Preparation of ZnO Nanomaterials and Their Photocatalytic Degradation of Organic Pollutants
XUHuanhuan,XULan(徐岚),2
1 College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, China2 National Engineering Laboratory for Modern Silk, Soochow University, Suzhou 215123, China
Abstract: Nano zinc oxide (ZnO) has excellent performance and low cost, but ZnO has a wide band gap and its electron-hole is difficult to achieve effective separation, which greatly limits its photocatalytic activity. This paper introduces the structural properties of ZnO and the mechanism of photocatalytic degradation of organic pollutants, and summarizes the preparation of ZnO nanomaterials and the application studies in improving its photocatalytic properties, in order to promote the research and development of ZnO nanomaterials.
Key words: zinc oxide (ZnO); nanomaterials; preparation method; degradation property; photocatalysis
Introduction
In recent years, the energy crisis and environmental problems have continuously troubled people’s modern life. Therefore, the research on sustainable resources and technologies has gradually increased. The use of photocatalytic technology to degrade and remove organic pollutants is an effective and environmentally friendly way for wastewater treatment, which makes many researches focus on the development of various high-efficiency photocatalysts. Nano-semiconductor photocatalysts, such as TiO2, ZnO, Fe2O3and ZnS, have been widely concerned and studied because of their high efficiency, low energy consumption and non-toxicity in sewage treatment. ZnO with a large energy band gap[1]is ann-type semiconductor material, which has high electron transport efficiency and exhibits high activity in photocatalytic reactions. It has excellent stability and biocompatibility. Moreover, it is easy to crystallize, which is an efficient and inexpensive inorganic functional material[2]. As a catalyst, ZnO has a large specific surface area, good stability, simple preparation[3], and high absorption efficiency in most of the solar spectrum, which has attracted more and more attention. However, ZnO also has some shortcomings that limit its photocatalytic application. For example, the wide band gap of ZnO causes it to mainly absorb ultraviolet (UV) light, but it can hardly use visible light. It is easier to recombine electrons (e-) and holes (h+) after being excited by light[4]. In this regard, this article introduces the research progress of nano ZnO in the preparation, strategies of promoting photocatalytic performance and applications, which has a reference significance for the research and innovation of nano ZnO materials in the field of photocatalytic degradation of organic pollutants.
1 Structure and Property of ZnO
ZnO is a type II-VI semiconductor compound, and belongs to a wide band gap semiconductor material. Under natural conditions, ZnO has a higher exciton binding energy and a deeper UV or boundary UV absorptive capacity[5]. Due to its excellent thermal stability, ZnO can achieve high-efficiency exciton emission even at higher temperature, which causes its potential to be further developed and applied to UV semiconductor optoelectronic devices[6]. ZnO is yellow at high temperature and becomes white after cooling. In addition, it has excellent electrical and thermal conductivities, high chemical stability and photoelectric stability[7].
The crystal structure of ZnO is various, including tetragonal rocksalt, cubic zincblende and hexagonal wurtzite, as shown in Fig. 1[8]. Under natural conditions, ZnO mostly exists as a hexagonal wurtzite structure, which has higher thermodynamic stability than the other two structures[1]. It can be seen from Fig.1 that each hexagonal wurtzite zinc oxide sub-lattice contains four Zn2+surrounded by four O2- [9], forming a tetrahedral crystal structure. This central asymmetric coordination structure leads to the formation of polar symmetry, which produces piezoelectric and spontaneous polarization effects in the zinc oxide wurtzite crystal, affecting the growth of ZnO and thus producing a variety of different morphological structures.

Fig. 1 ZnO crystal morphology: (a) tetragonal rocksalt;(b) cubic zincblende;(c) hexagonal wurtzite
In Fig. 1, the yellow balls represent Zn2+and the blue balls represent O2-. The main physical parameters of wurtzite ZnO are displayed in Table 1. Compared with some semiconductor metal oxides, ZnO can absorb a wider solar spectrum and more photons. However, the wide band gap energy and low photocorrosion resistance limit the absorption of ZnO in the visible light region, leading to the rapid recombination of photogenerated charges and lower photocatalytic efficiency, which hinders its practical application[10]. ZnO has good piezoelectric effect and photoelectric performance, and also has excellent chemical, mechanical and thermal stabilities. It has a wide application prospect, which can be used to manufacture solar cells, transparent electrodes, sensors, light-emitting devices, and catalysts to degrade various pollutants,etc.[11]Accordingly, ZnO nanomaterials can be an ideal material for developing green functional products.
