Improved Fibroblast Adhesion and Proliferation by Controlling Multi-level Structure of Polycaprolactone Microfiber
JIAOYongjie1,2,LIChaojing1,2,LIQiwei1,2,LIULaijun1,2,WANGFujun1,2,WANGLu(王璐)1,2
1 Key Laboratory of Textile Science and Technology, Ministry of Education, Donghua University, Shanghai 201620, China2 College of Textiles, Donghua University, Shanghai 201620, China
Abstract: Improving wound healing efficiency is a key issue for high performance dressings. The surface topology of fibers in wound dressings plays an important role in regulating cell behaviors during the regeneration. Herein, a polycaprolactone (PCL) scaffold with a shish-kebab structure was prepared by electrospinning and solution-induced crystallization. L929 cells were used to investigate the behavior of fibroblasts on the multi-level microfiber. The results showed that the shish-kebab fiber-based scaffold enhanced the cell proliferation when compared with the normal fiber and the fiber with a porous structure. Protein absorption, cell adhesive force, and cell modulus also increased by the shish-kebab fiber. The shish-kebab fiber-based scaffold with improved fibroblast-regulation ability can be applied in rapid wound healing.
Key words: cell-regulation; multi-level microfiber; shish-kebab structure; electrospinning; surface
Introduction
Wound healing is a complex process which is composed of four phases, namely hemostasis, inflammation, proliferation, and remodeling[1]. It is a time-consuming process if the repair relies on the self-healing ability of the human body. Thus, how to promote soft tissue regeneration is an important research direction in the field of the wound dressing[2].
Electrospinning attracts great interests in the field of wound healing, since the electrospun fibers provide physical protection for wounds, and mimic the extracellular matrix (ECM) structure which provides a proper environment for cell proliferation and differentiation during the regeneration[3]. Cells sense the topography and chemical constituents of the ECM and their behaviors are regulated by those signals. Consequently, the proliferation, the differentiation, the migration and the collagen deposition behavior of cells change. Traditional electrospun nanofibers own a smooth surface, which are different from the natural collagen fibers in the ECM. The shish-kebab structure is discovered and used for tissue regeneration due to its collagen-like surface[4]. Guoetal.[5]studied the influence of the shish-kebab polycaprolactone (PCL) structure on endothelial cells, indicating that cell proliferation and migration were enhanced. With the understanding of coagulation, we believe that the shish-kebab structure may be more suitable for wound healing than for blood-contacting. It is because that the topography and the stiffness of the shish-kebab structure could cause coagulation and promote endothelial cells to differentiate into myofibroblasts, while myofibroblasts have a faster rate of proliferation than fibroblasts which fit right for enhancing wound healing[6-7].
This research prepared PCL microfiber scaffolds with a shish-kebab structure. Microfiber scaffolds with a smooth surface structure and even a porous surface structure were used as references. Mouse fibroblasts were used forinvitroexperiments to explore the structural-induced mechanism for potential applications in wound healing.
1 Materials and Methods
1.1 Materials
PCL (Mn= 80 kDa) was purchased from Sigma-Aldrich, USA. Polyvinylpyrrolidone (PVP,Mn= 1 300 kDa) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd., China. Chloroform (CF), methanol, calcium chloride (CaCl2), and glacial acetic acid were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd., China. Albumin solution (BSA) and bicinchoninic acid (BCA) protein assay kits were purchased from Shanghai Sangon Biotech Co., Ltd., China. Roswell Park Memorial Institute (RPMI) 1640 medium was purchased from GE Healthcare, USA. Fetal bovine serum (FBS), penicillin/streptomycin (P/S) and sodium pyruvate were purchased from ThermoFisher Scientific, USA. Cell counting kit-8 (CCK-8), tetramethylrhodamine (TRITC) phalloidin, 4′,6-diamidino-2-phenylindole (DAPI) and Triton X-100 solution were purchased from Yeasen Biotech Co., Ltd., China. The fibroblast cell lines (L929 cells) were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, China.
1.2 Preparation of scaffold
1.2.1ElectrospinningprocessforPCLscaffoldandPCL-Porous(PCL-P)scaffold
The PCL particles were dissolved in the CF/methanol solvent with magnetic stirring for 24 h to obtain a stable solution. The volume fraction of CF in the solution was 75% and the volume fraction of methanol was 25%. The solution was transferred into a 10 mL syringe (19G blunt stainless-steel needle). The applied voltage was 15 kV, the feed rate was 1 mL/h, and the distance from the needle to the collector was 20 cm. The ambient temperature was maintained at 25 ℃, and the humidity was maintained at about 30%. After electrospinning, the prepared PCL scaffold was placed into a vacuum oven for 24 h.
