微小RNA介导意大利蜜蜂工蜂对东方蜜蜂微孢子虫的跨界调控
杜宇,范小雪,蒋海宾,王杰,冯睿蓉,张文德,余岢骏,隆琦,蔡宗兵,熊翠玲,郑燕珍,2,陈大福,2,付中民,2,徐国钧,2,郭睿,2
微小RNA介导意大利蜜蜂工蜂对东方蜜蜂微孢子虫的跨界调控
杜宇1,范小雪1,蒋海宾1,王杰1,冯睿蓉1,张文德1,余岢骏1,隆琦1,蔡宗兵1,熊翠玲1,郑燕珍1,2,陈大福1,2,付中民1,2,徐国钧1,2,郭睿1,2
1福建农林大学动物科学学院(蜂学学院),福州 350002;2福建农林大学蜂疗研究所,福州 350002
【】东方蜜蜂微孢子虫()感染意大利蜜蜂(,简称意蜂)导致蜜蜂微孢子虫病。本研究结合前期已获得的miRNA和mRNA组学数据,通过生物信息学方法对意蜂工蜂中肠的差异表达miRNA(differentially expressed miRNA,DEmiRNA)靶向结合的东方蜜蜂微孢子虫的mRNA和差异表达mRNA(DEmRNA)进行预测、数据库注释和调控网络分析,以期在组学水平解析miRNA介导意蜂工蜂对东方蜜蜂微孢子虫的跨界调控机制。通过比较东方蜜蜂微孢子虫侵染7 d和10 d的意蜂工蜂中肠(AmT1、AmT2)和未受侵染的工蜂中肠(AmCK1、AmCK2)的miRNA组学数据筛选出宿主的显著性DEmiRNA,通过比较侵染意蜂工蜂中肠的东方蜜蜂微孢子虫(NcT1、NcT2)和东方蜜蜂微孢子虫纯净孢子(NcCK)的mRNA数据筛选出病原的DEmRNA。利用TargetFinder软件预测宿主显著性DEmiRNA靶向结合的病原mRNA和DEmRNA。利用相关生物信息学工具对上述靶DEmRNA进行GO和KEGG数据库注释。结合前期研究结果筛选出孢壁蛋白、极管蛋白、蓖麻毒素B凝集素、ABC转运蛋白、ATP/ADP移位酶和糖酵解/糖异生途径等毒力因子和能量代谢通路相关的病原DEmRNA及与其存在靶向结合关系的宿主显著性DEmiRNA,并构建和分析二者的调控网络。AmCK1 vs AmT1比较组中宿主的48条显著上调miRNA和36条显著下调miRNA分别靶向病原的1 345和1 046条mRNA;进一步分析发现,宿主的47条显著上调miRNA和34条显著下调miRNA可分别靶向NcCK vs NcT1比较组中病原的584条显著下调mRNA和265条显著上调mRNA,它们可分别注释到19和22个功能条目以及66和64条通路。AmCK2 vs AmT2比较组中宿主的56条显著上调miRNA和51条显著下调miRNA分别靶向病原的1 260和1 317条mRNA;进一步分析发现,宿主的52条显著上调miRNA和49条显著下调miRNA可分别靶向NcCK vs NcT2比较组中病原的587条显著下调mRNA和336条显著上调mRNA,它们可分别注释到20和23个功能条目以及64和65条通路。AmCK1 vs AmT1和AmCK2 vs AmT2比较组的8条共同显著上调miRNA和1条共同显著下调miRNA分别靶向NcCK vs NcT1和NcCK vs NcT2比较组中的144条共同显著下调和10条共同显著上调mRNA,可分别注释到18和13个功能条目以及38和7条通路。此外,AmCK1 vs AmT1和AmCK2 vs AmT2比较组中宿主的显著上调miRNA可靶向结合NcCK vs NcT1和NcCK vs NcT2比较组中与RNAi途径,孢壁蛋白和蓖麻毒素B凝集素等毒力因子,糖酵解/糖异生途径以及MAPK信号通路相关的病原下调表达mRNA。在东方蜜蜂微孢子虫的侵染过程中,意蜂工蜂中肠的DEmiRNA与病原的DEmRNA之间存在复杂的靶向结合关系以及潜在的跨界调控关系;宿主的DEmiRNA可能通过抑制或降解病原的RNAi途径、毒力因子、糖酵解/糖异生通路、ATP/ADP移位酶、ABC转运蛋白及MAPK信号通路相关靶DEmRNA影响病原的侵染和增殖。
意大利蜜蜂;东方蜜蜂微孢子虫;微小RNA;跨界调控;调控网络;免疫防御
0 引言
【研究意义】意大利蜜蜂(,简称意蜂)作为重要的经济昆虫和授粉昆虫在养蜂生产、科学研究和生态多样性维持等方面具有不可替代的价值[1]。作为群居性昆虫,蜜蜂易遭受多种病原微生物的侵袭,其中东方蜜蜂微孢子虫()是一种专性侵染成年蜜蜂中肠上皮细胞的单细胞真菌病原,可导致蜜蜂微孢子虫病,该病原还能与其他生物因子或非生物因子共同胁迫蜜蜂,严重危害蜜蜂健康和养蜂生产[2]。人们对于蜜蜂与东方蜜蜂微孢子虫的相互作用进行了较多研究[3-5],但对背后的分子机制还知之甚少。因此,探究微小RNA(microRNA,miRNA)介导意蜂工蜂对东方蜜蜂微孢子虫的跨界调控,不仅可为明确相关分子机制提供理论依据,也能加深对二者间互作的理解。【前人研究进展】在蜂群中,东方蜜蜂微孢子虫的孢子通过粪-口或口-口途径被蜜蜂宿主摄入体内,病原增殖高度依赖宿主细胞的物质和能量供应[3-5]。长期的协同进化使二者间形成了独特的互作关系,东方蜜蜂微孢子虫能抑制蜜蜂的免疫反应,引起消化系统紊乱,缩短蜜蜂寿命,并影响其定位、学习记忆和归巢能力等[3]。但对于蜜蜂能否跨界调控东方蜜蜂微孢子虫,相关研究还很滞后。miRNA是一类长度约为18—25 nt的高度保守的单链非编码RNA(non-coding RNA,ncRNA),可通过靶向mRNA的3′ UTR抑制mRNA的翻译或使其降解,从而发挥转录后水平的调控作用[6]。近期研究发现,miRNA不仅在原生细胞中发挥功能,还能在物种之间相互传播,促进不同物种之间的串扰、交流或信号干扰[7-10]。2012年,ZHANG等首次证实植物来源的miR-168a可通过胃肠道吸收进入哺乳动物的肝细胞,通过抑制小鼠低密度脂蛋白受体的表达适配器蛋白1(LDLRAP1),减弱血浆中低密度脂蛋白的清除[7]。该团队还发现植物蜂粮来源的miR-162a通过抑制蜜蜂幼蜂的卵巢和整体的生长发育,阻止幼虫分化为蜂王并诱导趋向工蜂的发育过程[8]。目前被广泛认可的外源RNA介导的调控机制主要分为两种,一种是在秀丽隐杆线虫()[11]、赤拟谷盗()[12]和褐飞虱()[13]等物种体内的系统性RNA干扰(RNAi)缺陷(SID)跨膜通道介导的远源dsRNA摄取,进而导致体内基因表达沉默,例如XU等[13]发现褐飞虱中外源性dsRNA通过siRNA途径触发基因沉默,SID-1是褐飞虱系统性RNAi必需的蛋白;另一种是miRNA可通过脱落囊泡(SV)、外泌体及凋亡小体等微囊泡(MV)腔室的包裹作用,以保护其在另一物种体内不被外源RNase酶降解,并进入到细胞体内发挥跨界调控基因表达的作用[14-15]。MiRNA作为关键的效应因子在宿主-病原互作中扮演关键角色[16-17]。CUI等[9]发现球孢白僵菌()可将bba-milR-1装载进囊泡并转运到斯氏按蚊()的细胞内,跨界调控宿主基因和的表达,球孢白僵菌在侵染前期通过下调的表达抑制Toll信号通路,而在侵染后期通过下调的表达以逃避黑化反应。MAYORAL等[10]通过印迹杂交证实沃尔巴克氏体()的miRNA存在于埃及伊蚊()纯化的细胞层中,并作为效应因子调节埃及伊蚊表达,促进自身增殖。蜜蜂与微孢子虫的跨界调控研究迄今仅有一例报道,HUANG等[18]合成东方蜜蜂微孢子虫的siRNA并饲喂给被该病原侵染的西方蜜蜂(),通过深度测序和比较分析发现在侵染后1—6 d分别有7条宿主miRNA和5条病原miRNA发生差异表达,进一步推测东方蜜蜂微孢子虫miRNA可能被转运到宿主细胞质调控宿主的新陈代谢和免疫应答。笔者团队前期已对东方蜜蜂微孢子虫侵染意蜂工蜂过程中宿主的侵染应答机制和病原的侵染机制进行了进行一系列探索,系统解析了意蜂工蜂中肠的mRNA差异表达谱和免疫应答[19],miRNA差异表达谱及调控网络[20],差异表达lncRNA的多种调控方式及潜在功能[21],以及东方蜜蜂微孢子虫的高表达基因[22]、可变剪接基因[23]、差异基因[24]、差异miRNA的表达谱[25]。【本研究切入点】目前,有关意蜂与东方蜜蜂微孢子虫之间的跨界调控研究极为有限。笔者团队前期已对东方蜜蜂微孢子虫侵染意蜂工蜂过程中宿主的miRNA差异表达谱和病原的mRNA差异表达谱分别进行解析,可为进一步探究宿主差异表达miRNA(differentially expressed miRNA,DEmiRNA)跨界调控病原差异表达mRNA(DEmRNA)提供必要的数据基础。【拟解决的关键问题】通过生物信息学方法预测意蜂工蜂中肠DEmiRNA靶向结合的东方蜜蜂微孢子虫DEmRNA,对靶DEmRNA进行数据库注释和相关分析,进一步构建宿主DEmiRNA与病原DEmRNA的调控网络,并对调控网络中的病原DEmRNA进行分析和探讨,以期在组学水平解析DEmiRNA介导意蜂工蜂对东方蜜蜂微孢子虫的跨界调控,为阐明背后的分子机制打下基础。
