Effects of electroacupuncture pretreatment on M1 polarization of alveolar macrophages in rats with acute lung injury
ZHANG Yi (张毅), SU Jingchao (苏景超), CHENG Chen (程晨), WANG Caiyun (王彩云), MIAO Qing (苗青),ZHANG Jingtao (张景涛), ZHANG Xinfang (张新芳), XIANG Shuiying (项水英), LIU Zibing (刘自兵)
1 Graduate School, Anhui University of Chinese Medicine, Hefei 230012, China
2 Jingxian County Hospital of Traditional Chinese Medicine, Jingxian 242500, China
3 School of Integrated Traditional Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei 230012, China
4 Institute of Acupuncture and Meridian, College of Acupuncture-moxibustion and Tuina, Anhui University of Chinese Medicine, Hefei 230012, China
Abstract
Keywords: Acupuncture Therapy; Electroacupuncture; Acute Lung Injury; Macrophages, Alveolar; Tumor Necrosis Factoralpha; NF-kappa B; Signal Transduction; Rats
Acute lung injury (ALI) is due to the accumulation of inflammation and protein exudates in the lung tissue caused by pathogenic factors inside and outside the lung,and the decrease of blood oxygen binding capacity is a critical symptom, which can develop into acute respiratory distress syndrome (ARDS)[1]. There are many kinds of immune cells involved in the pathological changes of ALI, including macrophages, neutrophils, Tlymphocytes, etc. Studies have shown that alveolar macrophages (AMs) play an important role in ALI[2].Macrophages are highly plastic and can be polarized into M1 phenotype with pro-inflammatory properties and M2 phenotype with anti-inflammatory properties after external stimulation, so as to maintain the homeostasis of lung environment[3-4]. Acupuncture can reduce the degree of lung injury in patients with ALI, as it can increase oxygenation index, down-regulate pulmonary inflammatory factors such as tumor necrosis factor(TNF)-α, and up-regulate the expression of interleukin(IL)-10, thus reducing inflammatory reaction and improving lung function[5]. Electroacupuncture (EA) can down-regulate the expression of M1 macrophages, thus improving chronic diseases such as chronic obstructive pulmonary disease[6], spinal cord injury[7], ischemic stroke[8]. However, there are few reports about acupuncture alleviating lung injury of ALI by regulating the polarization of M1 macrophages. In ALI, Toll-like receptor (TLR) 4/nuclear factor (NF)-κB p65 signaling pathway is activated, which polarizes AM towards M1[9-11]. Therefore, it is important for ALI to reduce lung injury by inhibiting TLR4/NF-κB p65 signaling pathway to prevent AMs from polarizing to M1[12-13]. The purpose of this study is to investigate whether EA pretreatment can inhibit the polarization of AMs to M1 in ALI rats through TLR4/NF-κB p65 signaling pathway, reduce the inflammatory reaction, and exert a protective effect on the lung.
1 Materials and Methods
1.1 Experimental animals and grouping
Forty 8-week-old adult specific-pathogen-free male Sprague-Dawley rats, weighing (230±20) g, were provided by Jinan Pengyue Experimental Animal Breeding Co., Ltd., China [License No. SCXK (Lu)20190003]. Rats were raised in separate cages with free drinking and eating, under the controlled environment with humidity of 60.0%-70.0% and temperature at 25 ℃.After one-week adaptive feeding, the rats were randomly divided into a normal group, a model group and three groups of EA pretreatment [a Chize (LU5)group, a Zusanli (ST36) group and a Chize (LU5) plus Zusanli (ST36) group], with eight rats in each group. The rats in the normal group did not receive any treatment,and the treatment of animals during the experiment was in line with theGuiding Opinions on the Treatment of Experimental Animalsissued by the Ministry of Science and Technology of the People’s Republic of China in September 2006, and this study was approved by the Animal Ethics Committee of Anhui University of Chinese Medicine (Ethical Review No. AHUM-rats-2020009).
1.2 Main reagents
Lipopolysaccharide (LPS, Article No. L2880, Sigma Co.,Ltd., USA); TNF-α, IL-1β, myeloperoxidase (MPO)enzyme-linked immunosorbent assay (ELISA) kit (Wuhan Genmei Science and Technology Co., Ltd., China); rabbit anti-rat clusters of differentiation 86 (CD86) first antibody (Batch No. AF07132755p, Beijing Biosynthesis Biotechnology Co., Ltd., China); mouse anti-rat inducible nitric oxide synthase (iNOS) first antibody (Batch No.GR3183074-12, Abcam Co., Ltd., UK); rabbit anti-rat TLR4 first antibody (Batch No. CN89330, Bioword Co., Ltd.,USA); rabbit anti-rat NF-κB p65 first antibody (Batch No.8242T, CST Co., Ltd., USA); fluorescent quantitative polymerase chain reaction (PCR) kit (Batch No. E096-01B,Suzhou Novoprotein Scientific Inc., China); reverse transcription kit (Batch No. RR047A, Thermo Scientific Co., Ltd., USA); PCR primers [Batch No. 111781060,Sangon Biotech (Shanghai) Co., Ltd., China].