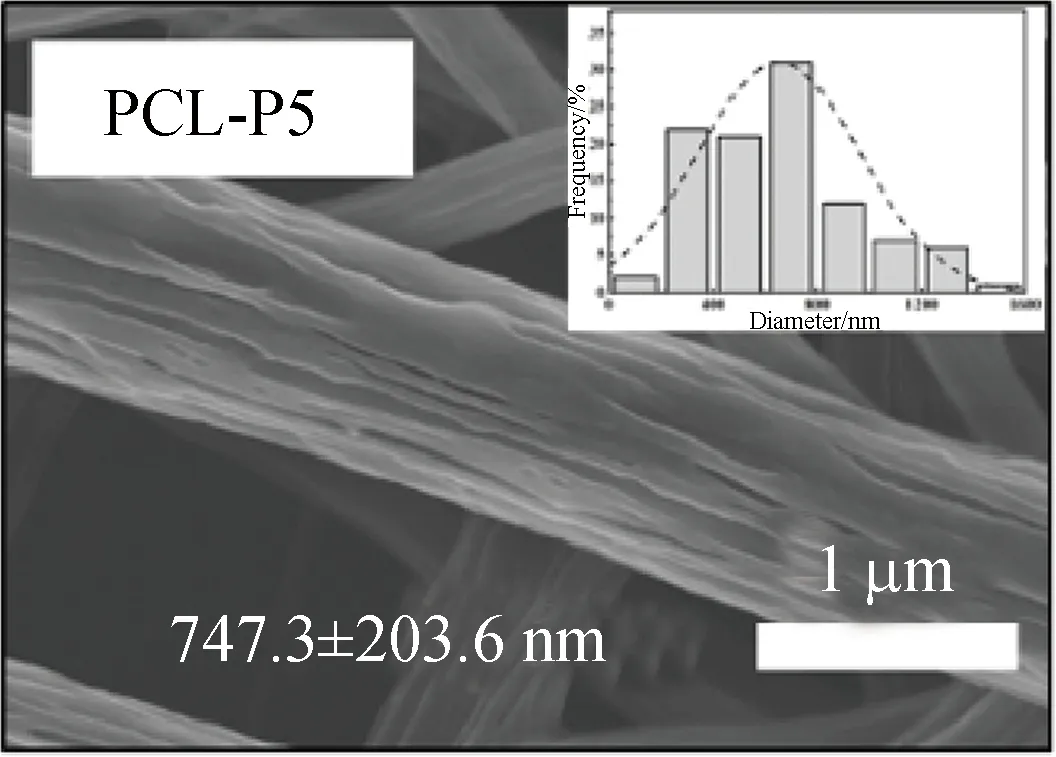
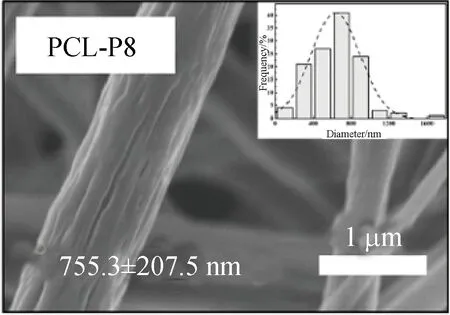
PCL was mixed with PVP and dissolved in the CF/methanol solvent with magnetic stirring for 24 h. The volume ratio of PCL to PVP is 8∶2 or 5∶5. The electrospinning parameters for the PCL-P scaffold were the same as those for the PCL scaffold, except that the ambient temperature was maintained at 25-30 ℃, and the humidity was maintained at about 30%-40%. After electrospinning, the prepared scaffold was placed into a vacuum oven for 24 h. The PCL-P scaffold was obtained by soaking the samples in 1 000 mL deionized water for 24 h to remove PVP and dried in a vacuum oven. PCL-P scaffolds were named as PCL-P8 and PCL-P5 according to the contents of PCL in the following study.
1.2.2PreparationofPCL-shish-kebab(PCL-SK)scaffold
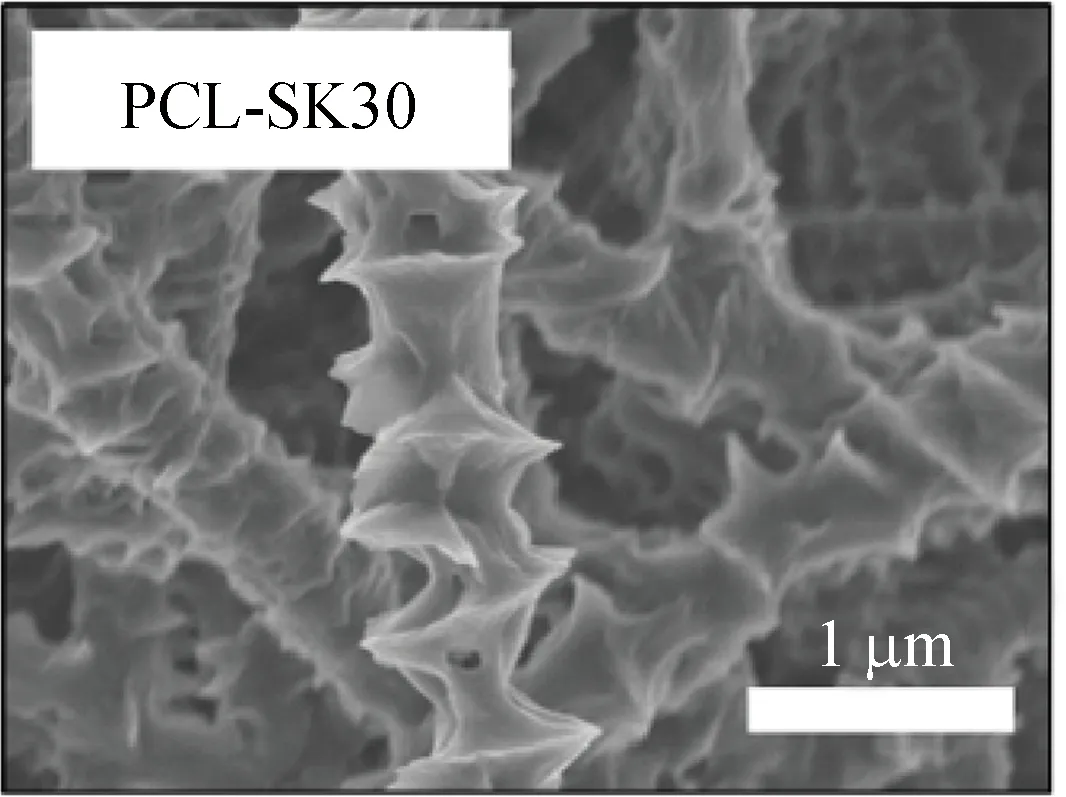
The PCL-SK scaffold was prepared by solution-induced crystallization. Firstly, a PCL scaffold was obtained from electrospinning as mentioned above. Secondly, PCL particles were dissolved in 75% acetic acid solution at 60 °C with a mass-to-volume ratio of 1%. After being cooled to room temperature, PCL fiber mats were immersed into the PCL/acetic acid solution mentioned above for 5 min and 30 min, respectively, to obtain a shish-kebab structure with different kebab sizes. Finally, PCL-SK scaffolds were obtained after being rinsed with the acetic acid solution for three times to remove the residual PCL and being dried in a vacuum oven for 24 h. PCL-SK scaffolds were named as PCL-SK5 and PCL-SK30 according to incubation times.
1.3 Characterization of scaffold structure
Morphology of scaffolds was observed by a field emission scanning electron microscope (FE-SEM) (SU8010, Hitachi, Japan) at a 20 kV beam voltage. The kebab size and the kebab periodic distance were calculated from FE-SEM images using the ImageJ software (National Institutes of Health, USA). The crystallinity was determined by X-ray diffraction (XRD, D/max-2550VB, Rigaku, Japan) equipped with a Cu Kα radiation source (λ= 0.154 2 nm) at 40 mA and 45 kV and the scanning speed was 5 (°)/min, in a 2θrange from 30° to 70°.
1.4 Characterization of protein adsorption
Protein adsorption on scaffolds was measured by the BCA method. Scaffolds were cut into 14 mm × 14 mm square, put into a 24-well plate, and fixed with steel rings. Samples were soaked with 75% alcohol for 30 min, and then washed in phosphate-buffered saline (PBS) for three times (30 min each time). After being removed the residual liquid, 1 mL BSA solution at a concentration of 1 mg/mL was added to each well, and the 24-well plate was placed in a constant temperature shaker at 37 ℃. After