1 材料与方法
试验于2017年9月至2019年10月在福建农林大学动物科学学院(蜂学学院)蜜蜂保护实验室完成。
1.1 供试生物材料
意蜂工蜂取自福建农林大学动物科学学院(蜂学学院)教学蜂场。东方蜜蜂微孢子虫感染的意蜂外勤蜂取自福州市闽侯县荆溪源安养蜂场。
1.2 意蜂工蜂中肠的miRNA组学数据来源
笔者团队前期通过Percoll不连续密度梯度离心法对东方蜜蜂微孢子虫孢子进行纯化,并对意蜂工蜂进行饲喂接种及中肠样品的剖取[21]。前期已分别抽提东方蜜蜂微孢子虫侵染7 d和10 d的工蜂中肠样品(AmT1、AmT2)和未受侵染的工蜂中肠样品(AmCK1、AmCK2)的总RNA,并委托广州基迪奥生物科技有限公司通过Illumina MiSeq平台对建好的cDNA文库进行单端测序。
笔者团队已对测序数据进行过滤和质控[26]:(1)剔除原始读段(raw reads)中含5′接头序列、含polyA、低质量的reads和剪切掉3′接头序列后的<18或>30个核苷酸的序列,得到高质量的有效序列标签(clean tags);(2)利用Bowite软件[27]将获得的clean tags比对GeneBank及Rfam(11.0)数据库,过滤比对上rRNA、scRNA、snoRNA和tRNA的clean tags,得到未注释的tags(unannotated tags);(3)比对东方蜜蜂微孢子虫参考基因组(assembly ASM98816v1)(https://www.ncbi.nlm.nih.gov/genome/931?genome_assembly_id=230435),去除比对上的数据(即为东方蜜蜂微孢子虫的数据);(4)将剩余数据继续比对西方蜜蜂参考基因组(assembly Amel_4.5)(http://www. ncbi.nlm.nih.gov/genome/48?genome_assembly_id=22683),剔除比对上基因组外显子、内含子和重复序列的clean tags,剩余比对上的数据(mapped tags)可用于后续分析。
笔者团队前期已利用miRDeep2软件[28]将上述剩余的mapped tags与miRBase数据库中收录的miRNA前体序列进行比对,获得已知miRNA序列。同时,将未比对上的tags比对基因组,得到可能的前体序列,根据tags在前体序列上的分布信息和前体结构能量信息,采用贝叶斯模型经打分实现novel miRNA的鉴定。利用每百万标签序列(tags per million,TPM)公式(TPM=T×106/N,T表示miRNA的tags,N表示总miRNA的tags)对miRNA进行表达量的归一化处理。按照|log2fold change (FC)|≥1且≤0.05的标准筛选AmCK1 vs AmT1和AmCK2 vs AmT2比较组的显著性DEmiRNA,用于本研究中靶向东方蜜蜂微孢子虫的mRNA和DEmRNA的预测和分析。
1.3 东方蜜蜂微孢子虫的mRNA组学数据来源
笔者团队前期按照1.2中的方法对意蜂工蜂进行饲喂接种及中肠样品制备,并利用基于链特异性cDNA建库的RNA-seq技术对接种的中肠样品进行测序,得到同时包含宿主数据和病原数据的混合mRNA组学数据[29]。将上述混合数据连续比对核糖体数据库、西方蜜蜂基因组(assembly Amel_4.5)和东方蜜蜂微孢子虫基因组(assembly ASM98816v1),筛滤得到处于侵染过程的病原mRNA组学数据[20]。其中,将侵染8日龄(即侵染后7 d)工蜂中肠内的东方蜜蜂微孢子虫设为NcT1(NcT1-1、NcT1-2和NcT1-3为3个生物学重复),侵染11日龄(即侵染后10 d)工蜂中肠内的东方蜜蜂微孢子虫设为NcT2(NcT2-1、NcT2-2和NcT2-3为3个生物学重复)。笔者团队前期也已利用基于链特异性cDNA建库的RNA-seq技术对东方蜜蜂微孢子虫的纯净孢子(NcCK:NcCK-1、NcCK-2和NcCK-3)进行深度测序,获得了高质量的mRNA组学数据[30]。测序原始数据已上传NCBI SRA数据库,Bioproject号分别为PRJNA395264(NcCK)和PRJNA406998(NcT1和NcT2)。
笔者团队前期已采用FPKM(Fragments Per Kilobase of transcript per Million fragments mapped)算法计算和归一化基因表达量;利用edgeR软件[31]筛选NcCK vs NcT1和NcCK vs NcT2比较组的显著性DEmRNA,筛选标准为|log2FC|≥1且≤0.05。上述病原DEmRNA可用于本研究中宿主显著性DEmiRNA的靶向预测及分析。
1.4 意蜂工蜂中肠显著性DEmiRNA靶向东方蜜蜂微孢子虫mRNA和DEmRNA的预测及分析
利用TargetFinder软件[32]预测AmCK1 vs AmT1和AmCK2 vs AmT2比较组中显著性DEmiRNA靶向结合的东方蜜蜂微孢子虫mRNA,以及NcCK vs NcT1和NcCK vs NcT2比较组的显著性DEmRNA,采用默认参数。利用OmicShare在线工具集合(www. omicshare.com)的相关工具对上述靶标mRNA和DEmRNA进行GO(Gene Ontology)和KEGG(Kyoto Encyclopedia of Genes and Genomes)数据库注释,采用默认参数。
1.5 意蜂工蜂中肠显著性DEmiRNA与东方蜜蜂微孢子虫mRNA和DEmRNA的调控网络构建及分析
根据1.4中预测出的意蜂工蜂中肠显著性DEmiRNA与东方蜜蜂微孢子虫mRNA和显著性DEmRNA的靶向结合关系,构建二者之间的调控网络,并利用Cytoscape软件[33]可视化调控网络。根据前人在微孢子虫和笔者所在课题组在东方蜜蜂微孢子虫方面的研究结果[34-39],孢壁蛋白、极管蛋白、蓖麻毒素B凝集素、糖酵解/糖异生途径以及ABC转运蛋白和ATP/ADP转位酶与微孢子虫的侵染和增殖活动关系密切,筛选与上述蛋白和途径相关的病原DEmRNA及存在靶向结合关系的宿主显著性DEmiRNA,并构建、分析及可视化调控网络。
2 结果
2.1 意蜂工蜂中肠DEmiRNA靶向东方蜜蜂微孢子虫mRNA和DEmRNA的预测及分析
AmCK1 vs AmT1比较组中84条显著性DEmiRNA靶向结合东方蜜蜂微孢子虫的1 620条mRNA,其中宿主的48条显著上调miRNA和36条显著下调miRNA分别靶向病原的1 345和1 046条mRNA。进一步分析发现,宿主的47条显著上调miRNA可靶向NcCK vs NcT1比较组中病原的584条显著下调mRNA,34条显著下调miRNA可靶向病原的265条显著上调mRNA(图1-A、1-B)。AmCK2 vs AmT2比较组中107条显著性DEmiRNA共靶向结合东方蜜蜂微孢子虫的1 717条mRNA,其中宿主的56条显著上调miRNA和51条显著下调miRNA分别靶向病原的1 260和1 317条mRNA。进一步分析发现,宿主的52条显著上调miRNA可靶向NcCK vs NcT2比较组中病原的587条显著下调mRNA,49条显著下调miRNA可靶向病原的336条显著上调mRNA(图1-C、1-D)。
进一步分析发现,AmCK1 vs AmT1和AmCK2 vs AmT2比较组包含8条共同显著上调miRNA,可靶向NcCK vs NcT1和NcCK vs NcT2比较组中92条共同显著上调mRNA和144条共同显著下调mRNA;此外,1条共同显著下调miRNA可靶向病原的10条共同显著上调mRNA和16条共同显著下调mRNA(图2)。