1.3 Main instruments
Hwato brand acupuncture needles (0.30 mm in diameter and 25 mm in length, Suzhou Tianxie Acupuncture Equipment Co., Ltd., China); SDZ-V-type multi-function EA instrument (Suzhou Medical Appliance Factory, China); ani-Res2005 small animal respiratory function tester (Beijing Bellambo Technology Co., Ltd.,China); K 8160 gel imaging system (Beijing Kechuang Ruixin Biotechnology Co., Ltd., China); Image J biomedical image analysis software (National Institutes of Health, USA); PCR instrument and the software(Thermo Scientific Co., Ltd., USA).
1.4 EA pretreatment
Acupoints: Bilateral Chize (LU5) and Zusanli (ST36).
Methods:According to theExperimental Acupuncture Science[14], the acupoints of Chize (LU5) and Zusanli (ST36)were located. Chize (LU5) is located at the lateral depression of the transverse elbow striation of the forelimb and punctured perpendicularly for 3 mm.Zusanli (ST36) is located between the tibia and fibula approximately 5 mm lateral to the anterior tubercle of the tibia, and punctured perpendicularly for 5 mm. The needle handles were connected to the EA instrument.The EA treatment was performed by a well-trained acupuncturist. According to the previous studies[15-16], EA was applied for 30 min every day for a total of 6 d, with an alternating frequency of 2 Hz/15 Hz, and a current of 1-2 mA. After EA, ALI was induced by LPS on the seventh day in all EA pretreatment groups as well as in the model group.
1.5 Establishment of animal models
The ALI rat model was established by LPS tracheal perfusion method[17], with 3% pentobarbital sodium[1 mL/(kg·bw)] for anesthesia, and fixed on the rat fixator.After the trachea was exposed along the neck midline,the rat tracheas were perfused with LPS by syringe[2 mg/(kg·bw)], and then the incision was sutured. The pulmonary function testing and specimen collection were conducted 3 h later.
1.6 Test items and methods
1.6.1 Pulmonary function test
The rats were anesthetized and intubated after tracheotomy to keep the airway unobstructed. The rats were placed in a small animal respiratory function tester in a supine position, connected to a ventilator and a signal conditioner through a catheter. With the pulmonary function testing software, all rats were measured for the forced vital capacity (FVC), forced expiratory volume in 0.1 s (FEV0.1), the ratio of FEV0.1 to FVC (FEV0.1/FVC), forced expiratory volume in 0.3 s(FEV0.3), and the ratio of FEV0.3 to FVC (FEV0.3/FVC).
1.6.2 Ratio of the lung wet weight to dry weight
After the rats were sacrificed by bleeding from the abdominal aorta, the left lung was taken, placed on filter paper to remove excess water, and weighed to obtain the wet weight (W). Then the lung tissue together with filter paper was put into a constant temperature drying box at 60 ℃. After 48 h of drying, the left lung was taken out together with the filter paper, weighed and obtained the dry weight (D). The W/D was calculated to evaluate pulmonary edema.
1.6.3 Hematoxylin-eosin (HE) staining and lung injury score
The middle lobe of the right lung was fixed with 4%paraformaldehyde solution, embedded in paraffin,sectioned at 4 μm, dewaxed with xylene, and stained with HE. Three visual fields were randomly selected from each specimen under a light microscope, and the pathological changes and lung injury score were observed with a magnification of 200 times[18], including alveolar wall thickness, lung tissue destruction, and inflammatory cell infiltration. Each sample was scored on a five-point scale: 0 point as minimal injury, 1 point as mild injury, 2 points as moderate injury, 3 points as severe injury, and 4 points as maximum injury. The lung injury score was the average score of the three items.
1.6.4 Extraction of AMs from rats
After thoracotomy, the mediastinum was separated,and the right common bronchus was ligated. Normal saline of 5 mL was used for the lavage of the left lung three times. The left main bronchoalveolar lavage fluid(BALF) was extracted and centrifuged at 1 500 r/min for 5 min, and the supernatant was obtained. The cell sediment was rinsed with PBS, centrifuged, added with RPMI1640 medium, diluted to 1×106cells/mL,transferred to a 96-well plate, and incubated at 37 ℃with 5% CO2incubator for 2 h. AMs were obtained after the plate was rinsed with warm RPMI16 medium for one or two times, the non-adherent cells were then removed,and the adherent cells were collected.