being reached the predetermined time point (3, 6 and 12 h), samples were rinsed in PBS for three times to remove the free protein and weak adhesion protein. Then 1 mL sodium dodecyl sulfate (SDS) solution was added to each well. Incubated in a constant temperature shaker at 37 ℃ for 4 h to strip the protein from samples, the protein-containing supernatant was pipetted into a 96-well plate and was added with the configured BCA working solution. After half an hour of reaction, the absorbance was measured at 562 nm using a microplate reader (Multiskan FC, Thermo, USA), and the protein adhesion amount was obtained according to the standard curve.
1.5 Cell adhesion and proliferation analysis
L929 cells were cultured in a complete medium consisting of 89% RPMI 1 640 medium, 10% FBS, 0.5% P/S, and 0.5% sodium pyruvate. All of the cells were cultured in 5% CO2at 37 ℃. The scaffolds were seeded with L929 cells at a density of 2 × 104cell/well.
After being cultured for 1 d and 7 d, cell proliferation was examined using a CCK-8 according to instructions. The absorbance at 450 nm was measured with a microplate reader (Multiskan FC, Thermo, USA). The average and the standard deviation were calculated for each sample.
Cell morphology was determined by TRITC phalloidin and DAPI staining. Scaffolds with cells were rinsed with PBS and fixed in 4% paraformaldehyde for 4 h. After fixation, scaffolds were rinsed with PBS three times for 10 min each time. Triton X-100 solution (0.5%) was added at room temperature for 5 min to permeate, and then rinsed with PBS. The 200 μL TRITC phalloidin was added to stain the cytoskeleton, and incubated for 30 min in the dark. After being rinsed with PBS for 5 min, 200 μL DAPI solution was added to counterstain the nucleus for 30 s. Fluorescence images were obtained using a fluorescence microscope (Ti-S, Nikon, Japan). The excitation wavelengths were 545/570 nm for TRITC and 364/454 nm for DAPI.
Cell adhesive forces on the scaffold were tested as follows. L929 cells were seeded on the scaffolds at 4 × 104cell/well. After being cultured for 12 h, pre-heated sterile PBS was used to wash away non-adherent cells. The sample was fixed to the bottom of the centrifuge tube, and the centrifuge tube was filled with PBS and centrifuged at several different speeds for 5 min. The suspended cells were removed by rinsing after centrifugation. CCK-8 assay was used to determine the optical density of the remaining cells before and after centrifugation using a microplate reader (Multiskan FC, Thermo, USA) at 450 nm, and the residual adhesion rate of the cells on the scaffold was calculated to characterize the size of the cell adhesion.