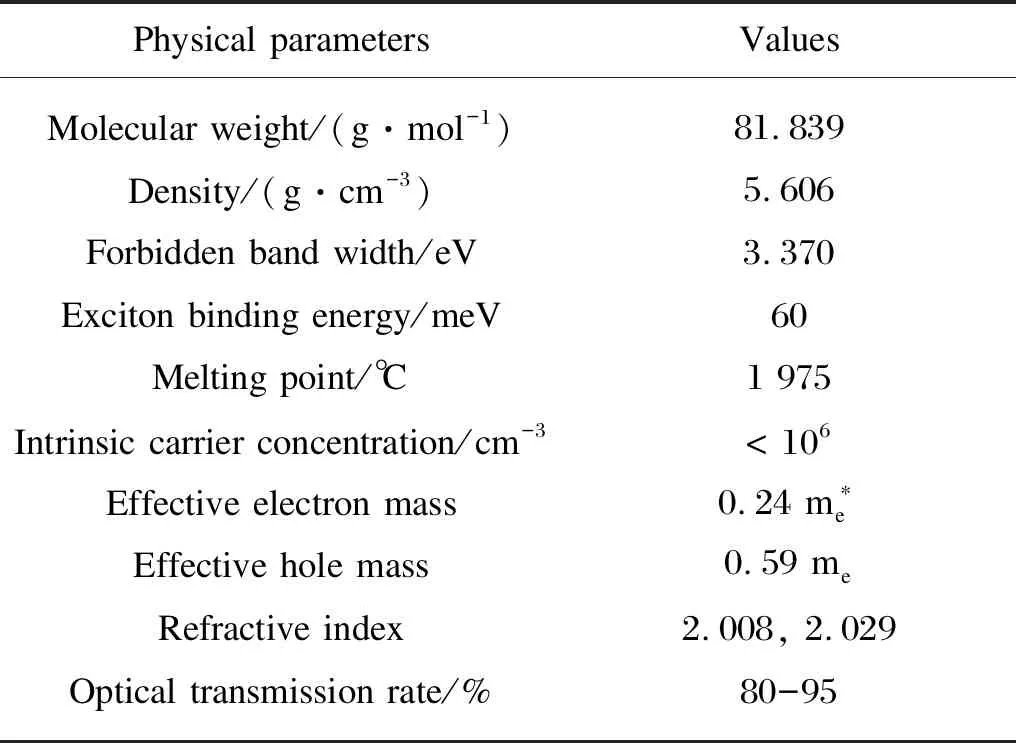
Table 1 Physical properties of wurtzite ZnO
2 Photocatalytic Degradation Mechanism of Organic Pollutants Using ZnO
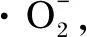


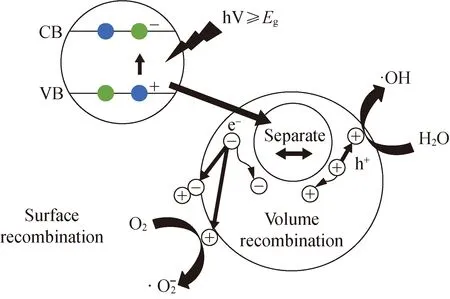
Fig. 2 Photocatalytic degradation mechanism
3 Preparation Methods of ZnO Nanomaterials
Common preparation methods of ZnO nanomaterials can be divided into two categories: the physical method and the chemical method. Compared with the two methods, the former is not easy to obtain products with high purity and good quality, while the latter has a relatively better synthetic effect. Among them, the chemical method can be divided into the gas phase method, the solid phase method and the liquid phase method. A brief introduction to these chemical methods is as follows.
3.1 Gas phase method
The gas phase method is to convert ZnO crystals into the gas phases under certain conditions, and then condense and crystallize to obtain ZnO nanoparticles (NPs). The gas phase methods for preparing ZnO nanomaterials include physical vapor deposition methods (such as the thermal evaporation method, the pulse laser deposition method and the magnetron sputtering) and the chemical vapor deposition method[13]. The ZnO nanomaterials manufactured by the gas phase method have the advantages of high purity, good crystallinity and adjustable size, but the disadvantage is that the reaction temperature is high. Lee and Kim[14]synthetized ZnO thin films by the metal organic chemical vapor deposition and the ultrasonic atomization at 275 ℃, and studied the influence of film thickness on their electrical and optical properties, as well as the microstructures.