1.6.5 ELISA
The contents of TNF-α, IL-1β, and MPO in the BALF of rats were detected by the double-antibody sandwich ELISA method. The sample was detected strictly according to the instructions of ELISA kits. The optical density (OD) value was determined by an enzymelabeling instrument, and the concentration of the actual sample was calculated.
1.6.6 Real-time quantitative PCR (RT-qPCR)
This method was used to detect the mRNA expression levels of CD86, iNOS, TLR4, and NF-κB p65 in the alveoli.The suspension of alveoli was cleaved by 1 mL trizol and the total RNA was extracted. According to the instructions of the kit, those mRNAs were reversely transcribed into cDNA. β-actin was used as the internal reference to detect CD86, iNOS, TLR4, and NF-κB p65 by RT-qPCR. All primers were determined for RT-qPCR assay(Table 1). The PCR reaction conditions were designed as follows. Pre-denaturation at 95 ℃ for 1 min, 95 ℃ for 20 s, 60 ℃ for 1 min, for a total of 40 cycles. The relative expression levels of CD86, iNOS, TLR4, and NF-κB p65 were calculated by 2-ΔΔCtmethod.
1.6.7 Western blot (WB)
The protein expression levels of CD86, iNOS, TLR4, and NF-κB p65 in the alveoli were detected by WB. The collected alveoli were cleaved with 1 mL radio immunoprecipitation assay (RIPA) lysate, and the total protein was obtained after centrifugation. The buffer of 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was added to the protein samples at 1:4. Heated in boiling water for 10 min, the protein was fully denatured. Cooled at room temperature, the protein samples were put into the hole of SDS-PAGE glue, with 10 μL in each hole. The proteins were separated by gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane, with the glue concentrated at 80 V for 30 min, and the glue separated at 120 V for 1 h. After the transfer of the membrane,Western blocking solution was added and sealed at room temperature for 2 h. According to the instructions, the primary antibodies were added at an appropriate proportion of dilution [CD86 (1:1 000), iNOS (1:1 000),TLR4 (1:500), NF-κB p65 (1:1 000)], shaken slowly and incubated overnight at 4 ℃. The second antibodies were added at 1:20 000 and incubated at room temperature for 1.2 h. The images were collected with electro-chemi-luminescence (ECL) photoluminescence solution, with β-actin used as the internal reference, and the relative expression of the target protein was determined by the ratio of the gray value of the target protein to the internal reference.
1.7 Statistical analysis
All data were analyzed by SPSS version 23.0 statistical software, expressed as mean ± standard deviation(±s). The one-way analysis of variance was used for the comparisons among multiple groups, and the least significant difference test was used for the comparisons between two groups.P<0.05 indicated statistically significant.
2 Results
2.1 Comparison of the pulmonary function
There was no significant difference in the FVC among all groups (P>0.05). Compared with the normal group,the FEV0.1, FEV0.1/FVC, FEV0.3, and FEV0.3/FVC decreased significantly in the model group (P<0.01).Compared with the model group, the levels of FEV0.1,FEV0.1/FVC, FEV0.3, and FEV0.3/FVC in the Chize (LU5)group, Zusanli (ST36) group, and Chize (LU5) plus Zusanli(ST36) group were significantly higher (P<0.01). There were no significant differences in all indicators among the Chize (LU5) group, Zusanli (ST36) group, and Chize(LU5) plus Zusanli (ST36) group (P>0.05), (Figure 1 and Figure 2).

