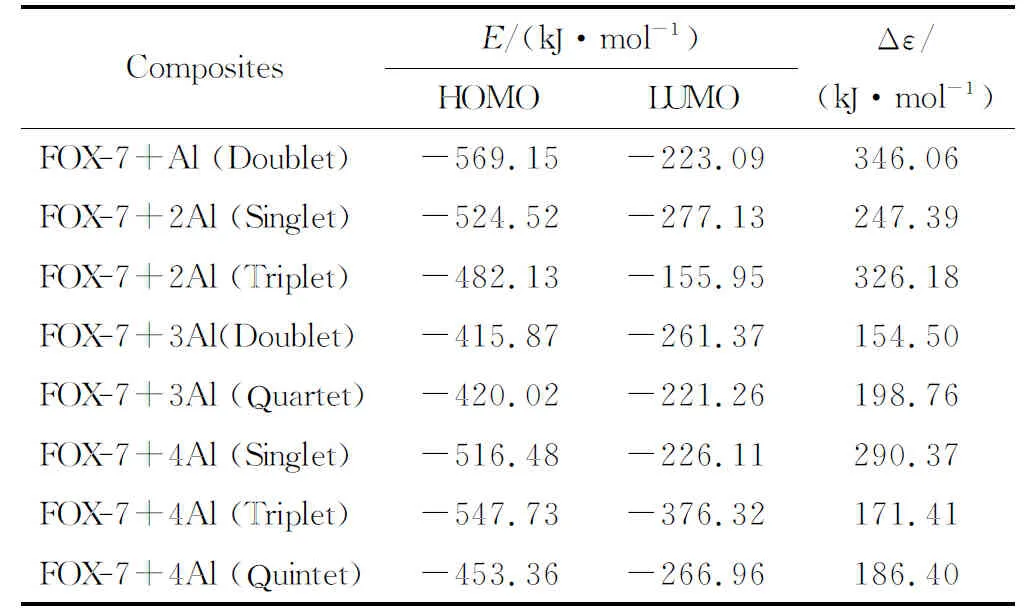
Table 1 displays some thermodynamic properties of the composites considered. Note that the multiplicities have some influence on these properties so that lower state for the same number of aluminum atoms is more favorable. WhereasCvvalue is higher as the multiplicity gets higher keeping the number of aluminum atoms the same.Table 1 also shows some energies of the composites whereE, ZPE andEcare the total electronic energy, zero point vibrational energy and corrected (for ZPE) total electronic energy, respectively.
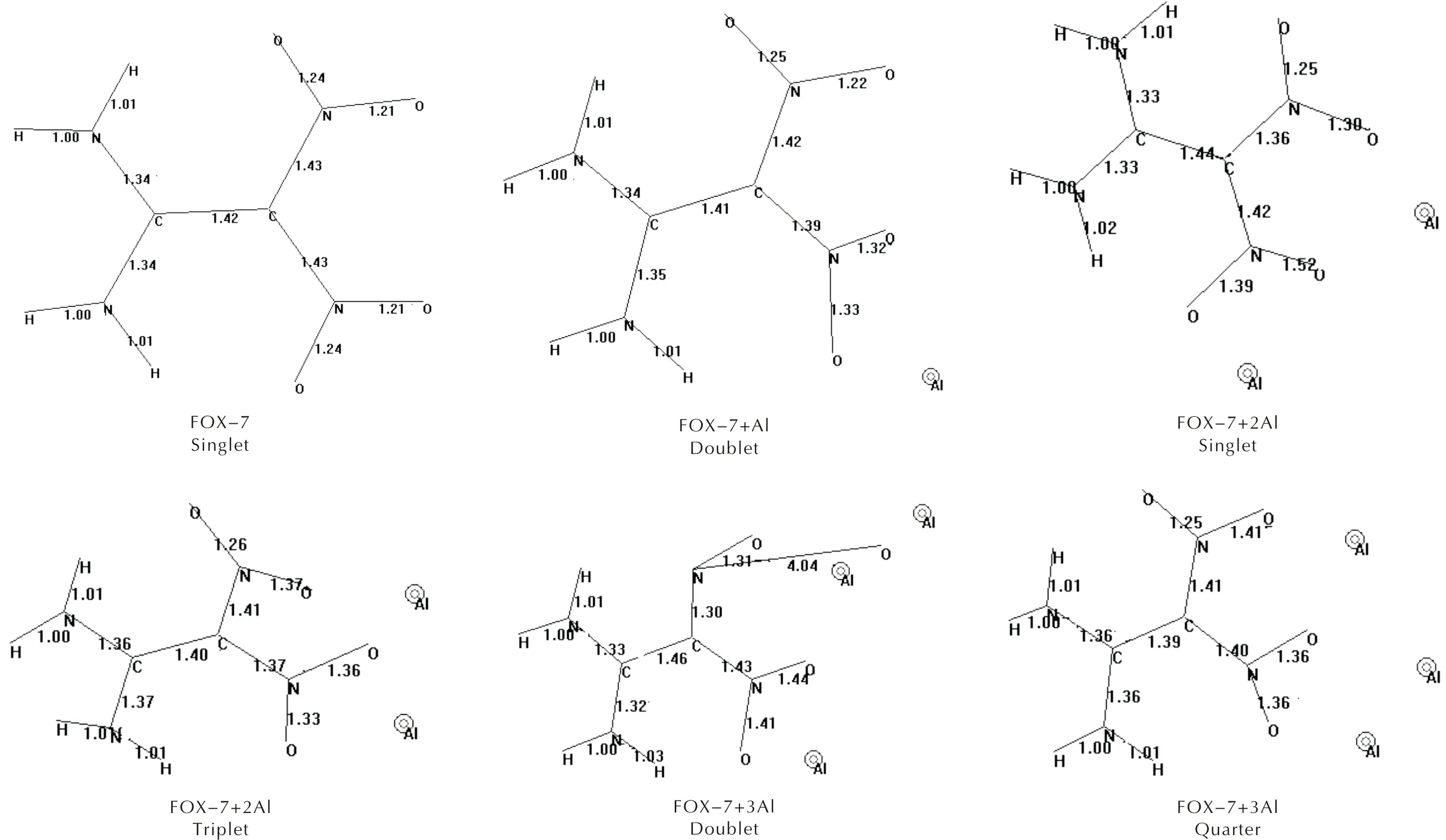

Fig.2 Bond lengths /distances(Angstörm) of FOX-7 and some of its aluminized composites(n=1-4)
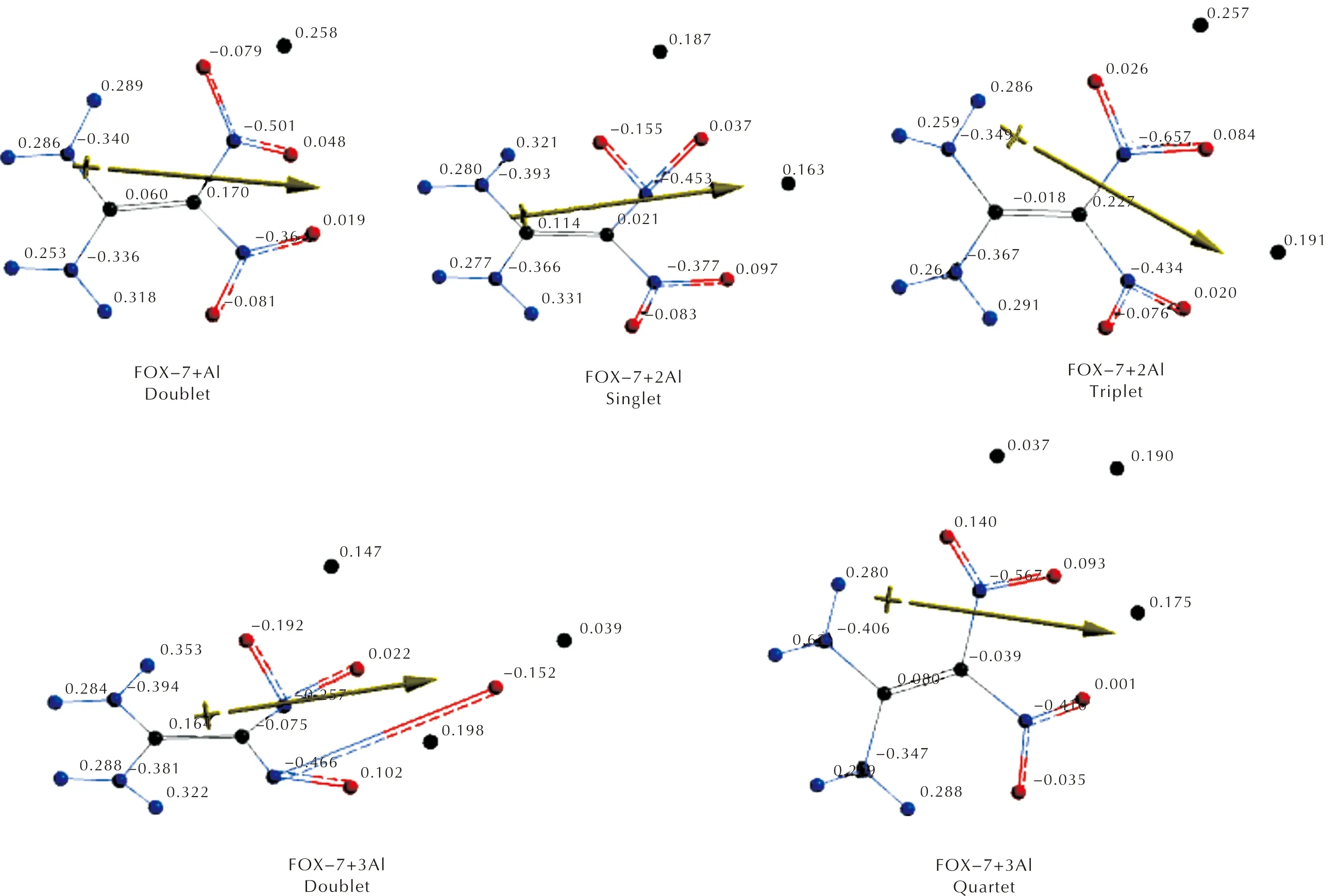
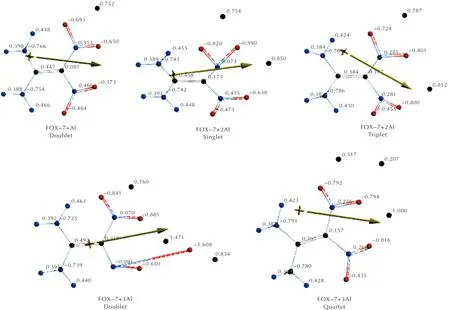
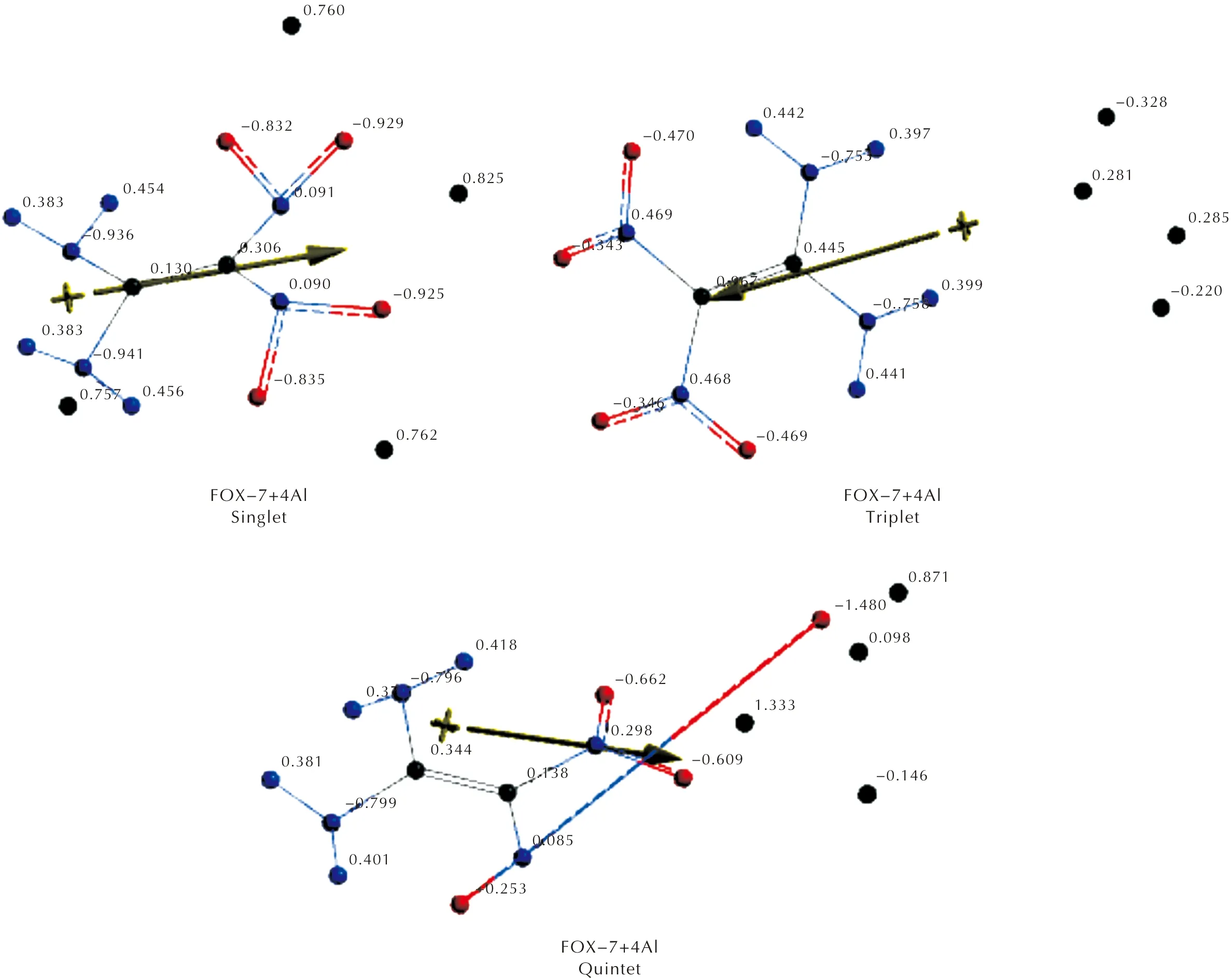