3.2 Solid phase method
The solid phase method is a method that the zinc salt or ZnO is mixed in proportion and uniformly ground, and then is subjected to heat treatment to obtain NPs. The cost of this method is relatively low, and the operation process is relatively simple. The reaction process does not require any solvent, and has a good application prospect. The solid phase method mainly includes the ball milling method, the high-temperature solid phase reaction method, the thermal decomposition method, and the reduction reaction method. The preparation of nano ZnO by the solid phase method requires the high uniformity of mixed raw materials and the proper control of calcination temperature. Therefore, the raw materials should be fully mixed and the processing temperature of the precursor should be regulated reasonably in the solid phase reaction. The disadvantage is that the incomplete reaction will cause materials to liquefy, resulting in the waste of resources. Divya and Pradyumnan[15]fabricated ZnO crystals doped with different contents of erbium by the solid phase reaction method. The results showed that doping erbium could improve the photocatalytic performance of ZnO, and the catalyst exhibited an excellent stability in the degradation of methylene blue under UV.
3.3 Liquid phase method
The liquid phase method is to dissolve soluble metal salts into a solution, and uniformly precipitate or crystallize metal ions by using a precipitant or via evaporation, sublimation, hydrolysis and other operations, and then thermally decompose to form nanomaterials. The solution precipitation method, the hydrothermal method, the sol-gel method, the electrospinning method, the microemulsion method, and the ultrasonic synthesis method are common liquid phase methods used to prepare ZnO nanomaterials. The liquid phase preparation of nanomaterials has the characteristics of cheap, simple, low energy consumption, and good controllability of chemical composition and shape, and the prepared nanomaterials have excellent surface activity.
3.3.1Directprecipitationmethod
The principle of the direct precipitation method is to add a precipitant containing OH-, C2O42-, CO32-,etc. into a soluble salt solution, resulting in generating precipitates under certain conditions and obtaining Zn NPs after washing, drying, and thermal decomposition. The direct precipitation method is simple in operation, low in cost, and low in equipment and process requirements, but the particle size distribution is not concentrated, the dispersibility is poor, and the anions in the solution are difficult to be removed. Chenetal.[16]prepared the nano ZnO particles by a direct precipitation method. The precipitation was produced via the chemical reaction between zinc nitrate and ammonium carbonate in aqueous solutions. The precursor precipitates were calcinated and finally formed the nano-sized ZnO particles. The X-ray diffraction (XRD) and Brunner-Emmet-Teller (BET) measurement results exhibited that the synthesized ZnO nanoparticle sizes had the inconsistency, which might be due to the emergency of conglomeration. Therefore, improving experimental conditions of direct precipitation process should be considered.
3.3.2Homogeneousprecipitationmethod
In the homogeneous precipitation method, the crystal particles are slowly and uniformly precipitated from the solution, the precipitates are gradually and uniformly formed, and finally ZnO is obtained by thermal decomposition. This is because the precipitation precursors such as urea added during the reaction induce the precipitation agent to precipitate out slowly in the whole solution. The particles size distribution of the ZnO nanomaterials prepared by the homogeneous precipitation method is relatively narrow. It is difficult to cause agglomeration, and the dispersion is favourable. Compared with the direct precipitation method, the reactants can be uniformly mixed and the reaction rate is more controllable, but the removal of anions is still difficult. Aietal.[17]prepared flower-like and spherical-like ZnO nanoparticals through simple hydrothermal and homogeneous precipitation methods. The ZnO powders prepared by homogeneous precipitation exhibited a better photocatalytic effect than the flower-like ZnO prepared by hydrothermal synthesis under the same conditions in degrading Rhodamine B and phenol under UV.