2.2 意蜂工蜂中肠DEmiRNA靶向东方蜜蜂微孢子虫mRNA和DEmRNA的功能注释
GO数据库注释结果显示,AmCK1 vs AmT1比较组中显著性DEmiRNA靶向东方蜜蜂微孢子虫的mRNA可注释到25个功能条目,包括代谢进程(312)、催化活性(279)和结合(274)等;AmCK2 vs AmT2比较组中显著性DEmiRNA靶向东方蜜蜂微孢子虫的mRNA可注释到25个功能条目,包括代谢进程(322)、催化活性(284)和细胞进程(277)等。
AmCK1 vs AmT1中显著上调miRNA靶向NcCK vs NcT1中的584条显著下调mRNA,涉及代谢进程(84)和催化活性(82)等19个功能条目;宿主的显著下调miRNA靶向病原的265条显著上调mRNA,涉及代谢进程(70)和结合(63)等22个功能条目。AmCK2 vs AmT2中显著上调miRNA靶向NcCK vs NcT2中病原的587条显著下调mRNA,涉及代谢进程(76)和催化活性(75)等20个功能条目;宿主的显著下调miRNA靶向病原的336条显著上调mRNA,涉及代谢进程(76)和结合(69)等23个功能条目。进一步分析结果显示,AmCK1 vs AmT1和AmCK2 vs AmT2比较组8条共同显著上调miRNA可靶向NcCK vs NcT1和NcCK vs NcT2比较组中144条共同显著下调mRNA,涉及代谢进程(20)等18个功能条目(图3);而1条宿主的共同显著下调miRNA可靶向10条病原的共同显著上调mRNA,涉及代谢进程(4)等13个功能条目(图3)。括号内的数字表示注释在该条目的mRNA数量。
2.3 意蜂工蜂中肠显著性DEmiRNA靶向东方蜜蜂微孢子虫mRNA和显著性DEmRNA的通路注释
KEGG数据库注释结果显示,AmCK1 vs AmT1比较组中显著性DEmiRNA靶向东方蜜蜂微孢子虫的mRNA可注释到84条通路,包括代谢途径(107)、次生代谢物的生物合成(40)和核糖体(39)等;AmCK2 vs AmT2比较组中显著性DEmiRNA靶向东方蜜蜂微孢子虫的mRNA可注释到84条通路,包括代谢途径(118)、核糖体(50)和次生代谢物的生物合成(48)等。
AmCK1 vs AmT1中显著上调miRNA靶向NcCK vs NcT1中的显著下调mRNA可注释到代谢途径(35)和核糖体在真核生物中的生物合成(17)等66条通路;宿主的显著下调miRNA靶向病原的显著上调mRNA可注释到代谢途径(29)和次生代谢物的生物合成(16)等64条通路。AmCK2 vs AmT2中显著上调miRNA靶向NcCK vs NcT2中的显著下调mRNA可注释到代谢途径(34)和细胞周期(15)等64条通路;宿主的显著下调miRNA靶向病原的显著上调mRNA可注释到代谢途径(35)和次生代谢物的生物合成(19)等65条通路。此外,AmCK1 vs AmT1和AmCK2 vs AmT2比较组的8条共同显著上调miRNA可靶向NcCK vs NcT1和NcCK vs NcT2比较组中144条共同显著下调mRNA,可注释到代谢途径(10)和RNA转运(5)等38条通路;1条宿主的共同显著下调miRNA可靶向10条病原的共同显著上调的mRNA,可注释到代谢途径(2)和抗生素的生物合成(1)等7条通路。括号内的数字表示注释在该通路的mRNA数量。
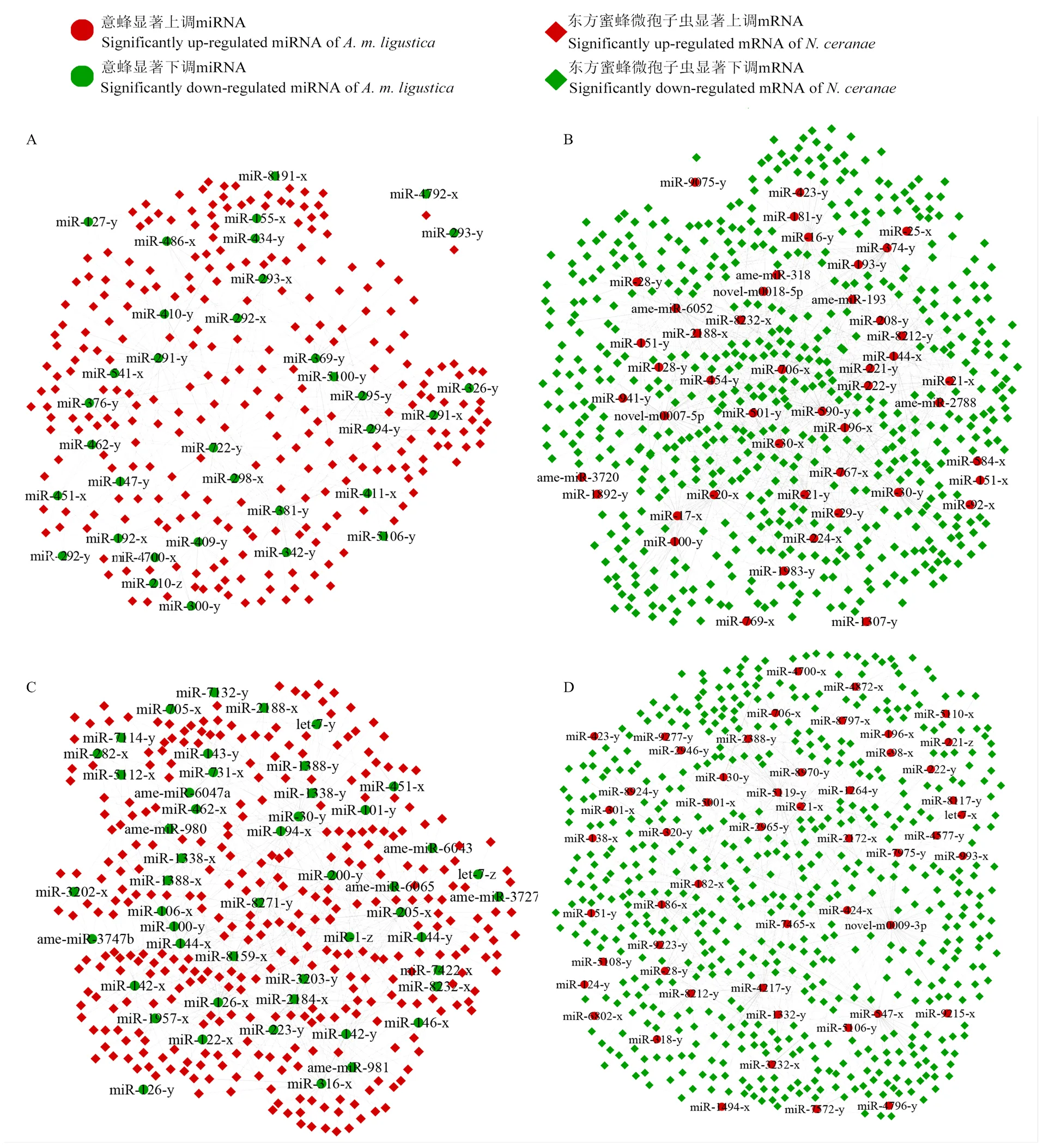
A:AmCK1 vs AmT1中显著下调miRNA及其靶向NcCK vs NcT1中显著上调mRNA的调控网络Regulatory network of significantly down-regulated miRNAs in AmCK1 vs AmT1 and their target significantly up-regulated mRNAs in NcCK vs NcT1;B:AmCK1 vs AmT1中显著上调miRNA靶向NcCK vs NcT1中显著下调mRNA的调控网络Regulatory network of significantly up-regulated miRNAs in AmCK1 vs AmT1 and their target significantly down-regulated mRNAs in NcCK vs NcT1;C:AmCK2 vs AmT2中显著下调miRNA靶向NcCK vs NcT2中显著上调mRNA的调控网络Regulatory network of significantly down-regulated miRNAs in AmCK2 vs AmT2 and their target significantly up-regulated mRNAs in NcCK vs NcT2;D:AmCK2 vs AmT2中显著上调miRNA靶向NcCK vs NcT2中显著下调mRNA的调控网络Regulatory network of significantly up-regulated miRNAs in AmCK2 vs AmT2 and their target significantly down-regulated mRNAs in NcCK vs NcT2

图2 AmCK1 vs AmT1和AmCK2 vs AmT2比较组的共同显著上调(下调)miRNA靶向NcCK vs NcT1和NcCK vs NcT2比较组的共同显著下调(上调)mRNA的调控网络
2.4 意蜂工蜂中肠显著性DEmiRNA及其靶向的东方蜜蜂微孢子虫毒力因子/侵染因子相关DEmRNA的调控网络
在AmCK1 vs AmT1比较组中,分别有13条显著上调miRNA(miR-8212-y、miR-374-y和miR-590-y等)和9条显著下调miRNA(miR-291-y、miR-409-y和miR-326-y等)靶向4条ABC转运蛋白编码基因相关的显著DEmRNA,分别有7条显著上调miRNA(ame-miR-6052、miR-501-y和miR-767-x等)和1条显著下调miRNA(miR-381-y)靶向3条ATP/ADP转位酶编码基因相关的显著DEmRNA(图4-A、表1、表2)。分别有9条显著上调miRNA(miR-193-y、ame-miR-193和miR-590-y等)和1条显著下调miRNA(miR-451-x)靶向NcCK vs NcT1比较组中的3条极管蛋白编码基因相关的显著DEmRNA,分别有5条显著上调miRNA(miR-941-y、miR-16-y和novel-m0007-5p等)和1条显著下调miRNA(miR-291-y)靶向1条孢壁和锚定吸盘复合蛋白编码基因和1条孢壁蛋白编码基因相关的显著DEmRNA,分别有8条显著上调miRNA(ame-miR-6052、miR-8212-y和miR-144-x等)和6条显著下调miRNA(miR-409-y、miR-294-y和miR-291-x等)靶向7条蓖麻毒素B凝集素编码基因相关的显著DEmRNA(图4-B、表1、表2)。分别有21条显著上调miRNA(miR-8232-x、miR-144-x和miR-767-x等)和10条显著下调miRNA(miR-291-y、miR-381-y和miR-462-y等)靶向10条糖酵解/糖异生途径相关的显著DEmRNA(图4-C、表1、表2)。此外,分别有7条显著上调miRNA(miR-224-x、novel-m0007-5p和miR-16-y等)和2条显著下调miRNA(miR-155-x和miR-291-x)靶向MAPK信号通路相关的5条显著DEmRNA(表1、表2)。