Fig.4 Electrostatic charges (natural) on the atoms of composites(n=1-4) considered
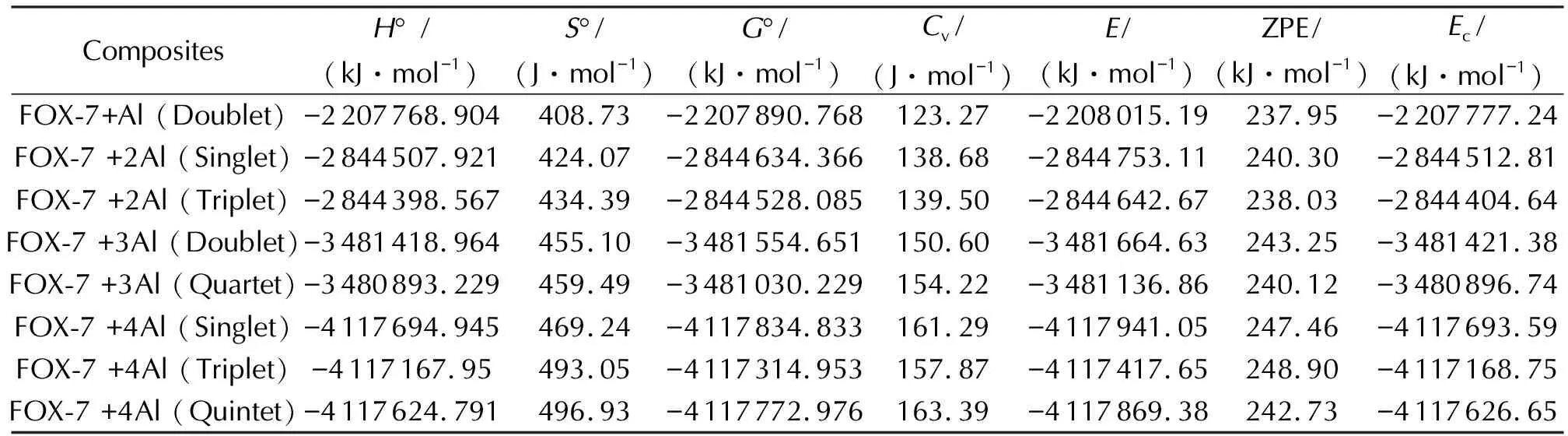
CompositesH° /(kJ·mol-1)S°/(J·mol-1)G°/(kJ·mol-1)Cv/(J·mol-1)E/(kJ·mol-1)ZPE/(kJ·mol-1)Ec/(kJ·mol-1)FOX-7+Al (Doublet)-2207768.904408.73-2207890.768123.27-2208015.19237.95-2207777.24FOX-7 +2Al (Singlet)-2844507.921424.07-2844634.366138.68-2844753.11240.30-2844512.81FOX-7 +2Al (Triplet)-2844398.567434.39-2844528.085139.50-2844642.67238.03-2844404.64FOX-7 +3Al (Doublet)-3481418.964455.10-3481554.651150.60-3481664.63243.25-3481421.38FOX-7 +3Al (Quartet)-3480893.229459.49-3481030.229154.22-3481136.86240.12-3480896.74FOX-7 +4Al (Singlet)-4117694.945469.24-4117834.833161.29-4117941.05247.46-4117693.59FOX-7 +4Al (Triplet)-4117167.95493.05-4117314.953157.87-4117417.65248.90-4117168.75FOX-7 +4Al (Quintet)-4117624.791496.93-4117772.976163.39-4117869.38242.73-4117626.65