3.3.3Microemulsionmethod
In the microemulsion method, two immiscible solvents form the emulsion under the action of the surfactant, and solid phases are precipitated from the emulsion and interacts. After nucleation, coalescence, agglomeration, and heat treatment, NPs are obtained. The nanomaterials prepared by the microemulsion method have the advantages of the small particle size, concentrated distribution, controllable size, good uniformity, simple equipment and wide application. Nano ZnO prepared by the microemulsion method also has excellent optical, electrical, and magnetic properties, but the presence of surfactants in the microemulsion will reduce the purity of the NPs. Shahietal.[18]used the microemulsion method to prepare vanadium doped ZnO NPs which was used to degrade methylene blue. The results identified that the doped vanadium had obviously improved the degradation effect of the ZnO NPs.
3.3.4Sol-gelmethod
The sol-gel method is to hydrolyze and condense the uniformly mixed metal alkoxide Zn(OR)2in an organic medium, so that the solution is gelled by the sol gelation process, and then dried and calcined into nano powders. The sol-gel method has the advantages of short time and low reaction temperature. Different materials can be prepared under controlled conditions, but the price is relatively expensive, so it is not suitable for expanding production. Balchaetal.[19]manufactured ZnO NPs by the precipitation and the sol-gel method. The XRD pattern displayed the structure of hexagonal wurtzite. The results identified that the photocatalytic effect of ZnO nanostructures prepared by the sol-gel method was superior to the precipitation method. Yusoffetal.[20]prepared ZnO NPs by the sol-gel method and used it to photodegrade phenol under sunlight. It was found that under acidic conditions, ZnO could photodegrade phenol, and the removal rate of phenol reached 100% after 2 h reaction.
3.3.5Hydrothermalmethod
The hydrothermal method is to synthesize ZnO NPs by heating the precursor solution of soluble zinc salt and lye to a certain temperature in a closed autoclave. The hydrothermal reaction method for preparing ZnO nanomaterials is simple and controllable, and can obtain the particles with good dispersion and crystallization. However, the hydrothermal method has higher requirements for experimental equipment. Bazazietal.[21]prepared ZnO NPs with various shapes through the hydrothermal method and the ball milling-hydrothermal method. The NPs with the quasi-prismatic structure manufactured by the ball milling-hydrothermal method showed better photocatalytic effect compared with other ZnO nanostructures. Saleh[22]employed the hydrothermal method to prepare ZnO nanospheres which were used to degrade methyl orange under UV radiation, displaying excellent photocatalytic degradation activities.
3.3.6Electrospinningdevice

Fig. 3 Electrospinning device
The electrospinning device is mainly made of a high-voltage power supply, an injection device and a receiving device, as shown in Fig. 3[23]. Under the action of electric field force, the spinning solution sprayed by the injection device overcomes the surface tension to form a Taylor cone, and forms uniform nanofibers attached on the receiving device through diffusion drawing. The electrospun nanofibers have large specific surface area, nanoporous structure, and good uniformity, which are easy to recycle and reuse and are more environmentally friendly. Shahetal.[24]prepared polyacrylonitrile (PAN) nanofibers loaded with ZnO nanorods (NRs) by the electrospinning method. ZnO loaded evenly on the nanofiber membrane, which was propitious to the recovery and recycle of powder catalysts. A series of characterizations and testings showed that ZnO NRs loaded on the nanofiber membrane had excellent orientation, and PAN/ZnO composite nanofiber membranes (CNFMs) had a larger specific surface area and better optical properties. The CNFMs exhibited the excellent degradation effects of cationic malachite green dye and anionic methyl orange dye in the visible light and recyclability. Pantetal.[25]utilized the electrospinning to mix fly ash particles with ZnO nanofibers. The electrospun nanofibers provided a large loaded area for fly ash, and the composites had outstanding adsorption and photocatalytic performance, which had a deep influence on the wastewater treatment.
4 Modification Method for Improving Photocatalytic Efficiency of ZnO
4.1 Element doping
Element doping can change the coordination environment of zinc in the lattice, and affect the morphology and crystal structure of ZnO, thereby influencing the electronic energy band structure of ZnO[4], narrowing its forbidden band width and generating more electrons-holes, which causes the red shift of the band gap of ZnO, reduces the absorption edge to a lower energy, and greatly improves the photocatalytic activity in the visible light region[26]. Element doping of ZnO can be divided into doping metal elements (such as Al[27], Ag[28], Fe[29], and Cu[30]) and doping nonmetal elements such as C[31], S[32], and N[33]. Figure 4 displayed the energy band structure of pure ZnO, metal-doped ZnO, and nonmetal-doped ZnO, respectively[4]. The element doped ZnO forms different impurity energy levels within the forbidden band. The impurity energy levels of metal-doped ZnO are close to the CB, and the impurity energy levels of nonmetal doped ZnO are close to the VB. This band structure can improve the photoelectric properties of ZnO materials, and capture photogenerated charges, which greatly enhances the photocatalytic performance of ZnO during the photocatalytic reaction.