Table 1. Primer sequences in the PCR reaction
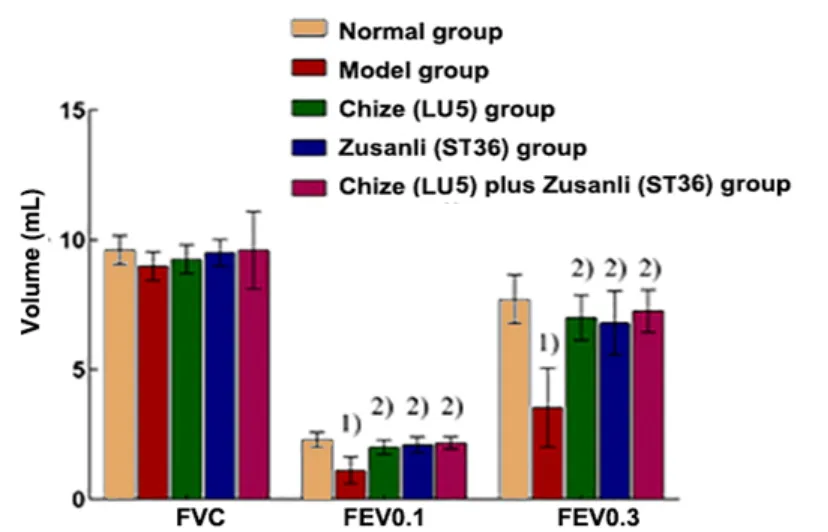
Figure 1. Pulmonary function in different groups (n=8)
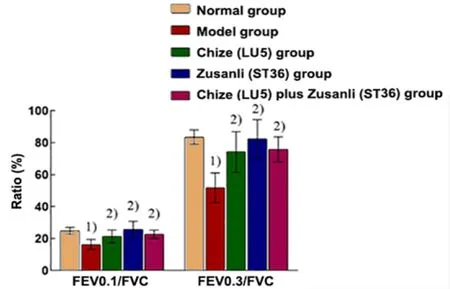
Figure 2. Ratios of pulmonary function in different groups (n=8)
2.2 Comparison of W/D
Compared with the normal group, the W/D was significantly increased in the model group (P<0.01). The W/D in the Chize (LU5) group, Zusanli (ST36) group, and Chize (LU5) plus Zusanli (ST36) group was significantly lower than that in the model group (P<0.05 orP<0.01).There was no significant difference among the Chize (LU5)group, Zusanli (ST36) group, and Chize (LU5) plus Zusanli(ST36) group (P>0.05), (Figure 3).
2.3 Comparisons of the lung pathology and lung injury score
HE staining showed that in the normal group, the alveoli were continuous and uniform in size, and there was no obvious inflammatory cell infiltration. But in the model group, there were obvious edema in parenchyma,significant thickening of alveolar septum, exudation of many red blood cells, and obvious infiltration of inflammatory cells. The thickening of alveolar septum was slightly alleviated, and there was still significant inflammatory cell infiltration in the Chize (LU5) group.The alveolar septum became thinner and more continuous, and a small number of inflammatory cells were gathered in the Zusanli (ST36) group and Chize (LU5)plus Zusanli (ST36) group (Figure 4).
The lung injury score in the model group was higher than that in the normal group (P<0.01), and the lung injury score in the Chize (LU5) group, Zusanli (ST36)group, and Chize (LU5) plus Zusanli (ST36) group was significantly lower than that in the model group (P<0.01).The score in the Chize (LU5) plus Zusanli (ST36) group was higher than that of the other two EA pretreatment groups (P<0.01), (Figure 5).
2.4 Comparisons of the TNF-α, IL-1 β and MPO contents in the BALF
The contents of TNF-α, IL-1β, and MPO in the model group were higher than those in the normal group(P<0.01). These factors in the Chize (LU5) group, Zusanli(ST36) group and Chize (LU5) plus Zusanli (ST36) group were significantly lower than those in the model group(P<0.01). The contents in the Zusanli (ST36) group and Chize (LU5) plus Zusanli (ST36) group were significantly lower than those in the Chize (LU5) group (P<0.01).Compared with the Zusanli (ST36) group, the contents in the Chize (LU5) plus Zusanli (ST36) group were significantly decreased (P<0.01), (Figure 6-Figure 8).