As seen in Table 1,in the case ofn=2, the singlet composite is more stable than the triplet, whereas forn=3 case the doublet composite possesses a broken N—O bond and itsG° value is more favorable than the respective value of the quartet case. As for the case ofn=4, the overall stabilities of the composites follow the order of singlet>quintet>triplet. However, note that the quintet has a broken N—O bond. Ye et al., investigated the decomposition of FOX-7 for Al13 cluster and the results indicated the cleavage of N—O bond. However, as presently indicated , even a much smaller number of Al atoms may cause the decomposition of FOX-7.
Figure 5 shows the IR spectra of the composites (n=1-4). In the case of FOX-7+Al (doublet) the peaks at 3683 and 3455cm-1stand for asymmetric N—H stretchings whereas the peak at 3481cm-1is for symmetrical one. All the peaks below 1660cm-1are due to various bendings. FOX-7+2Al (singlet) possesses a peak at 1639cm-1which is the N—H bendings and C—N stretching. In general, it is to be noted that the N—H stretchings change their positions depending on the state of the composite. It is also true for the fingerprint region of the composites. The aluminum atoms affect bond strengths in FOX-7, thus IR characteristics of the systems highly depend on the states.
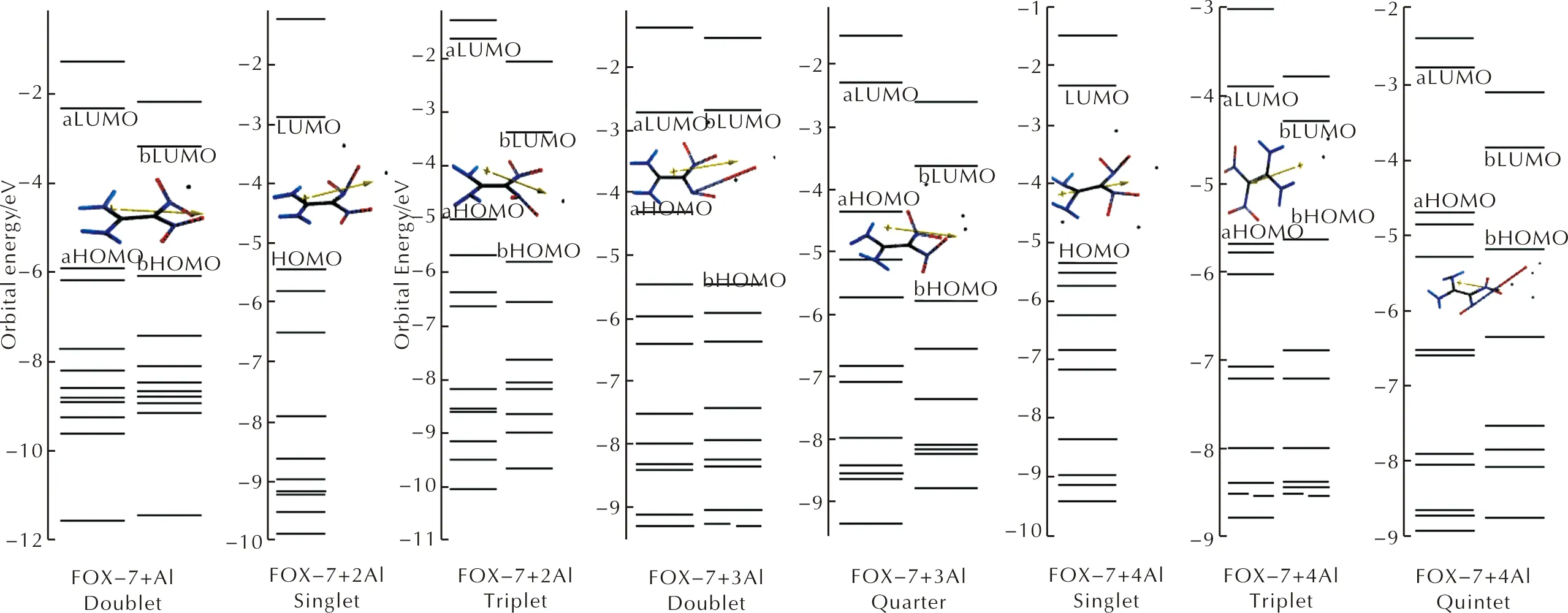
Figure 6 shows some of the molecular orbital energy levels of the composites. Note that for open-shell systems, unrestricted level of calculations yieldα-andβ-type orbitals (in the figure indicated as a-and b-). Generally, in these open-shell compositesα-HOMO energy level is higher thanβ-HOMO energy level, butβ-LUMO energy level is lower than the energy level ofα-LUMO.