Cell modulus was determined by an atomic force microscope (AFM, 5500 AFM-SPM, Keysight, USA). The detected region (100 μm×100 μm) was scanned using the contact mode, and the stiffness of the probe was 0.2 N/m. The data were analyzed by the Hertz model[8].
(1)
whereFis the applied force,δis the indentation depth of the sample which can be obtained from the force-displacement curves,υis the Passion’s ratio (0.5 for cells),αis the half-opening angle of the cone of the probe, which is 17.5°, andEis the cell modulus.
1.6 Statistical analysis
All experiments were conducted with at least three samples. The data were presented as mean values ± standard deviation (SD). One-way analysis of variance (ANOVA) with the LSD test was performed using the SPSS software (version 16.0). The data were considered to be significant whenP<0.05(*).
2 Results and Discussion
2.1 Morphological properties
FE-SEM images showed that three kinds of scaffolds, which were PCL, PCL-P and PCL-SK scaffolds, were prepared successfully. Among them, PCL-P and PCL-SK scaffolds included two kinds of scaffolds, respectively. As shown in Figs. 1 (a)-(e), the average fiber diameters of PCL and PCL-P scaffolds were homogeneous (about 700 nm). There were narrow pores on the surface of PCL-P scaffolds, which were caused by the extraction of PVP, and the pores on PCL-P5 scaffolds were longer than those on PCL-P8 scaffolds. Due to the large molecular weight of PVP, PVP was continuously pulled in the electric field, forming an elongated and narrow porous surface. As the contents of PVP increased, the number of pores increased. For the PCL-SK scaffolds,
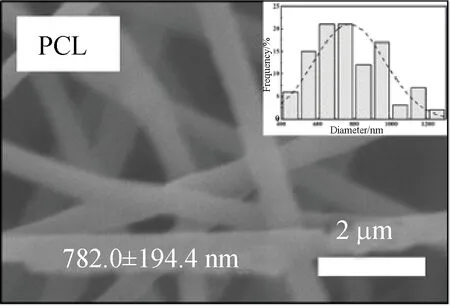
(a)
(b)
(c)

(d)
(e)

(f)
both PCL-SK5 and PCL-SK30 owned obvious shish-kebab structures. As the crystallization time increased, the kebab size and the kebab periodic distance increased as shown in Fig. 1(f), which ranged from 158 nm to 365 nm and 375 nm to 725 nm, respectively. The results showed that the shish-kebab structure could be regulated by the crystallization time.