1:代谢进程Metabolic process;2:细胞进程Cellular process;3:单一组织进程Single-organism process;4:应激反应Response to stimulus;5:信号Signaling;6:生物调节进程Biological regulation process;7:定位Localization;8:生物调节Biological regulation;9:细胞成分组织或生物合成Cellular component organization or biogenesis;10:细胞Cell;11:细胞组件Cell part;12:细胞器Organelle;13:细胞膜组件Cell membrane part;14:细胞膜Cell membrane;15:高分子复合物Macromolecular complex;16:细胞器组件Organelle part;17:催化活性Catalytic activity;18:结合Binding

A:宿主的显著性DEmiRNA与病原的ABC转运蛋白、ATP/ADP转位酶相关DEmRNA的调控网络Regulatory network of hostsignificant DEmiRNAs and pathogen DEmRNAs associated with ABC transporter and ATP/ADP translocase;B:宿主的显著性DEmiRNA与病原的蓖麻毒素B凝集素、孢壁蛋白和极管蛋白相关DEmRNA的调控网络Regulatory network ofhost significant DEmiRNAs and pathogen DEmRNAs associated with ricin B lectin, spore wall protein and polar tube protein;C:宿主的显著性DEmiRNA与病原的糖酵解/糖异生途径相关DEmRNA的调控网络Regulatory network of hostsignificant DEmiRNAs and pathogen DEmRNAs associated with glycolysis/gluconeogenesis pathway

表2 靶向NcCK vs NcT1中病原毒力因子/侵染因子相关下调DEmRNA的AmCK1 vs AmT1中宿主显著上调miRNA的信息概要
在AmCK2 vs AmT2比较组中,分别有8条显著上调miRNA(miR-28-y、miR-8924-y和miR-8212-y等)和10条显著下调miRNA(miR-142-y、miR-8159-x和miR-2184-x等)可靶向3条ABC转运蛋白编码基因相关的显著DEmRNA;分别有6条显著上调miRNA(novel-m0009-3p、miR-706-x和miR-1332-y等)和7条显著下调miRNA(miR-462-x、miR-144-x和miR-8159-x等)可靶向3条ATP/ADP转位酶编码基因相关的显著DEmRNA(图5-A)。分别有8条显著上调miRNA(miR-424-x、miR-318-y和miR-4217-y等)和7条显著下调miRNA(miR-144-x、miR-8159-x和miR-142-x等)可靶向NcCK vs NcT2比较组中的3条极管蛋白编码基因相关的显著DEmRNA;分别有7条显著上调miRNA(miR-182-x、miR-5119-y和miR-138-x等)和9条显著下调miRNA(miR-462-x、miR-142-y和miR-5112-x等)可靶向1条孢壁和锚定吸盘复合蛋白编码基因、2条孢壁蛋白前体编码基因及1条孢壁蛋白编码基因相关的显著DEmRNA;分别有5条显著上调miRNA(miR-547-x、miR-4577-y和miR-8212-y等)和9条显著下调miRNA(miR-144-x、miR-142-y和miR-223-y等)可靶向6条蓖麻毒素B凝集素编码基因相关的显著DEmRNA(图5-B)。分别有14条显著上调miRNA(miR-222-y、miR-221-z和miR-1332-y等)和19条显著下调miRNA(miR- 8159-x、miR-2184-x和miR-8271-y等)可靶向病原的12条糖酵解/糖异生途径相关的显著DEmRNA(图5-C)。此外,分别有5条显著上调miRNA(miR-4796-y、miR-1332-y和miR-4217-y等)和5条显著下调miRNA(miR-142-y、miR-8159-x和miR-731-x等)可靶向MAPK信号通路相关的5条显著DEmRNA。

A:宿主的显著性DEmiRNA与病原的ABC转运蛋白、ATP/ADP转位酶相关DEmRNA的调控网络Regulatory network of hostsignificantDEmiRNAs and pathogen DEmRNAs associated with ABC transporter and ATP/ADP translocase;B:宿主的显著性DEmiRNA与病原的蓖麻毒素B凝集素、孢壁蛋白和极管蛋白相关DEmRNA的调控网络Regulatory network ofhost significant DEmiRNAs and pathogen DEmRNAs associated with ricin B lectin, spore wall protein and polar tube protein;C:宿主的显著性DEmiRNA与病原的糖酵解/糖异生途径相关DEmRNA的调控网络Regulatory network of hostsignificant DEmiRNAs and pathogen DEmRNAs associated with glycolysis/gluconeogenesis pathway
3 讨论
随着相关研究的增多和深入,有关miRNA在宿主和病原互作中的媒介作用被广泛报道[16-17,40-42]。前期研究中,笔者团队一方面解析了意蜂工蜂中肠响应东方蜜蜂微孢子虫侵染的miRNA差异表达谱及DEmiRNA介导的宿主免疫应答[20];另一方面解析了东方蜜蜂微孢子虫侵染意蜂工蜂过程中的mRNA差异表达谱以及毒力因子、侵染因子及相关DEmRNA在病原侵染中的作用[24]。利用已获得的高质量miRNA和mRNA组学数据,本研究进一步探究miRNA介导意蜂工蜂与东方蜜蜂微孢子虫间的相互作用。
东方蜜蜂微孢子虫能够控制蜜蜂的物质代谢、能量代谢和免疫防御等[43]。但关于蜜蜂是否能够通过差异表达miRNA调控东方蜜蜂微孢子虫基因表达的研究未见报道。本研究中,AmCK1 vs AmT1比较组中的显著上调miRNA靶向NcCK vs NcT1比较组中的显著下调mRNA可注释到11条碳水化合物代谢通路、8条脂质代谢通路、3条氨基酸代谢通路及2条能量代谢通路。AmCK2 vs AmT2比较组中显著上调miRNA靶向NcCK vs NcT2比较组中的显著下调mRNA可注释到10条碳水化合物代谢通路、8条脂质代谢通路、2条氨基酸代谢通路及2条能量代谢通路。此外,AmCK1 vs AmT1和AmCK2 vs AmT2比较组的共同显著上调miRNA靶向NcCK vs NcT1和NcCK vs NcT2比较组的共同显著下调mRNA可注释到8条碳水化合物代谢通路、2条脂质代谢通路以及2条能量代谢通路。以上结果表明被东方蜜蜂微孢子虫感染的意蜂工蜂中肠可能通过合成与分泌miRNA跨界抑制或降解病原相关mRNA,进而影响中肠细胞内寄生的病原的糖类、脂质、蛋白和遗传物质等物质代谢途径,以及氧化磷酸化和甲烷代谢等能量代谢途径,体现出二者之间存在密切的相互作用。
RNAi是一种RNA介导的特异性基因沉默机制,已被大量研究证实在动物、植物、昆虫和微孢子虫等生物中发挥基因表达调节作用,具有防控病虫害的应用潜力[12-13,44-45]。多数微孢子虫在进化过程已失去RNAi途径,但东方蜜蜂微孢子虫保留了该途径的3个关键基因即、和()的同源序列[46]。HUANG等[18]针对设计特异性siRNA,并混入饲料对东方蜜蜂微孢子虫感染的西方蜜蜂工蜂进行饲喂,发现病原的孢子载量显著降低,并且超过10%的病原蛋白编码基因发生显著性差异表达。本研究发现,在AmCK1 vs AmT1比较组中,宿主的miR-30-x、miR-30-y和miR-196-x显著上调表达且靶向NcCK vs NcT1比较组中病原2个显著下调表达的-1,包括XM_002995496.1(=3.68E-34, log2FC=-2.6)和XM_002996709.1(=3.39E-34, log2FC=-3.0),说明意蜂工蜂中肠可能通过差异表达相应的miRNA对病原的RNAi途径相关的部分mRNA进行表达调控,进而影响东方蜜蜂微孢子虫的RNAi途径。