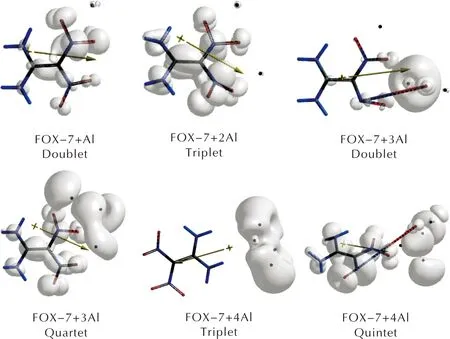
Table 2 shows the HOMO, LUMO energies and the interfrontier molecular orbital gap values (Δε) for the composites considered. The order of HOMO energy levels isn=1 (doublet) It has been reported that as the HOMO-LUMO energy gap (Δε) decreases, the explosive becomes more sensitive to impact[58-62]. Hence, the composite having three aluminum atoms is expected to be more sensitive than the composites having lesser number of aluminum atoms. Note that forn=3, the quartet state of the composite is stable but the doublet decomposes. Fig.5 IR spectra of the composites(n=1-4) considered Fig.6 Some of the molecular orbital energy levels of the composites(n=1-4) Table 2 The HOMO and LUMO energies and Δε values of the composites considered Figure 7 shows the spin densities on the