2.2 Crystallization analysis
The crystallinity of material relates to the surface rigidity of the scaffold, which affects the fibroblastic behavior[9]. The crystallinities of scaffolds were presented in Fig. 2. In general, the crystallinity of the PCL-SK30 scaffold (51.82%) was the highest among all kinds of scaffolds, since the integrity of lamellae attached to the surface increased crystallinity and the crystallinity was positively related to the lamella sizes[4]. The crystallinity of the PCL scaffold (39.02%) was higher than that of PCL-P8 scaffolds (37.97%) and PCL-P5 scaffolds (28.72%), because the high contents of PVP provided steric hindrance which affected the tight packing of PCL macromolecular chains[10].
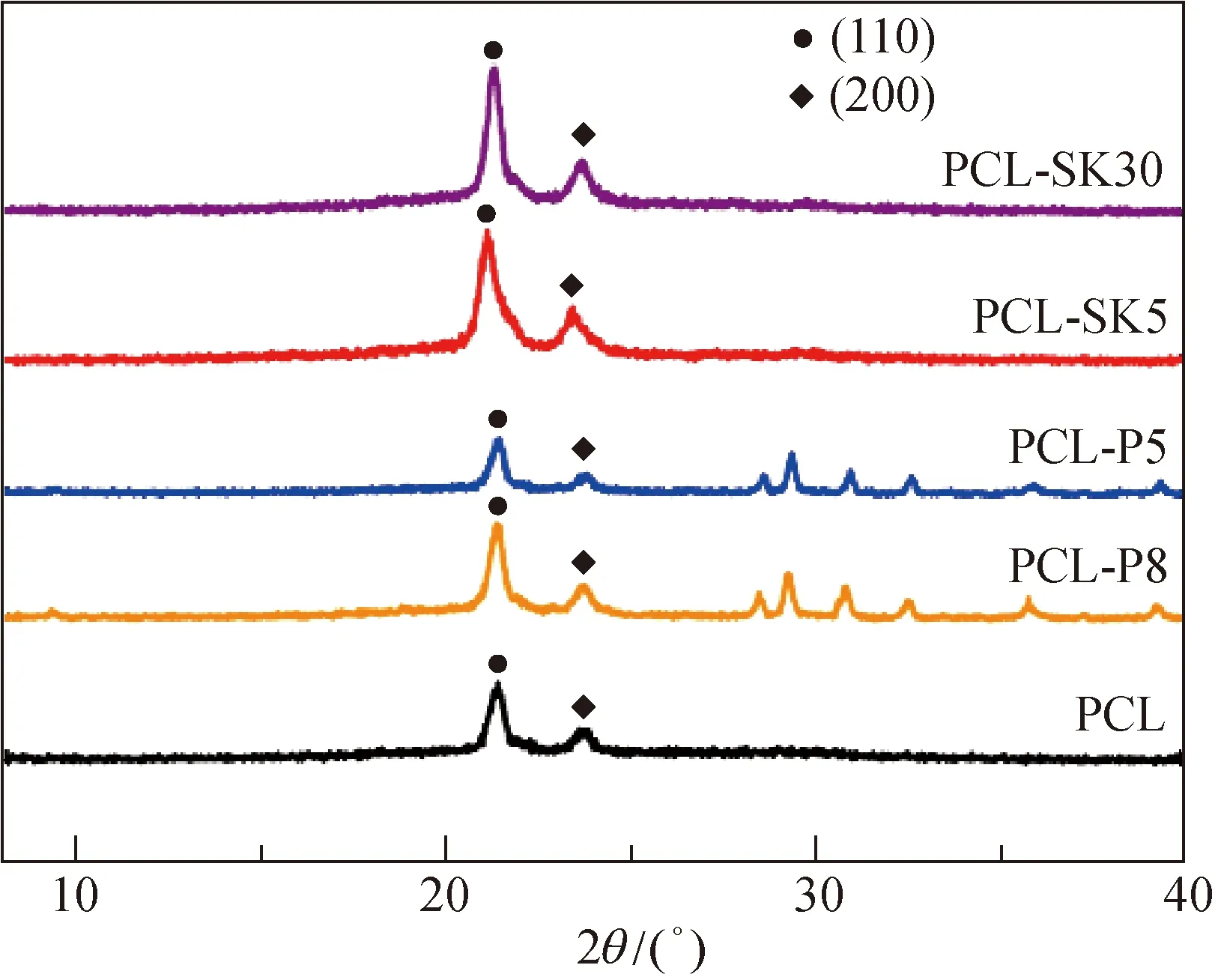
Fig. 2 XRD spectra
2.3 Bioactivity of scaffolds
2.3.1Cellproliferation
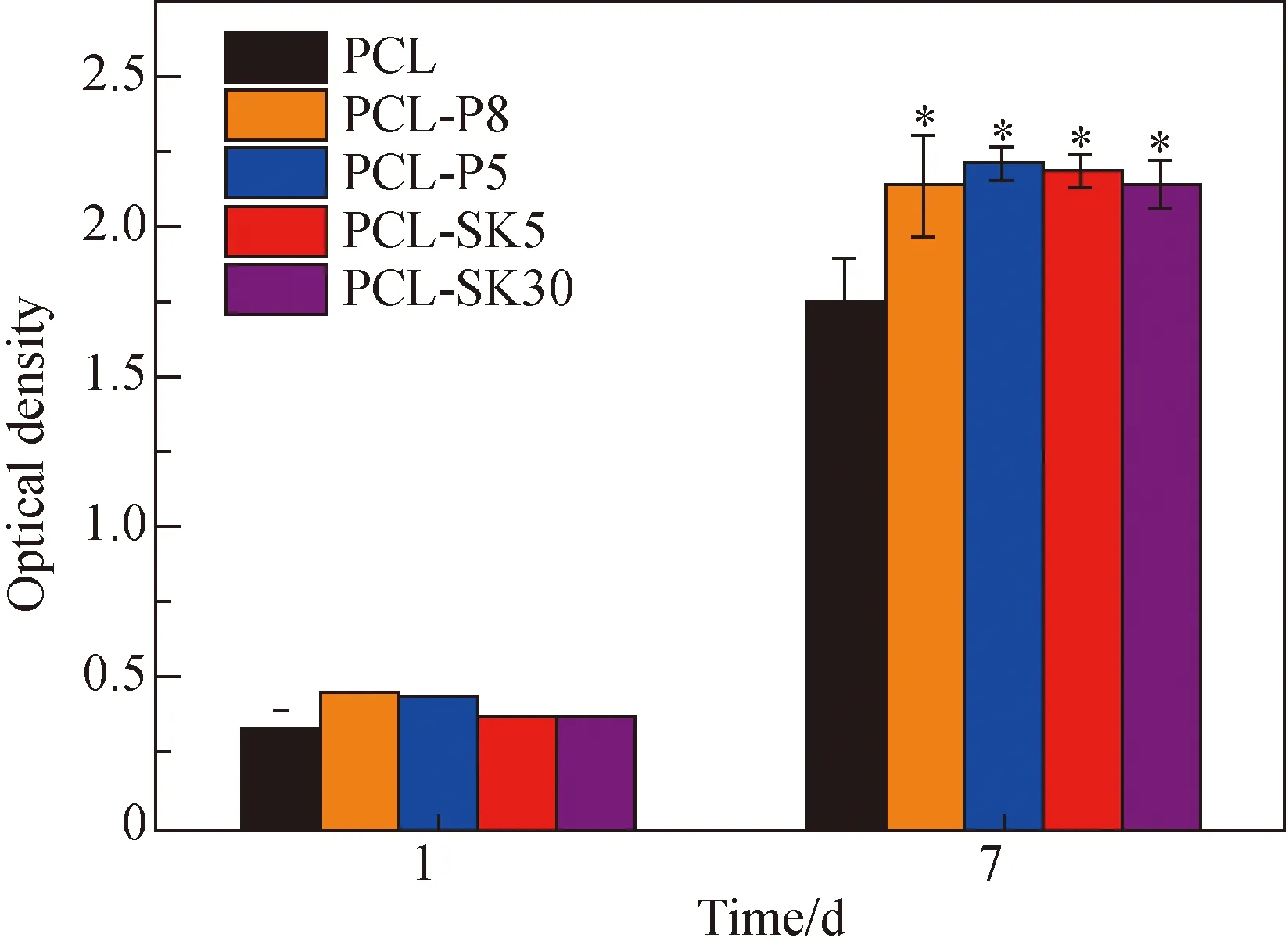
Fig. 3 CCK-8 assay of cell proliferation
L929 cell proliferation on various scaffolds was presented in Fig. 3. There was no significant difference on day 1. After 7 days’ culture, the optical densities of cells on the PCL-P5 scaffold (2.137), the PCl-P8 scaffold (2.209), the PC-SK5 scaffold (2.184) and the PCL-SK30 (2.141) scaffold were significantly higher than the optical density on the PCL scaffold (1.755) because the topology brought the fiber larger surface area. Noting that cell proliferation on the PCL-SK5 scaffold (2.184) was higher than that on the PCL-SK30 scaffold (2.141), it could be speculated that the large size of kebabs was not beneficial to cell proliferation. Considering that PCL-P5 and PCL-SK5 scaffolds led to higher cell proliferation compared with PCL-P8 and PCL-SK30 scaffolds, respectively, we chose these two scaffolds for following experiments.
2.3.2Proteinadsorptionontoscaffolds