孢壁蛋白与微孢子虫的孢子发芽与侵染能力密切相关,并通过促使孢子黏附宿主细胞以调节家蚕微孢子虫()的感染过程[47-48]。本研究发现,AmCK2 vs AmT2比较组中宿主的2条显著上调miRNA靶向NcCK vs NcT2比较组中病原显著下调的孢壁蛋白9编码基因(图5),暗示被东方蜜蜂微孢子虫侵染的意蜂工蜂中肠可能通过上调部分miRNA的表达量加强对东方蜜蜂微孢子虫孢壁蛋白编码基因的抑制,从而一定程度上限制病原侵染和增殖。
蓖麻毒素B凝集素是一种通过识别并结合宿主细胞表面多糖蛋白配体和糖脂的半乳糖残基的凝集素蛋白,并促进病原体附着或感染宿主细胞[49-50]。LIU等[51]研究证实家蚕微孢子虫的蓖麻毒素在孢子黏附宿主细胞和提高孢子的感染性等方面扮演重要角色。本研究发现,AmCK1 vs AmT1和AmCK2 vs AmT2比较组中宿主的miR-196-x显著上调且均靶向NcCK vs NcT1和NcCK vs NcT2比较组中下调表达的蓖麻毒素B凝集素编码基因XM_002996297.1(表1、表2),暗示意蜂工蜂中肠在东方蜜蜂微孢子虫侵染的过程中通过上调表达miR-196-x对靶向的XM_002996297.1进行持续抑制,在病原侵染的不同时间点通过选择性差异表达不同的miRNA影响病原的蓖麻毒素B凝集素编码基因的表达,从而阻遏东方蜜蜂微孢子虫对宿主细胞的黏附和侵染。
在长期的协同进化过程中,微孢子虫的线粒体已逐渐退化消失,取而代之的是一种称为纺锤剩体(mitosome)的细胞器[35]。微孢子虫在寄主细胞中生存和增殖需要源源不断的能量和物质供给,既能通过糖酵解/糖异生途径将葡萄糖转化为丙酮酸[52],还可以通过ATP/ADP转位酶和ABC转运蛋白窃取宿主合成的能量和物质供自身能量需求[36]。本研究发现,AmCK1 vs AmT1比较组中宿主的miR-222-y等10条显著上调miRNA靶向NcCK vs NcT1比较组中病原的糖酵解/糖异生途径相关的2条显著下调mRNA(乙酰辅酶A合成酶编码基因XM_002995703.1和磷酸丙糖异构酶编码基因XM_002996794.1)(表1、表2、图4);AmCK2 vs AmT2比较组中宿主的miR-222-y和miR-221-z显著上调表达且均靶向NcCK vs NcT2比较组中病原的糖酵解/糖异生途径相关的显著下调表达的XM_002995703.1(图5)。上述结果表明在东方蜜蜂微孢子虫的侵染过程中,意蜂工蜂中肠可能通过上调miR-222-y和miR-221-z等miRNA的表达量,加强对病原糖酵解/糖异生途径中乙酰辅酶A合成酶编码基因(XM_002995703.1)的抑制,从而限制病原的能量代谢,影响病原的侵染与增殖。
ATP/ADP转位酶和ABC转运蛋白参与微孢子虫对宿主物质和能量的窃取[36]。ABC转运蛋白广泛存在于真核细胞,能够利用ATP的能量对胞内的糖、核苷酸、氨基酸、多肽、蛋白质等进行跨膜转运[53]。PALDI等[36]研究发现通过RNAi敲减东方蜜蜂微孢子虫的ATP/ADP转位酶基因可导致病原的增殖水平下降。本研究发现,AmCK1 vs AmT1比较组中宿主的miR-454-y和miR-144-x显著上调且靶向NcCK vs NcT1比较组中显著下调表达的ATP/ADP转位酶编码基因(XM_002996538.1)(表1、表2、图4);AmCK2 vs AmT2比较组中显著上调表达的miR-1332-y等4条miRNA靶向结合NcCK vs NcT2比较组中显著下调表达的ATP/ADP转位酶编码基因(XM_002996538.1)(图5)。此外,AmCK1 vs AmT1比较组中宿主的miR-16-y等9条显著上调miRNA靶向NcCK vs NcT1比较组中病原的3条显著下调表达的ABC转运蛋白编码基因(XM_002995069.1、XM_002996253.1和XM_002996675.1)(表1、表2、图4);AmCK2 vs AmT2比较组中宿主的6条显著上调miRNA靶向NcCK vs NcT2比较组中病原的2条显著下调表达的ABC转运蛋白编码基因(图5)。有趣的是,宿主的miR-28-y和miR-8212-y在2个比较组中均显著上调表达且均靶向2个比较组中病原的下调表达的ABC转运蛋白编码基因。以上结果说明意蜂工蜂中肠在东方蜜蜂微孢子虫侵染过程的不同时间点差异表达不同的miRNA对ATP/ADP转位酶编码基因进行跨界调控,从而抑制东方蜜蜂微孢子虫对宿主细胞的能量窃取;宿主也可能通过差异表达不同的miRNA、持续差异表达相同的miRNA对病原的ABC转运蛋白编码基因相关mRNA进行抑制,进而限制东方蜜蜂微孢子虫通过转运宿主的营养物质满足增殖所需。
MAPK信号通路与真菌的交配、菌丝侵染、附着胞形成、细胞壁完整性、胁迫反应和毒力等过程密切相关[54]。笔者团队前期研究发现蜜蜂球囊菌()在侵染不同抗性蜜蜂幼虫的过程中,MAPK信号通路及富集基因的活跃程度存在差异,中华蜜蜂()幼虫可能通过抑制该信号通路影响蜜蜂球囊菌的增殖,而蜜蜂球囊菌可能通过激活该通路促进对意蜂幼虫的侵染,体现了二者互作的复杂性[55-56]。本研究发现,AmCK1 vs AmT1比较组中宿主的3条显著上调miRNA(novel-m0007- 5p、miR-29-y和miR-16-y)靶向NcCK vs NcT1比较组中病原MAPK信号通路相关的2条显著下调mRNA(HECTD蛋白编码基因XM_002995842.1和磷脂酰肌醇-4-磷酸-5-激酶编码基因XM_002996061.1)(表1、表2);AmCK2 vs AmT2比较组中宿主显著下调表达的miR-8159-x和miR-316-x共同靶向NcCK vs NcT2比较组中病原显著下调表达的HECTD蛋白编码基因XM_002995842.1。上述结果说明意蜂工蜂中肠在东方蜜蜂微孢子虫侵染过程的不同时间点通过差异表达不同的miRNA抑制病原的MAPK信号通路,从而影响病原在宿主细胞内的环境适应,以及病原的细胞壁完整性和毒力等方面。
4 结论
在东方蜜蜂微孢子虫侵染意蜂工蜂中肠的过程中,宿主DEmiRNA与病原DEmRNA之间存在复杂的靶向结合关系和潜在的调控关系,宿主的DEmiRNA可能通过调控病原的RNAi途径、毒力因子、糖酵解/糖异生途径、ATP/ADP移位酶、ABC转运蛋白及MAPK信号通路相关DEmRNA的表达影响病原的侵染和增殖。
[1] GALLAI N, SALLES J M, SETTELE J, VAISSIERE B E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics, 2009, 68(3): 810-821.
[2] WITTNER M, WEISS L M. The Microsporidia and Microsporidiosis. John Wiley & Sons, Inc., 1999.
[3] MARTÍN-HERNÁNDEZ R, BARTOLOMÉ C, CHEJANOVSKY N, CONTE Y L, DALMON A, DUSSAUBAT C, GARCÍA-PALENCIA P, MEANA A, PINTO M A, SOROKER V, HIGES M.in: a 12 years postdetection perspective. Environmental Microbiology,2018, 20(4): 1302-1329.
[4] MAYACK C, NATSOPOULOU M E, MCMAHON D P.alters a highly conserved hormonal stress pathway in honeybees. Insect Molecular Biology, 2015, 24(6): 662-670.