composites having open-shell structures. Fig.7 Spin densities on the composites having stable open shell structures (n=1-4) As seen in Figure 7, FOX-7 moiety possesses some spin density depending on the state the composite system has. It is dictated by the number of Al atoms. However, FOX-7+3Al triplet has very little density on the remnant of FOX-7 but large on the oxygen atom originating from NO2group which oxidizes the nearby Al atoms effectively. The composite having triplet state ofn=4 case appears to have no spin density on the organic moiety indicating that FOX-7 structure in that composite remains as closed shell system. Based on density functional calculations at the level of unrestricted B3LYP/6-311++G(d,p) level, the effect of aluminum on FOX-7 structure has been investigated at the molecular level. The following main results are obtained. (1) Different multiplicity states arise depending on the number of aluminum atoms (n) per FOX-7 molecule which affects various properties of the composites. (2) Although the composites havingn=1-2 exhibit some conformational changes as compared to FOX-7 structure, the composites FOX-7 +3Al in the doublet state and FOX-7+4Al in the quintet state undergo decomposition by the cleavage of one of the N-O bonds. However, the quartet case ofn=3 and the singlet and triplet states ofn=4 are stable. (3) Depending on the multiplicity of the composition, Al atom(s) undergo some partial oxidation. (4) As the number of aluminum atoms increases the HOMO-LUMO energy gap of the composites generally decreases. Parallel to that the impact sensitivity of the composite is expected to increase.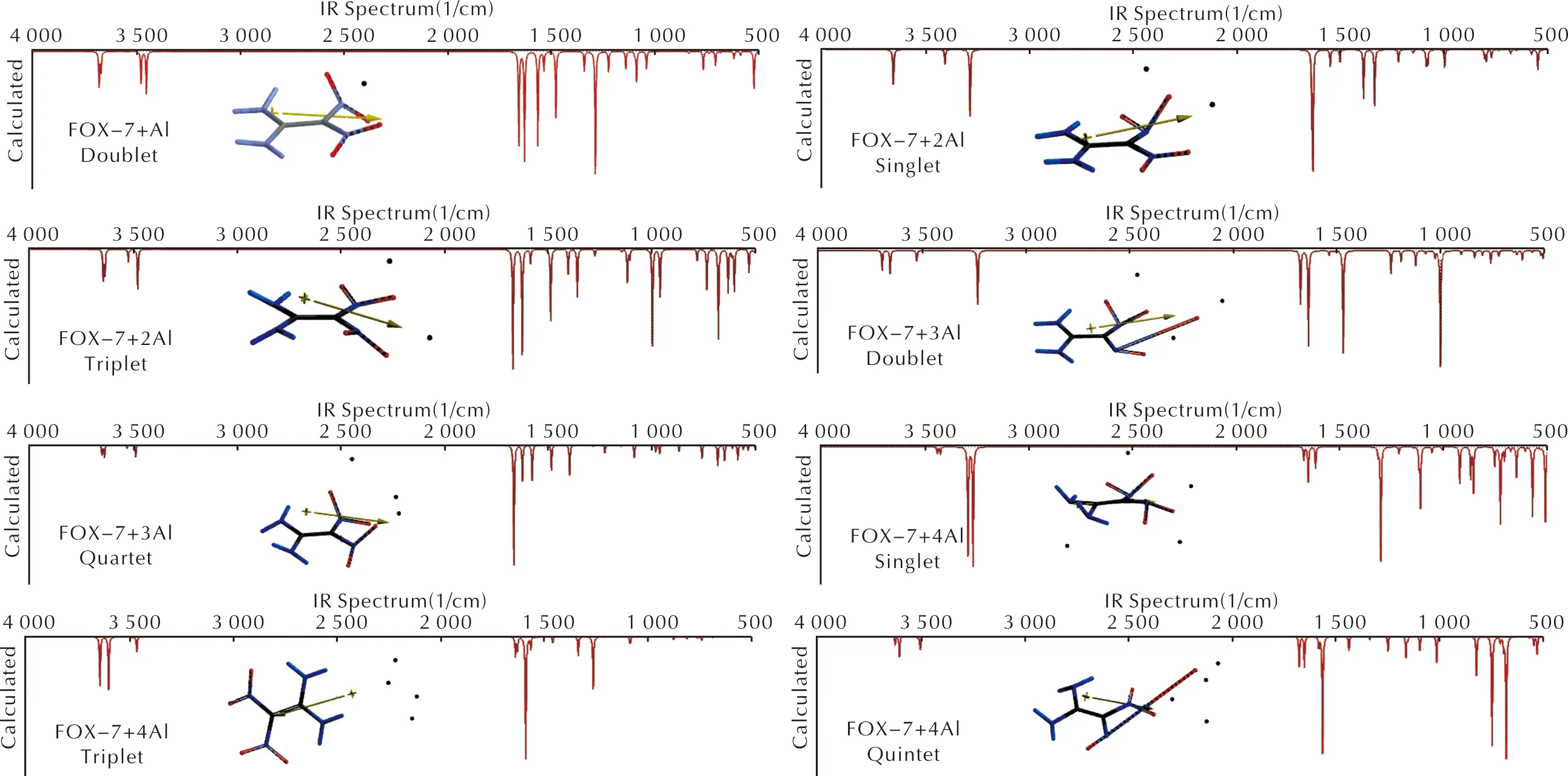
3 Conclusions