Cells interact with scaffolds via surface-bound proteins[11]. Therefore, protein adsorption on scaffolds was studied and presented in Fig. 4. The protein adsorption on the PCL-SK5 scaffold (13.708 4 μg/cm2) was the highest compared with that on other scaffolds at 6 h, over 1.5 times of that on the PCL scaffold (8.467 0 μg/cm2), indicating that different surface topographies influenced protein adsorption during the prometaphase adsorption process. The shish-kebab structure gave the fiber a larger specific surface area, which provided more protein adhesion sites. The rapid protein adsorption was caused by the specific surface area[12]. It was worth noting that there was no significant difference in the amount of protein adsorption on the PCL-SK5 scaffold at 6 h and at 12 h, while that on the PCL-P5 scaffold increased significantly. According to the results, the PCL-SK5 scaffold showed a faster protein adsorption rate than the PCL-P5 scaffold. The high protein adsorption amount on the PCL-SK5 scaffold in the first 6 h might benefit rapid wound healing.
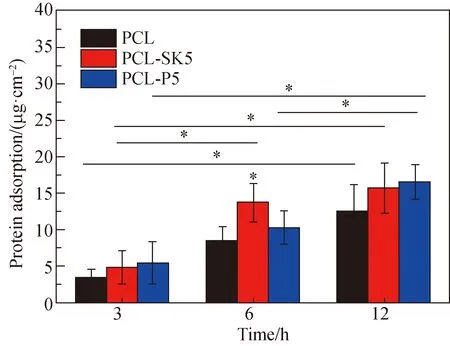
Fig. 4 Protein adsorption on PCL, PCL-SK5 and PCL-P5 scaffolds
2.3.3Cellmorphology
Cell morphology was shown in Fig. 5. By the time of 1 h, the cell adhered for a short time, showing a circular shape without spreading. By the time of 6 h, the spreading area increased. The spreading area of cells on the PCL-SK5 scaffold was higher, while the morphology of cells on the PCL-P5 scaffold was elongated. The number of adhesion sites on the PCL-SK5 scaffold was higher than that on other scaffolds, and the distribution of adhesion sites on the scaffold was more dispersed, thus causing a spreading cell morphology which was similar to the myofibroblast-like cell[13]. The elongated cells were caused by long and narrow pores on the surface of the PCL-P5 scaffold, indicating that the topography of the scaffold influenced the cell behavior.