Vallejoetal.[34]prepared the Ag-doped ZnO powders by the sol-gel method, and manufactured the film by employing the blade technique. Atomic force microscopy analysis exhibited that the film became smoother and the grain size decreased after being doped Ag. In addition, the optical characterization verified that the doped Ag had a positive impact on the optical performance of ZnO in the visible range, making the band gap of the film narrower. Moreover, the photocatalytic degradation results showed that compared with the ZnO film, the Ag-doped ZnO film had the better degradation efficiency for methylene blue under visible light. Yangetal.[35]synthesized layered flower-like ZnO nanostructures and S-doped porous flower-like layered ZnO nanostructures by the one-step hydrothermal method. It was proved that S and ZnO nanostructures had been completely mixed. Rietveld analysis displayed that the molar ratio of Zn to S obviously influenced the morphology and structure of ZnO. In addition, the photoluminescence (PL) spectrum showed that appropriate S doping improved the optical properties of ZnO nanostructures.
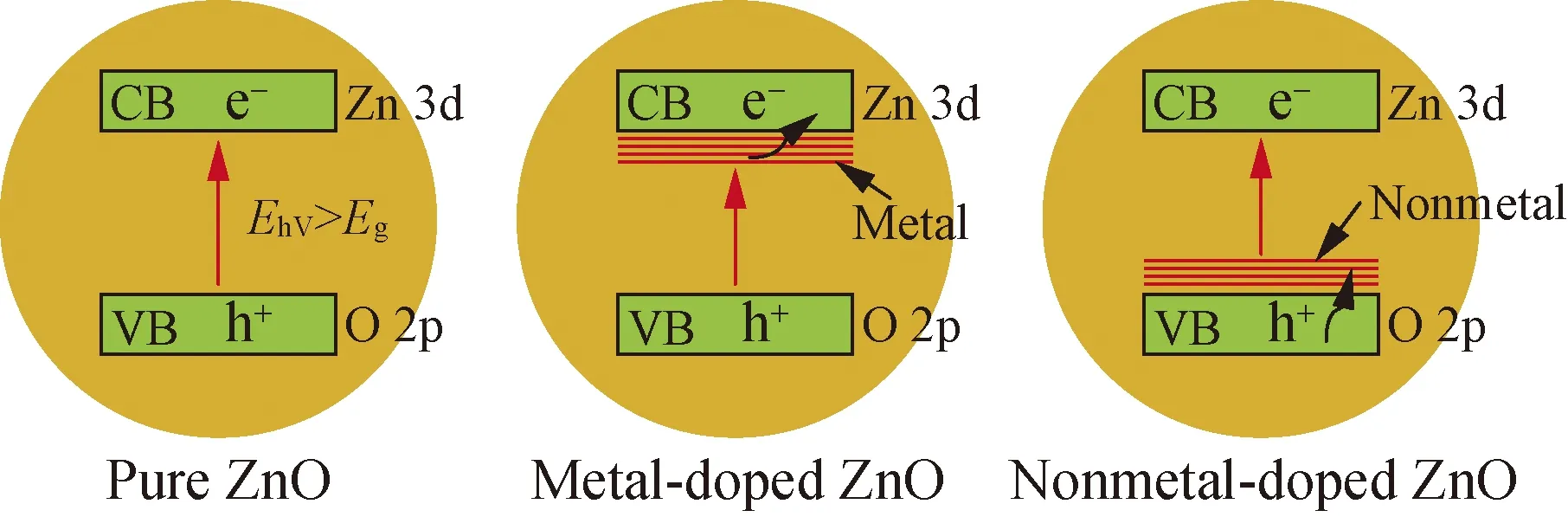
Fig. 4 Energy band structure of pure ZnO, metal-doped ZnO, and nonmetal-doped ZnO
4.2 Constructing heterojunctions
Two or more semiconductor materials with different forbidden band widths are combined to form a composite structure through physical or chemical methods, such as the hydrothermal method[37], the sol-gel method[47], and the electrospinning method[46], so that components with different structures and properties can synergistically improve the properties of ZnO nanomaterials. Heterogeneous recombination can expand the spectral response range of ZnO, and effectively promote the separation of photogenerated holes and electrons. The separation can also enhance the photoelectric properties and catalytic effects of ZnO, making the composite materials more widely used. The heterogeneous ZnO composite materials can be roughly divided into three types: the noble metal deposition, combining other metal semiconductors and coupling carbon materials.