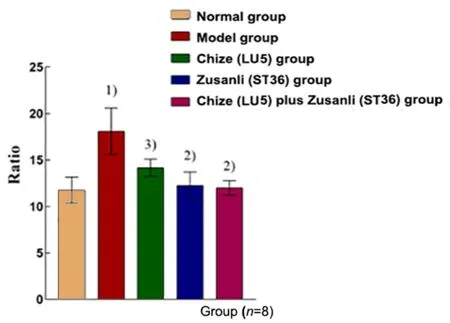
Figure 3. The ratio of the wet lung weight to dry weight in different groups
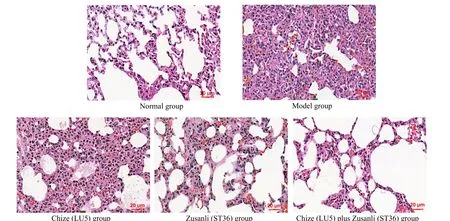
Figure 4. Lung pathology in different groups (HE staining, ×200)
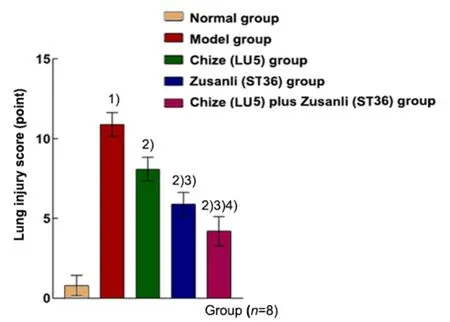
Figure 5. Lung injury score in different groups
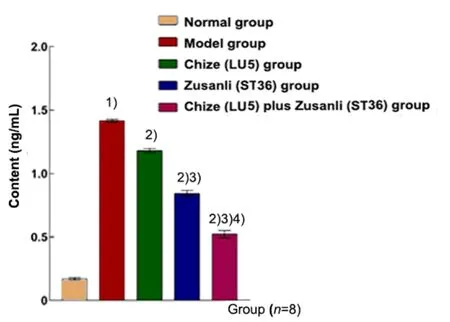
Figure 6. Content of TNF-α in the BALF in different groups
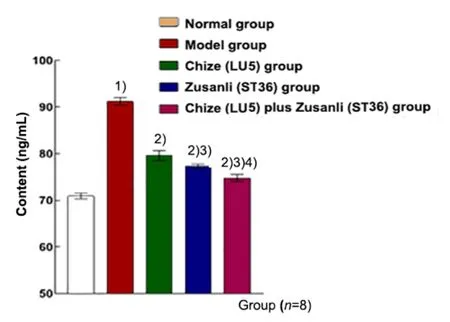
Figure 7. Content of IL-1β in the BALF in different groups
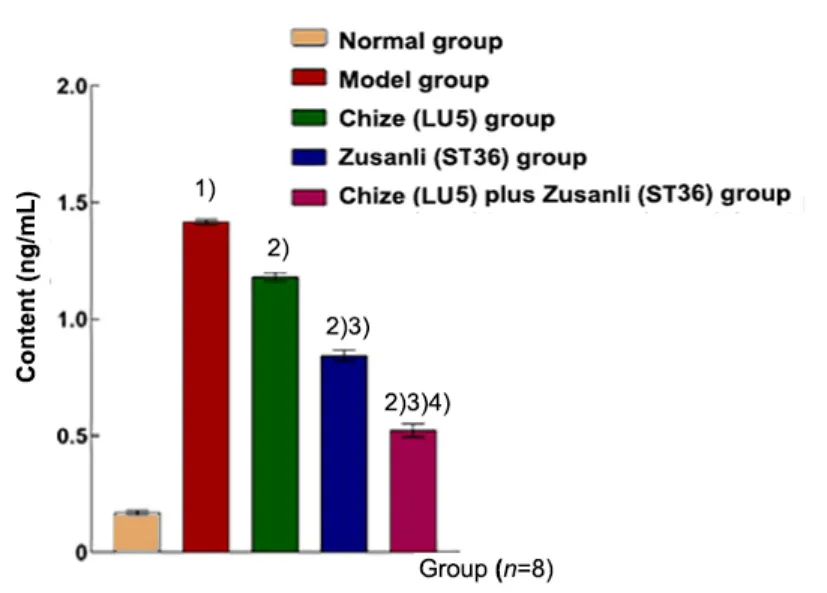
Figure 8. Content of MPO in the BALF in different groups
2.5 Comparisons of the CD86, iNOS, TLR4 and NF-κB p65 mRNA expression levels
Compared with the normal group, the mRNA expression levels of CD86, iNOS, TLR4, and NF-κB p65 in the alveoli were significantly increased in the model group (P<0.01). All the mRNA expression levels in the Chize (LU5) group, Zusanli (ST36) group, and Chize (LU5)plus Zusanli (ST36) group were lower than those in the model group (P<0.01). The mRNA expression level of iNOS in the Zusanli (ST36) group was lower than that in the Chize (LU5) group (P<0.05). All the mRNA expression levels in the Chize (LU5) plus Zusanli (ST36) group were significantly lower than those in the Chize (LU5) group and the Zusanli (ST36) group (P<0.05 orP<0.01),(Table 2).
2.6 Comparisons of the CD86, iNOS, TLR4, and NF-κB p65 protein expression levels
The relative expression levels of CD86, iNOS, TLR4, and NF-κB p65 proteins in the alveoli in the model group were significantly higher than those in the normal group(P<0.01). Compared with the model group, the relative expression levels of CD86, iNOS, TLR4, and NF-κB p65 proteins in the Chize (LU5) group, Zusanli (ST36) group,and Chize (LU5) plus Zusanli (ST36) group were significantly decreased (P<0.05 orP<0.01). There was no significant difference among the Chize (LU5) group,Zusanli (ST36) group, and Chize (LU5) plus Zusanli (ST36)group (P>0.05). There was no significant difference between the Chize (LU5) plus Zusanli (ST36) group and the Zusanli (ST36) group (P>0.05), (Figure 9).
3 Discussion
ALI is a common clinical disease, the main causes of which include infection, shock, trauma, postoperative immune stress, etc. Pathologically, it is characterized by increased pulmonary inflammation and diffuse pulmonary edema[19].