Fig. 5 Cell fluorescence staining images by the time of 1 h and by the time of 6 h (red means cytoskeleton and blue means nucleus)
2.3.4Celladhesiveforce
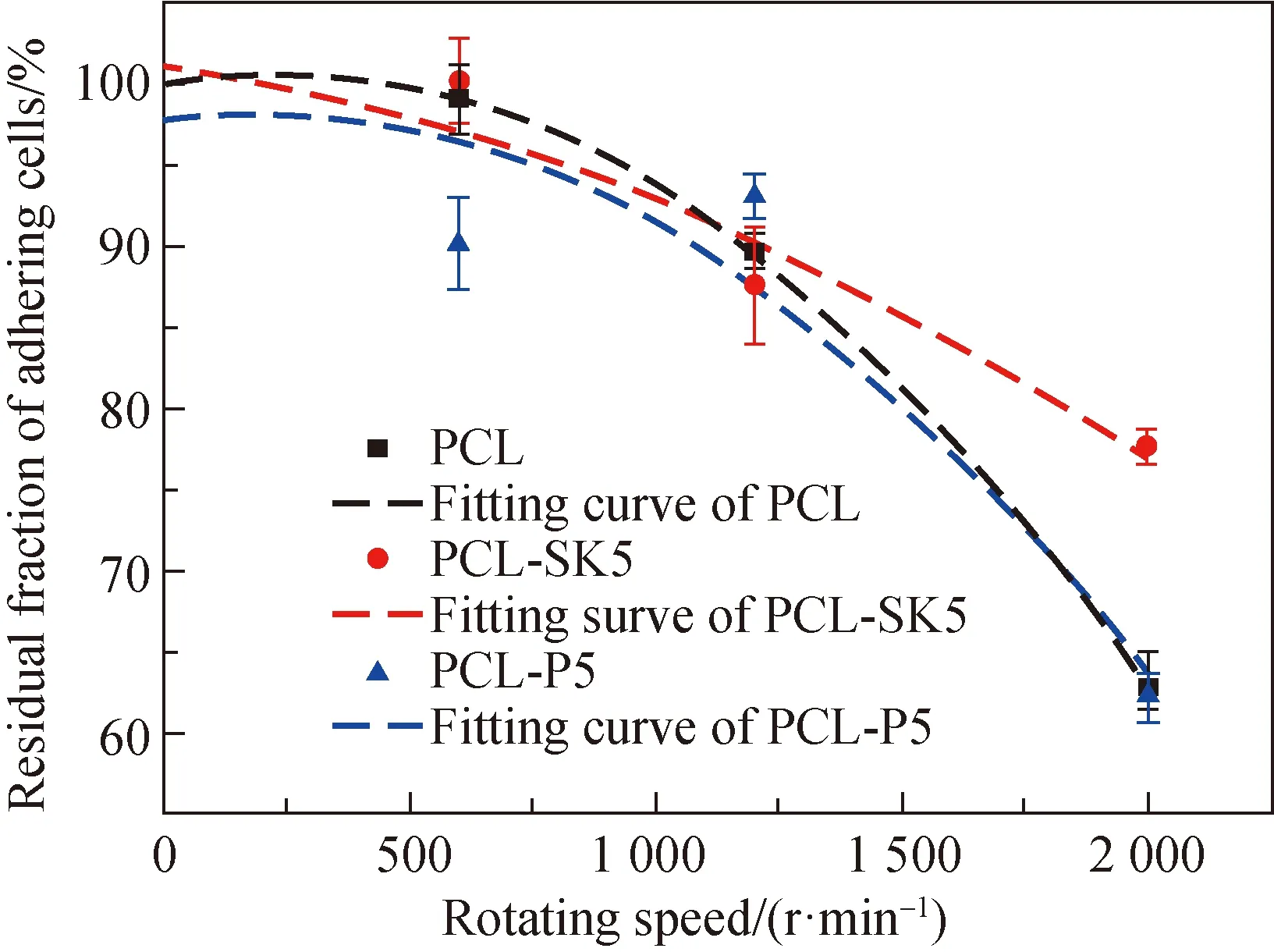
Fig. 6 Residual fraction of adhering cells
The buoyancy generated by the enhanced acceleration in the centrifuge shears the surface of scaffolds, and thus the residual fraction of adhering cells indirectly characterizes the size of the cell adhesive force at the same speed[14]. Three speeds (500, 1 200 and 2 000 r/min) were used to test the adhesive force of L929 cells after being cultured 12 h. The results showed that there was a significant difference in the residual fraction of adhering cells at 2 000 r/min (shown in Fig. 6). The residual fraction of adhering cells on the PCL-SK5 scaffold was over 80%, while that of PCL and PCL-P5 scaffolds was less than 62%. The shish-kebab structure increased the specific surface area and the stiffness, which affected the number of adhesive sites and adhesive plaque expression, and finally enhanced focal adhesion spreading and cell adhesive force. Cell adhesion was crucial for the early stages of wound healing. Large cell adhesive force contributed to the stability of wound regeneration. The increase of cell adhesive force indicated that the PCL-SK5 scaffold might be helpful for wound healing.
2.3.5Cellmodulus
Cells were cultured for 24 h and the cell modulus of the area around the nucleus was tested. The modulus of cells on the PCL-SK5 scaffold (1 567.2 Pa) was much higher than that on the PCL scaffold (747.1 Pa) and the PCL-P5 scaffold (886.2 Pa) (shown in Fig. 7). High crystallinity caused a rigid surface of the PCL-SK5 scaffold, which applied mechanical stimuli to fibroblasts. Cells produced more actin, and thus increased cell modulus, exhibiting a myofibroblast phenotype[15]. Considering that myofibroblast was helpful for wound healing[9, 16], we supposed that the shish-kebab structure could cause rapid wound healing.
It is known that cell behaviors are highly influenced by the pericellular microenvironment. The topography of the scaffold is one of the key factors which can regulate cell behaviors, because proteins can be bound to the surface of the scaffold and mediate the interaction between cells and scaffolds. The change of the topography influences the protein-binding sites, thus exerts effects on cells, and mediates actin production, cell proliferation, migration and differentiation[17]. In this study, the influences of the shish-kebab structure and the porous fiber morphology on proliferation and adhesion of L929 cells were investigated. We proved that the topography of the PCL fiber could bring those cells different adhesion and proliferation behaviors.