[5] EVANS J D, HUANG Q. Interactions among host-parasite microRNAs duringproliferation in. Frontiers in Microbiology, 2018, 9: 698.
[6] BARTEL D P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004, 116(2): 281-297.
[7] ZHANG L, HOU D X, CHEN X, LI D H, ZHU L Y, ZHANG Y J, LI J, BIAN Z, LIANG X Y, CAI X,. Exogenous plant miR168a specifically targets mammalian LDLRAP1: evidence of cross- kingdom regulation by microRNA. Cell Research, 2012, 22(1): 107-126.
[8] ZHU K, LIU M H, FU Z, ZHOU Z, KONG Y, LIANG H W, LIN Z G, LUO J, ZHENG H Q, WAN P,. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genetics, 2017, 13(8): e1006946.
[9] CUI C L, WANG Y, LIU J N, ZHAO J, SUN P L, WANG S B. A fungal pathogen deploys a small silencing RNA that attenuates mosquito immunity and facilitates infection. Nature Communications, 2019, 10(1): 4298.
[10] MAYORAL J G, HUSSAIN M, JOUBERT D A, ITURBE-ORMAETXE I, O’NEILL S L, ASGARI S.small noncoding RNAs and their role in cross-kingdom communications. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(52): 18721-18726.
[11] HINAS A, WRIGHT A J, HUNTER C P. SID-5 is an endosome- associated protein required for efficient systemic RNAi in. Current Biology, 2012, 22(20): 1938-1943.
[12] BUCHER G, SCHOLTEN J, KLINGLER M. Parental RNAi in(Coleoptera). Current Biology, 2002, 12(3): R85-R86.
[13] XU H J, CHEN T, MA X F, XUE J, PAN P L, ZHANG X C, CHENG J A, ZHANG C X. Genome-wide screening for components of small interfering RNA (siRNA) and micro-RNA (miRNA) pathways in the brown planthopper,(Hemiptera: Delphacidae). Insect Molecular Biology, 2013, 22(6): 635-647.
[14] CHENG L, SHARPLES R A, SCICLUNA B J, HILL A F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. Journal of Extracellular Vesicles, 2014, 3: 23743.
[15] VAN DER POL E, BOING A N, HARRISON P, STURK A, NIEUWLAND R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews, 2012, 64(3): 676-705.
[16] ZHANG T, ZHAO Y L, ZHAO J H, WANG S, JIN Y, CHEN Z Q, FANG Y Y, HUA C L, DING S W, GUO H S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nature Plants, 2016, 2(10): 16153.
[17] SANNIGRAHI M K, SHARMA R, SINGH V, PANDA N K, RATTAN V, KHULLAR M. Role of host miRNA hsa-miR-139-3p in hpv-16-induced carcinomas. Clinical Cancer Research, 2017, 23(14): 3884-3895.
[18] HUANG Q, LI W, CHEN Y, RETSCHNIG-TANNE G, YANEZ O, NEUMANN P, EVANS J D. Dicer regulatesproliferation in honeybees.Insect Molecular Biology, 2019, 28(1): 74-85.
[19] 付中民, 陈华枝, 刘思亚, 祝智威, 范小雪, 范元婵, 万洁琦, 张璐, 熊翠玲, 徐国钧, 陈大福, 郭睿. 意大利蜜蜂响应东方蜜蜂微孢子虫胁迫的免疫应答. 中国农业科学, 2019, 52(17): 3069-3082.
FU Z M, CHEN H Z, LIU S Y, ZHU Z W, FAN X X, FAN Y C, WAN J Q, ZHANG L, XIONG C L, XU G J, CHEN D F, GUO R. Immune responses oftostress. Scientia Agricultura Sinica, 2019, 52(17): 3069-3082. (in Chinese)
[20] 熊翠玲, 陈华枝, 祝智威, 王杰, 范小雪, 蒋海宾, 范元婵, 万洁琦, 卢家轩, 郑燕珍, 付中民, 徐国钧, 陈大福, 郭睿. 基于small RNA组学分析揭示意大利蜜蜂响应东方蜜蜂微孢子虫胁迫的免疫应答机制. 微生物学报, 2020, 60(7): 1458-1478.
XIONG C L, CHEN H Z, ZHU Z W, WANG J, FAN X X, JIANG H B, FAN Y C, WAN J Q, LU J X, ZHENG Y Z, FU Z M, XU G J, CHEN D F, GUO R. Unraveling the mechanism underlying the immune responses oftostress based on small RNA omics analyses. Acta Microbiologica Sinica, 2020, 60(7): 1458-1478. (in Chinese)
[21] CHEN D F, CHEN H Z, DU Y, ZHOU D D, GENG S H, WANG H P, WAN J Q, XIONG C L, ZHENG Y Z, GUO R. Genome-wide identification of long non-coding RNAs and their regulatory networks involved inresponse toinfection. Insects, 2019, 10(8): 245.