Table 2. The expression levels of CD86, iNOS, TLR4, and NF-κB p65 mRNAs in the alveoli of rats in different groups ( ±s)
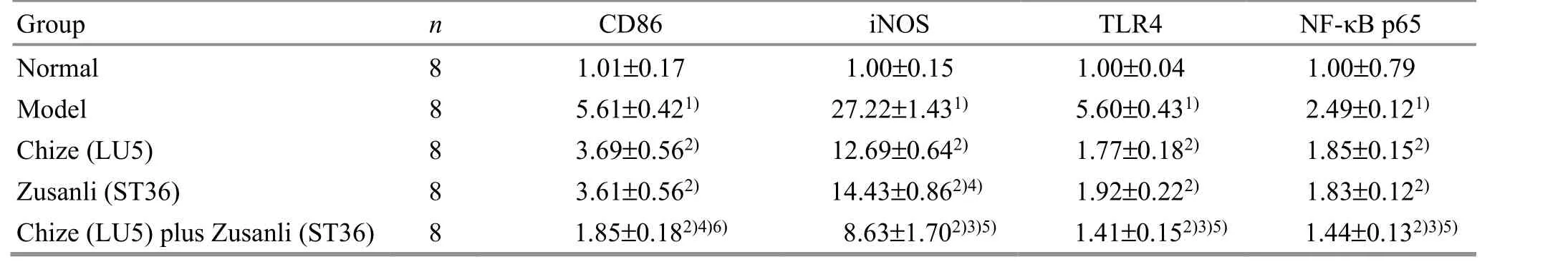
Table 2. The expression levels of CD86, iNOS, TLR4, and NF-κB p65 mRNAs in the alveoli of rats in different groups ( ±s)
Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01; compared with the Chize (LU5) group, 3)P<0.01, 4) P<0.05; compared with the Zusanli (ST36) group, 5) P<0.01, 6) P<0.05
TLR4 NF-κB p65 1.00±0.04 1.00±0.79 5.60±0.431) 2.49±0.121)1.77±0.182) 1.85±0.152)1.92±0.222) 1.83±0.122)1.41±0.152)3)5) 1.44±0.132)3)5)
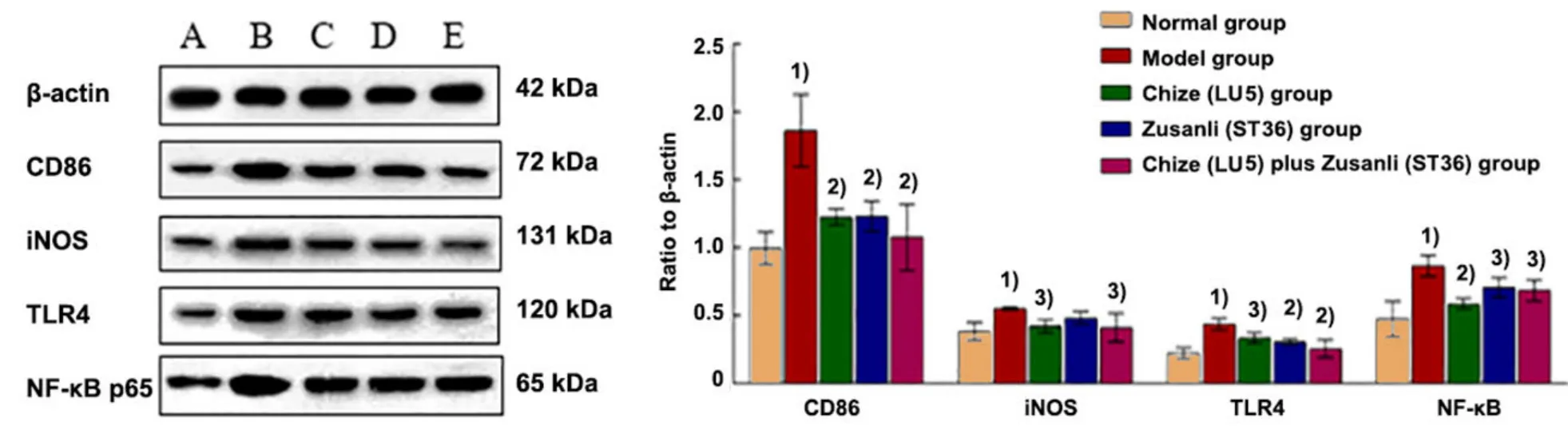
Figure 9. Comparison of the relative expression levels of CD86, iNOS, TLR4, and NF-κB p65 proteins in the alveoli of rats in different groups
As a traditional Chinese treatment method,acupuncture has the functions of tonifying the deficiency,reducing the excess, and improving body immunity[20].Clinical studies have shown that in patients with ALI caused by pancreatitis, acupuncture at Lieque (LU7),Chize (LU5) and Zusanli (ST36) on the basis of routine treatment can further increase the oxygenation index and reduce pulmonary inflammation[5]. EA pretreatment can decrease the contents of TNF-α and IL-1β in the serum of patients undergoing thoracic surgery, increase the content of IL-10, and reduce postoperative inflammatory reactions[21]. Perioperative EA preintervention in patients undergoing video-assisted thoracoscopic surgery can improve lung oxygenation and play a positive role in protecting the lung[22]. Animal experiments have confirmed that EA at Feishu (BL13)and Zusanli (ST36) can greatly improve the survival rate of ALI rabbits with LPS-induced endotoxic shock, downregulate the expression of pro-inflammatory factors and prevent the formation of pulmonary edema[23]. In ALI rats with sepsis induced by burns, EA at Zusanli (ST36)can inhibit neutrophil aggregation in the lungs and exert an effect of antioxidant stress[24]. The above clinical and experimental reports show that acupuncture and acupuncture pretreatment can be used for the prevention and treatment of ALI. The name of ALI has not been recorded in traditional Chinese medicine (TCM)documents, but the status of ALI patients has been described in detail.Huang Di Nei Jing(Yellow Emperor’sClassic of Internal Medicine) records some acute lung diseases such as serious coughs, gasps, spitting blood and wheezing. According to the main clinical manifestations of ALI, such as wheezing, mouth opening and shoulder lifting, nasal wing incitement and dyspnea,it should be considered as “sudden wheezing”,“wheezing”, “chest Bi-Impediment” or other categories of TCM[25]. According to TCM, the pathogenesis of ALI is mainly that the exogenous pathogens gradually injure the healthy Qi of the human body and the body lacks healthy Qi. Deficiency of Qi eventually destroys the human body. Many scholars have discussed that the TCM etiology