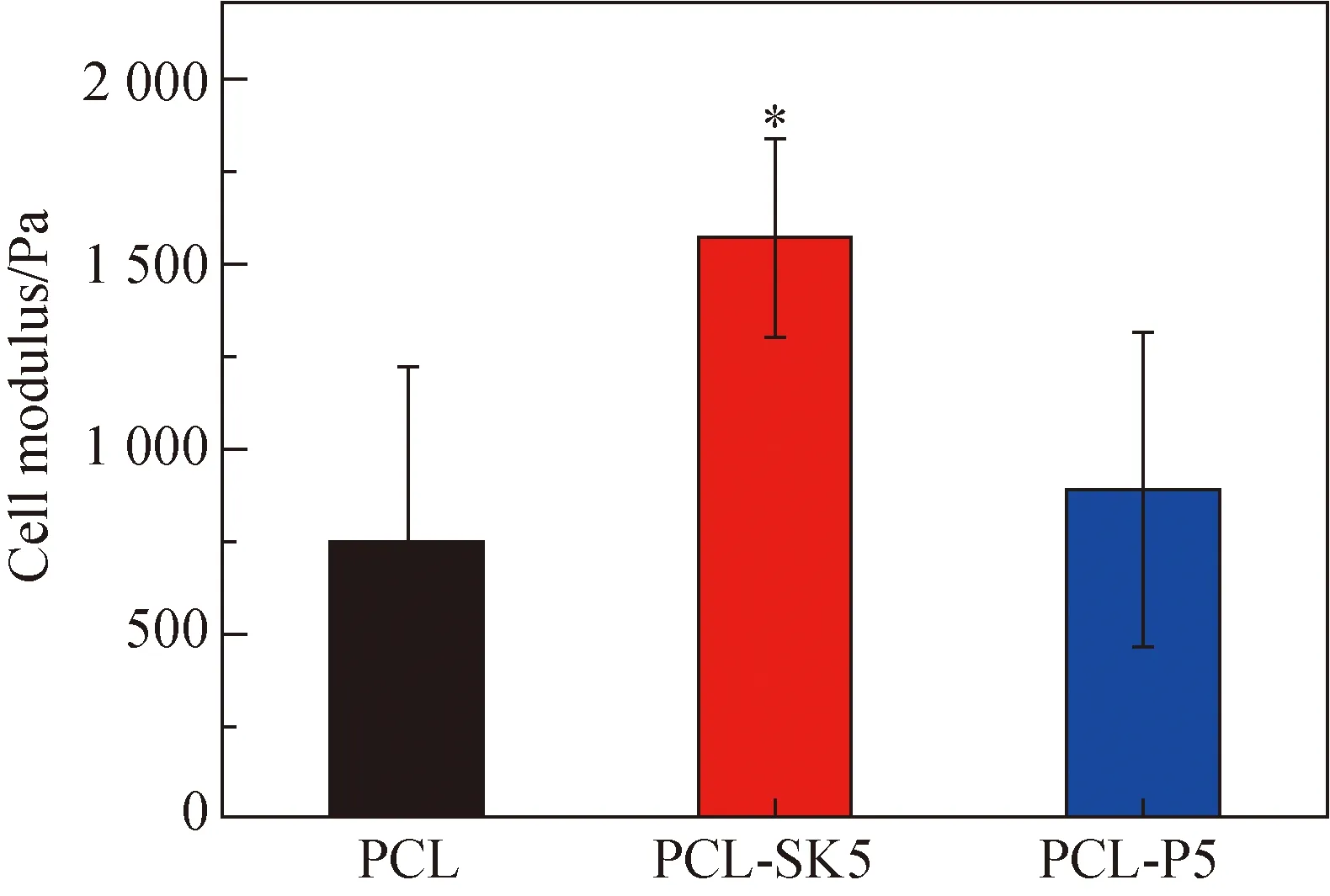
Fig. 7 Modulus of cells on scaffolds
3 Conclusions
Compared to smooth and porous fibers, the special topography and the shish-kebab fibers promoted the cell adhesive force and cell proliferation. Modulus of cells on shish-kebab fibers also increased, exhibiting a myofibroblast phenotype. The scaffold with a shish-kebab structure also caused rapid protein adsorption. All the results demonstrated that scaffolds with a shish-kebab structure might be helpful for rapid wound healing.
 Journal of Donghua University(English Edition)2020年4期
Journal of Donghua University(English Edition)2020年4期
- Journal of Donghua University(English Edition)的其它文章
- Preparation of ZnO Nanomaterials and Their Photocatalytic Degradation of Organic Pollutants
- Zero-Sequence Current Suppression Strategy for Open-End Winding Permanent Magnet Synchronous Motor Based on Model Predictive Control
- Preserving Data Privacy in Speech Data Publishing
- Method for Detecting Fluff Quality of Fabric Surface Based on Support Vector Machine
- Comprehensive Evaluation Method for Safety Performance of Automobile Textiles
- Undrained Stability Analysis of Three-Dimensional Rectangular Trapdoor in Clay