[22] 熊翠玲, 耿四海, 周丁丁, 石彩云, 郭意龙, 陈大福, 郑燕珍, 徐国钧, 张曦, 郭睿. 感染意大利蜜蜂工蜂的东方蜜蜂微孢子虫及其纯化孢子的高表达基因分析. 上海交通大学学报(农业科学版), 2019, 37(2): 6-13.
XIONG C L, GENG S H, ZHOU D D, SHI C Y, GUO Y L, CHEN D F, ZHENG Y Z, XU G J, ZHANG X, GUO R. Analysis of highly expressed genes ininfecting the midguts ofworker and purified fungal spores. Journal of Shanghai Jiaotong University (Agricultural Science), 2019, 37(2): 6-13. (in Chinese)
[23] 周倪红, 王海朋, 周丁丁, 付中民, 祝智威, 范元婵, 张曦, 熊翠玲, 郑燕珍, 陈大福, 郭睿. 意大利蜜蜂工蜂中肠响应东方蜜蜂微孢子虫胁迫的可变剪接基因分析. 福建农林大学学报(自然科学版), 2020, 49(3): 372-379.
ZHOU N H, WANG H P, ZHOU D D, FU Z M, ZHU Z W, FAN Y C, ZHANG X, XIONG C L, ZHENG Y Z, CHEN D F, GUO R. Analysis on the response of alternatively splicing genes inworkers’ midguts tostress. Journal of Fujian Agriculture and Forestry University (Natural Science Edition), 2020, 49(3): 372-379. (in Chinese)
[24] 耿四海, 周丁丁, 范小雪, 蒋海宾, 祝智威, 王杰, 范元婵, 王心蕊, 熊翠玲, 郑燕珍, 付中民, 陈大福, 郭睿. 转录组分析揭示东方蜜蜂微孢子虫侵染意大利蜜蜂的分子机制. 昆虫学报, 2020, 63(3): 294-308.
GENG S H, ZHOU D D, FAN X X, JIANG H B, ZHU Z W, WANG J, FAN Y C, WANG X R, XIONG C L, ZHENG Y Z, FU Z M, CHEN D F, GUO R. Transcriptomic analysis reveals the molecular mechanism underlyinginfection of.Acta Entomologica Sinica, 2020, 63(3): 294-308. (in Chinese)
[25] 耿四海, 石彩云, 范小雪, 王杰, 祝智威, 蒋海宾, 范元婵, 陈华枝, 杜宇, 王心蕊, 熊翠玲, 郑燕珍, 付中民, 陈大福, 郭睿. 微小RNA介导东方蜜蜂微孢子虫侵染意大利蜜蜂工蜂的分子机制. 中国农业科学, 2020, 53(15): 3187-3204.
GENG S H, SHI C Y, FAN X X, WANG J, ZHU Z W, JIANG H B, FAN Y C, CHEN H Z, DU Y, WANG X R, XIONG C L, ZHENG Y Z, FU Z M, CHEN D F, GUO R. The mechanism underlying microRNAs-mediatedinfection toworker. Scientia Agricultura Sinica, 2020, 53(15): 3187-3204. (in Chinese)
[26] 郭睿, 杜宇, 熊翠玲, 郑燕珍, 付中民, 徐国钧, 王海朋, 陈华枝, 耿四海, 周丁丁, 石彩云, 赵红霞, 陈大福. 意大利蜜蜂幼虫肠道发育过程中的差异表达microRNA及其调控网络. 中国农业科学, 2018, 51(21): 4197-4209.
GUO R, DU Y, XIONG C L, ZHENG Y Z, FU Z M, XU G J, WANG H P, CHEN H Z, GENG S H, ZHOU D D, SHI C Y, ZHAO H X, CHEN D F. Differentially expressed microRNA and their regulation networks during the developmental process oflarval gut. Scientia Agricultura Sinica,2018, 51(21): 4197-4209. (in Chinese)
[27] LANGMEAD B, TRAPNELL C, POP M, SALZBERG S L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology, 2009, 10(3): R25.
[28] Friedlander M R, Mackowiak S D, LI N, CHEN W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Research, 2012, 40(1): 37-52.
[29] CHEN H Z, DU Y, XIONG C L, ZHENG Y Z, CHEN D F, GUO R. A comprehensive transcriptome data of normal and- stressed midguts ofworkers.Data in Brief, 2019, 26: 104349.
[30] GUO R, CHEN D F, XIONG C L, HOU C S, ZHENG Y Z, FU Z M, LIANG Q, DIAO Q Y, ZHANG L, WANG H Q, HOU Z X, KUMAR D. First identification of long non-coding RNAs in fungal parasiteApidologie, 2018, 49: 660-670.
[31] ROBINSON M D, MCCARTHY D J, SMYTH G K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 2010, 26(1): 139-140.
[32] ALLEN E, XIE Z X, GUSTAFSON A M, CARRINGTON J C. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell, 2005, 121(2): 207221.
[33] SMOOT M E, ONO K, RUSCHEINSKI J, WANG P L, IDEKER T. Cytoscape 2.8: new features for data integration and network visualization.Bioinformatics, 2011, 27(3): 431432.
[34] HUANG Q, CHEN Y P, WANG R W, CHENG S, EVANS J D. Host-parasite interactions and purifying selection in a microsporidian parasite of honey bees. PLoS One, 2016, 11(2): e0147549.
[35] CORNMAN R S, CHEN Y P, SCHATZ M C, STREET C, ZHAO Y, DESANY B, EGHOLM M, HUTCHISON S, PETTIS J S, LIPKIN W I, EVANS J D. Genomic analyses of the microsporidian, an emergent pathogen of honey bees.PLoS Pathogens, 2009, 5(6): e1000466.
[36] PALDI N, GLICK E, OLIVA M, ZILBERBERG Y, AUBIN L, PETTIS J, CHEN Y P, EVANS J D. Effective gene silencing in a microsporidian parasite associated with honeybee () colony declines. Applied and Environmental Microbiology, 2010, 76(17): 5960-5964.
[37] PELIN A, SELMAN M, ARIS-BROSOU S, FARINELLI L, CORRADI N. Genome analyses suggest the presence of polyploidy and recent human-driven expansions in eight global populations of the honeybee pathogen. Environmental Microbiology, 2015, 17(11): 4443-4458.
[38] RODRÍGUEZ-GARCÍA C, EVANS J D, LI W, BRANCHICCELA B, LI J H, HEERMAN M C, BANMEKE O, ZHAO Y, HAMILTON M, HIGES M, MARTÍN-HERNÁNDEZ R, CHEN Y P. Nosemosis control in European honey bees,, by silencing the gene encodingpolar tube protein 3. Journal of Experimental Biology, 2018, 221(19): jeb184606.
[39] LIU H, LI M, HE X, CAI S, HE X, LU X. Transcriptome sequencing and characterization of ungerminated and germinated spores of. Acta Biochimica et Biophysica Sinica, 2016, 48(3): 246-256.
[40] CAI Y, SHEN J. Modulation of host immune responses toby microRNAs.Parasite Immunology, 2017, 39(2): 12417.
[41] ENTWISTLE L J, WILSON M S. MicroRNA-mediated regulation of immune responses to intestinal helminth infections. Parasite Immunology, 2017, 39(2): e12406.
[42] GARBIAN Y, MAORI E, KALEV H, shafir s, sela i. Bidirectional transfer of RNAi between honey bee and:gene silencing reducespopulation. PLoS Pathogens, 2012, 8(12): e1003035.
[43] VIDAU C, PANEK J, TEXIER C, BIRON D G, BELZUNCES L P, GALL M L, BROUSSARD C, DELBAC F, ALAOUI H E. Differentialproteomic analysis of midguts from-infected honeybees reveals manipulation of key host functionsJournal of invertebrate pathology,2014, 121: 89-96.
[44] FIRE A, XU S, MONTGOMERY M K, KOSTAS S A, DRIVER S E, mello c c. Potent and specific genetic interference by double- stranded RNA inNature, 1998, 391(6669): 806811.
[45] HANNON G J. RNA interference. Nature, 2002, 418(6894): 244-251.
[46] NDIKUMANA S, PELIN A, WILLIOT A, SANDERS J L, KENT M, CORRADI N. Genome analysis of: a microsporidian parasite of Zebrafish (). Journal of Eukaryotic Microbiology, 2017, 64(1): 18-30.
[47] 鲁兴萌, 汪方炜. 家蚕肠球菌对微孢子虫体外发芽的抑制作用. 蚕业科学, 2002, 28(2): 126-128.