and pathogenesis of ALI are related to heat,toxin, phlegm, and blood stasis[26-27]. Chize (LU5) and Zusanli (ST36) are the commonly used acupoints for clinical treatment of lung diseases[28-29]. Chize (LU5) can regulate lung Qi and is often used in the treatment of cough, asthma, etc. Zusanli (ST36) has the function of regulating and tonifying lung Qi, dispelling exogenous pathogens and strengthening the body’s immunity.Acupuncture at these two acupoints at the same time has the effect of benign two-way regulation of respiratory function. It has been reported that acupuncture at Chize (LU5) and Zusanli (ST36) in patients with ALI can regulate the balance of pro-inflammatory/anti-inflammatory factors and improve pulmonary ventilation function[30]. The previous studies of our group showed that EA at Zusanli (ST36) in ALI rats can effectively inhibit the expression of pro-inflammatory factors TNF-α and IL-1β, increase the expression of aquaporin 5, improve the metabolic ability of lung fluid,and reduce the degree of pulmonary edema[16]. In the lungs of ALI patients, there are a large number of M1 AMs playing a pro-inflammatory role, and the polarization of AMs to M1 is closely related to the prognosis of ALI[31]. Clinical research has shown that acupuncture can improve pulmonary ventilation and inhibit inflammation in patients with ALI[30]. This study assumes that acupuncture has an anti-inflammatory effect on ALI, and aims to discover whether it can inhibit the polarization of AMs to M1 by down-regulating the TLR4/NF-κB signaling pathway. Therefore, in this experiment, we selected Chize (LU5) and Zusanli (ST36)to pretreat ALI rats to explore the possible mechanism.
As the main component of endotoxin, LPS is the main pathogenic factor of ALI[32]. In order to study whether acupuncture pretreatment can alleviate ALI, we made ALI rat models by tracheal instillation of LPS[17]. After endotracheal instillation of LPS, the pulmonary function of rats was significantly weakened, and the pathological sections showed the characteristics of lung injuries,including the thickening of alveolar septum, interstitial edema, infiltration of many red blood cells and neutrophils in the alveoli, and a great increase in lung W/D. The results showed that the pulmonary edema in rats was serious. EA pretreatment significantly improved pulmonary function and alleviated the above pathological changes and lung injury score. The detection of inflammatory response in BALF showed that the content of neutrophil marker MPO was significantly increased, indicating that neutrophils gather in the lung,and the contents of inflammatory factors TNF-α and IL-1β increased significantly, indicating severe inflammatory reactions in the lung. EA pretreatment can significantly inhibit inflammatory reactions. The above indicators are based on the evaluation criteria of the 2011 official report of the American Thoracic Society on ALI in animals[33].