4.2.1Noblemetaldeposition
In the study of noble metal deposition of ZnO, noble metal particles (Ag, Au, Pt, Pd,etc.) are generally loaded on the surface of ZnO nanomaterials to form various heterostructure composites. Noble metals can expand the absorption of visible light by ZnO nanomaterials, and enhance the light absorption range of ZnO via the special plasmon resonance effect on the surface, thereby significantly improving the utilization of ZnO to sunlight[36]. Leetal.[37]synthesized a sea urchin-like ZnO nanostructure by the hydrothermal method, and improved its morphology by regulating hydrothermal temperature. Besides, ZnO nanomaterials loaded with plasma Au NPs had a wider absorption range of the visible light region, and the composite materials had excellent photocatalytic degradation property for methylene blue. Liuetal.[38]produced a series of flower-like Ag/ZnO composites with different contents of Ag NPs by the microwave-assisted one-pot method. Due to the special structure and the incorporation of Ag NPs, the light absorption performance and electron separation efficiency of the Ag/ZnO composites were greatly enhanced. Therefore, the deposition of Ag on the surface of ZnO can significantly increase the degradation efficiency of dyes and nitrogen oxides (NO) under the simulated sunlight.
4.2.2Combiningothermetalsemiconductors
The combination of ZnO and other metal semiconductors generally includes ZnO and broad band metal semiconductors as well as ZnO and narrow band metal semiconductors, such as CdS[39], SnO2[40], TiO2[41], BiVO4[42], and Fe3O4[43]. In the composites of ZnO and other semiconductors, the energy bands of the two semiconductors are staggered to form a heterojunction structure. In the photocatalysis process, because different semiconductors have CB structures with different energy levels, the photoexcited electrons can quickly move from one semiconductor to the adjacent semiconductors, accelerating the separation of electron-hole pairs and improving the photocatalytic performance[44]. The photo-generated charges exist in different semiconductor energy bands, and the effective separation of carriers can improve the photocatalytic activity. Shirzadi and Nezamzadeh-ejhieh[45]loaded ZnO-CuO onto clinoptilolite NPs for the degradation of mefenamic acid aqueous solution. The results verified the hybrid ZnO-CuO system loaded on clinoptilolite nanoparticles(CNPs) had a good degradation activity. It was found that the ternary heterostructure made up of ternary compound semiconductors could promote the separation and transfer of photogenerated electrons and holes in the materials, which had aroused great concerns. Sunetal.[46]successfully manufactured the ternary CdS/ZnS/ZnO (CZZ) heterostructure nanofiber composite through a series of processes. Because of the improvement of separation and transfer effects of photogenerated carriers, the hydrogen evolution rate of the fabricated CZZ ternary heterostructure under optimized conditions was higher than that of the original ZnO and ZnS/ZnO binary heterostructure prepared under the same conditions.
4.2.3Couplingcarbonmaterials
The carbon material has a remarkable porous structure, large specific surface area, high electrical, thermal and chemical stabilities, low price, non-toxic and harmless. The compounding of ZnO and carbon materials mainly includes compounding with graphene, carbon nanotubes(CNTs) and carbon quantum dots(CQDs). Due to the better carrier electron transport in them, these composites can promote the separation of electrons and holes and enhance the photocatalytic effect.
Graphene has wonderful adsorption performance and high reactivity, making the photocatalytic reaction proceed smoothly. Wangetal.[47]constructed reduced graphene (RGO)/ZnO core-shell nanostructures by the sol-gel method and heat treatment. The degradation results of methylene blue solution showed that the doping of RGO nanosheets significantly improved the absorption of visible light of ZnO nanostructures, and the enhancement degree was related to the degree of adding RGO. Wangetal.[48]synthesized porous graphene/ZnO nanocomposites through fine-tuning of local combustion. Methyl orange could be degraded completely within 150 min by using porous graphene/ZnO nanocomposites. It was proved that the prepared porous graphene/ZnO nanocomposites exhibited better photocatalysis effect under sunlight compared with pure ZnO NPs.