LU X M, WANG F W. Inhibition of cultured supernatant of enterococci strains on germination ofspores. Acta Sericologica Sinica, 2002, 28(2): 126-128. (in Chinese)
[48] YANG D L, PAN L X, PENG P, DANG X Q, LI C F, LI T, LONG M X, CHEN J, WU Y J, DU H H,. Interaction between SWP9 and polar tube proteins of the microsporidianand function of SWP9 as a scaffolding protein contribute to polar tube tethering to the spore wall. Infection and Immunity, 2017, 85(3): e00872-16.
[49] WEIS W, BROWN J H, CUSACK S, Paulson J C, Skehel J J, WileyD C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid.Nature, 1988, 333(6172): 426-431.
[50] RUOSLAHTI E, PIERSCHBACHER M D. New perspectives in cell adhesion: RGD and integrins. Science, 1987, 238(4826): 491-497.
[51] LIU H, LI M, CAI S, HE X, SHAO Y, LU X. Ricin-B-lectin enhances microsporidiainfection inN cells from silkworm. Acta Biochimica et Biophysica Sinica, 2016, 48(11): 1050-1057.
[52] 刘天明, 申玉龙, 刘庆军, 刘波. 古菌独特的脱氧酮糖酸(ED)葡萄糖酵解途径. 微生物学报, 2008, 48(8): 1126-1131.
LIU T M, SHEN Y L, LIU Q J, LIU B. The unique Entner-Doudoroff (ED) glycolysis pathway of glucose in Archaea—A review. Acta Microbiologica Sinica, 2008, 48(8): 1126-1131. (in Chinese)
[53] HIGGINS C F. ABC transporters: from microorganisms to man. Annual Review of Cell Biology, 1992, 8: 67-113.
[54] HAMEL L P, NICOLE M C, DUPLESSIS S, ELLIS B E. Mitogen- activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. The Plant Cell, 2012, 24(4): 1327-1351.
[55] 郭睿, 陈大福, 黄枳腱, 梁勤, 熊翠玲, 徐细建, 郑燕珍, 张曌楠, 解彦玲, 童新宇, 侯志贤, 江亮亮, 刀晨. 球囊菌胁迫中华蜜蜂幼虫肠道过程中病原的转录组学研究. 微生物学报, 2017, 57(12): 1865-1878.
GUO R, CHEN D F, HUANG Z J, LIANG Q, XIONG C L, XU X J, ZHENG Y Z, ZHANG Z N, XIE Y L, TONG X Y, HOU Z X, JIANG L L, DAO C. Transcriptome analysis ofstressing larval gut of. Acta Microbiologica Sinica, 2017, 57(12): 1865-1878. (in Chinese)
[56] 陈大福, 郭睿, 熊翠玲, 梁勤, 郑燕珍, 徐细建, 黄枳腱, 张曌楠, 张璐, 李汶东, 童新宇, 席伟军. 胁迫意大利蜜蜂幼虫肠道的球囊菌的转录组分析. 昆虫学报, 2017, 60(4): 401-411.
CHEN D F, GUO R, XIONG C L, LIANG Q, ZHENG Y Z, XU X J, HUANG Z J, ZHANG Z N, ZHANG L, LI W D, TONG X Y, XI W J. Transcriptomic analysis ofstressing larval gut of(Hyemenoptera: Apidae). Acta Entomologica Sinica, 2017, 60(4): 401-411. (in Chinese)
MicroRNA-mediated Cross-kingdom Regulation ofworker to
DU Yu1, FAN XiaoXue1, JIANG HaiBin1, WANG Jie1, FENG RuiRong1, ZHANG WenDe1, YU KeJun1, LONG Qi1, CAI ZongBing1, XIONG CuiLing1, ZHENG YanZhen1,2, CHEN DaFu1,2, FU ZhongMin1,2, XU GuoJun1,2, GUO Rui1,2
1College of Animal Sciences (College of Bee Science), Fujian Agriculture and Forestry University, Fuzhou 350002;2Apitherapy Research Institute, Fujian Agriculture and Forestry University, Fuzhou 350002
【】infectsand causes microsporidiosis. In this study, to reveal the mechanism of miRNA-mediated cross-kingdom regulation ofworker to, prediction, GO and KEGG database annotation as well as regulatory network analysis ofmRNAs and differentially expressed mRNAs (DEmRNAs) targeted by differentially expressed miRNAs (DEmiRNAs) ofworkers’ midguts were conducted by bioinformatic approaches based on previously gained miRNA and mRNA omics data【】Significant host DEmiRNAs were screened out by comparison of miRNA omics data fromworkers’ midguts at 7 d and 10 d postinfection (AmT1, AmT2) and corresponding uninfected midguts (AmCK1, AmCK2). DEmRNAs of pathogen were screened out through comparison of mRNA omics data frominfectingworker’s midgut (NcT1 and NcT2) and pure fungal spores (NcCK). mRNAs and DEmRNAs oftargeted by significant host DEmiRNAs were predicted using TargetFinder software. GO and KEGG database annotations of aforementioned targets were conducted using related bioinformatics tools. On basis of our previous findings, pathogen DEmRNAs associated with spore wall protein, polar tube protein, ricin B lectin, ATP/ADP translocase, ABC transporters and glycolysis/gluconeogenesis, and their target significant DEmiRNAs of host were filtered out, followed by construction and investigation of regulatory network.【】In AmCK1 vs AmT1 comparison group, 48 significantly up-regulated miRNAs and 36 significantly down-regulated miRNAs could respectively target 1 345 and 1 046 mRNAs of; additionally, 47 significantly up-regulated miRNAs and 34 significantly down-regulated miRNAs of host could target 584 significantly down-regulated mRNAs and 265 significantly up-regulated mRNAs in NcCK vs NcT1; these targets were involved in 19 and 22 functional terms as well as 66 and 64 pathways. In AmCK2 vs AmT2 comparison group, 56 significantly up-regulated miRNAs and 51 significantly down-regulated miRNAs could respectively target 1 260 and 1 317 mRNAs of, additionally, 52 significantly up-regulated miRNAs and 49 significantly down-regulated miRNAs could target 587 significantly down-regulated mRNAs and 336 significantly up-regulated mRNAs in NcCK vs NcT2, which were engaged in 20 and 23 functional terms as well as 64 and 65 pathways. Further, eight common significantly up-regulated miRNAs and one common significantly down-regulated miRNA in AmCK1 vs AmT1 and AmCK2 vs AmT2 comparison groups could respectively target 144 common significantly down-regulated mRNAs and 10 common significantly up-regulated mRNAs in NcCK vs NcT1 and NcCK vs NcT2 comparison groups, which could be annotated to 18 and 13 functional terms as well as38 and seven pathways. Moreover,host significantly up-regulated miRNAs in AmCK1 vs AmT1 and AmCK2 vs AmT2 could target pathogen significantly down-regulated mRNAs in NcCK vs NcT1 and NcCK vs NcT2, associated with RNAi, virulence factors such as polar tube protein, spore wall protein and ricin B lectin, glycolysis/gluconeogenesis and MAPK signal pathway.【】Complex target binding relationship and potential cross-kingdom regulatory relationship exist between host DEmiRNAs and pathogen DEmRNAs during the infection ofworker with; host DEmiRNAs are likely to inhibit or degrade pathogen DEmRNAs associated with RNAi, virulence factor/infection factor, glycolysis/gluconeogenesis pathway, ATP/ADP translocase, ABC transporters, and MAPK signal pathway to affectinfection and proliferation.
;;microRNA; cross-kingdom regulation; regulatory network; immune defense

10.3864/j.issn.0578-1752.2021.08.019
2020-06-02;
2020-06-22
国家现代农业产业技术体系建设专项(CARS-44-KXJ7)、福建农林大学杰出青年科研人才计划(xjq201814)、福建农林大学科技创新专项(CXZX2017342,CXZX2017343)、福建农林大学优秀硕士学位论文资助基金(杜宇)、福建省大学生创新创业训练计划(202010389016,202010389158)
杜宇,E-mail:m18505700830@163.com。范小雪,E-mail:imfanxx@163.com。杜宇和范小雪为同等贡献作者。通信作者郭睿,E-mail:ruiguo@fafu.edu.cn
(责任编辑 岳梅)