The most prominent pathological change of ALI is the acute inflammatory reaction of the lung. Inflammatory factors are mainly released by pulmonary inflammatory cells, taking part in and causing inflammatory response.TNF-α, IL-1β, and MPO are important inflammatory markers involved in the inflammatory response of ALI[34-35], and their contents are increased in the serum and lung tissue of patients with ALI[36-37]. They jointly participate in the formation of pulmonary edema and decrease the efficiency of pulmonary ventilation in ALI[31].As immune cells secrete these inflammatory factors,AMs participate in and play an important role[38-39].When stimulated by different cytokines, it can polarize into M1 (pro-inflammatory) macrophages, secreting TNF-α, iNOS, IL-8, etc., or M2 (anti-inflammatory)macrophages, secreting transforming growth factor-β,IL-10, and vascular endothelial growth factor[40-41]. M1 cell surface is characterized by high expression levels of CD86 and iNOS. The pathophysiological process of ALI is closely related to the immune response. The increased M1 macrophages further aggravate the inflammatory reactions in ALI[42]. It has been reported that LPS-induced ALI mouse model shows a significant increase in the expression of M1 macrophage marker iNOS[43]. Clinical report also shows that there are a large number of M1 macrophages in the BALF of early ARDS patients[31]. It has been found that AMs also play an important role in the migration of neutrophils to the lungs. With the increase of foreign antigens, the chemokines human macrophage chemoattractant protein-1 and macrophage inflammatory protein-2, secreted by M1, recruit a large number of monocytes and neutrophils from the systemic circulation into the lungs, aggravating and maintaining the inflammatory response, which is consistent with the increase of MPO content observed in the BALF[44]. It indicates that M1 macrophages are important immune cells involved in immune stress in ALI.
NF-κB inflammatory signaling pathway, under the action of endotoxin, binds to the specific recognition receptor TLR4 on the cell membrane. Through transmembrane transport, NF-κB phosphate dissociates into the nucleus and polarizes AMs to M1[45]. Animal experiment has confirmed that LPS can directly increase the expression of TLR4 and NF-κB in the lung tissue[46].When LPS binds to TLR4 to activate NF-κB signaling pathway and the related transcription factors, AMs are induced to polarize to M1. At present, it is known that there are five subunits of NF-κB, among which p65 and p50 are most closely related to macrophage polarization.P65 is involved in inducing macrophages to M1 polarization, while p50 is involved in inducing macrophages to M2 polarization[47]. Some scholars have pointed out that the inhibition of NF-κB p65 phosphorylation can reduce the polarization of AMs to M1[48]. NF-κB p65 is the main transcription factor in M1 polarization. Inhibition of TLR4/NF-κB p65 signaling pathway can inhibit the polarization of AMs to M1. The results have shown that activating the expression of TLR4/NF-κB p65 pathway proteins after intratracheal instillation of LPS can promote the polarization of AMs to M1.
Our previous work found that EA can down-regulate M1 polarization and inhibit the inflammatory response in rats with chronic obstructive pulmonary disease[6]. We found that after EA pretreatment, the inflammatory response and pulmonary ventilation function in the lung tissue of ALI rats were significantly improved, the contents of cytokines (TNF-α, IL-1β) and MPO in the BALF were significantly decreased, and the expression levels of M1 macrophage markers (CD86, iNOS) and their signaling pathway factors (TLR4, NF-κB p65) in the alveoli were decreased. The results suggest that EA pretreatment at Chize (LU5) and Zusanli (ST36) can reduce the inflammatory reaction of ALI and protect the lung, and the mechanism may be related to the downregulation of TLR4/NF-κB signaling pathway and inhibition of M1 polarization. The combination of Chize(LU5) and Zusanli (ST36) produces more significant effects than using these acupoints separately.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (国家自然科学基金项目, No.81373743); Project of Anhui Provincial Natural Science Foundation of China (安徽省自然科学基金项目, No.2008085MH267); Undergraduate Innovation Training Program of Anhui Province (安徽省省级大学生创新训练项目, No. 202006170243).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 6 September 2020/Accepted: 11 March 2021
 Journal of Acupuncture and Tuina Science2022年1期
Journal of Acupuncture and Tuina Science2022年1期
- Journal of Acupuncture and Tuina Science的其它文章
- Effects of electroacupuncture with different frequencies on hippocampal neuronal apoptosis and JNK signaling pathway in rats with vascular dementia
- Clinical observation on row needling at the Governor Vessel on the head for poststroke insomnia
- Effects of Tuina combined with graded motor imagery on the upper-limb motor function and quality of life of patients with hemiplegia after stroke
- Effects of acupuncture combined with Brunnstrom staging on upper-limb motor function, cerebral arterial blood flow velocity, and brain function remodeling after stroke
- Observation of the efficacy and safety of the Song-Relaxing and Zhen-Vibrating abdomen manipulation for patients with prediabetes
- Clinical study on Tuina plus Shen Ling Bai Zhu San in treating children with diarrhea due to spleen deficiency