CNT is a kind of carbon allotrope with good conductivity and unique hollow structure. The modification of photocatalyst by multi-walled CNT is usually considered as a reasonable synergistic effect, including enhancing light absorption, optimizing structure and improving charge transfer. Tieetal.[49]employed the one-step hydrothermal method to prepare ZnO/CNT heterostructure composites with adjustable photocatalytic efficiency through microstructure regulation. ZnO/CNT heterostructures displayed significant photocatalytic performance for degradation of Rhodamine B, which was attributed to adequate interfacial bonding between the ZnO nanostructure and the CNT, thereby obtaining more visible light absorption and more effective electron-hole pair separation. Oliveiraetal.[50]prepared vertically aligned CNTs with a height of 2.3 μm on Si/SiO2substrates by thermal chemical vapor deposition (TCVD), and applied atomic layer deposition (ALD) to coat ZnO on these CNTs. After 100 ALD cycles on these CNTs, the composites had the best degradation effect on Rhodamine B, which was due to the cooperation of CNT and ZnO.
CQDs are mainly composed of carbon with particle sizes of less than 10 nm, which are new fluorescent carbon nanomaterials[51]with a graphite crystal or diamond-like structure and a large number of oxygen-containing groups on the surface[52-53]. Studies have shown that CQDs have unique structures and photoelectric properties, such as a wide light absorption range, highly adjustable photoluminescence, and good photogenerated electron transport properties[54]. CQDs can be used as composite materials for semiconductor photocatalysts, and broaden the optical response range of semiconductors, resulting in the increase of the degradation rate of pollutants. Lietal.[55]provided a simple method to obtain CQDs, and synthetized ZnO/CQDs heterostructure by sol-gel method and a spin coating process. The results exhibited that the ZnO/CQDs heterostructure could significantly improve the efficiency of photocatalytic degradation of Rhodamine B. The number of layers of CQDs had the active influence on the photocatalytic reactivity due to the effective charge transfer between ZnO and CQDs. Yuetal.[56]fabricated ZnO/CQDs nanocomposites via hydrothermal synthesis. It was discovered that compared with pure ZnO NPs and N-doped TiO2, ZnO/CQDs nanocomposites displayed higher photocatalytic degradation efficiency to toxic gases (benzene and methanol) at room temperature under visible light irradiation.
5 Conclusions
This article briefly introduces and summarizes the preparation methods of nano ZnO and the strategies to improve the photocatalytic activity of nano ZnO in recent years. ZnO nanomaterials have excellent photocatalytic reactivity and other physical and chemical properties, but the utilization of visible light is minimal. In order to improve its performances, reduce the recombination opportunities of photo-generated carriers, expand the spectral response range, and achieve higher catalytic efficiency, the modification technology of ZnO becomes more and more diversified and mature, such as elements doping, noble metal deposition, semiconductor compounding, and carbon material coupling. At the same time, there are still some problems to be researched and solved in nano ZnO photocatalysts, such as selecting the appropriate composite elements or materials to optimize various modification methods, selecting the proper loading methods to solve the problem of recycling and reusing photocatalysts, improving the degradation types of organic pollutants, exploring the methods of batch preparation, promoting industrial production, and expanding the scope of applications. All of them need to be innovated and improved to develop new green photocatalysts with better performances.
 Journal of Donghua University(English Edition)2020年4期
Journal of Donghua University(English Edition)2020年4期
- Journal of Donghua University(English Edition)的其它文章
- Improved Fibroblast Adhesion and Proliferation by Controlling Multi-level Structure of Polycaprolactone Microfiber
- Zero-Sequence Current Suppression Strategy for Open-End Winding Permanent Magnet Synchronous Motor Based on Model Predictive Control
- Preserving Data Privacy in Speech Data Publishing
- Method for Detecting Fluff Quality of Fabric Surface Based on Support Vector Machine
- Comprehensive Evaluation Method for Safety Performance of Automobile Textiles
- Undrained Stability Analysis of Three-Dimensional Rectangular Trapdoor in Clay
