Biomass-derived carbon doping to enhance the current carrying capacity and flux pinning of an isotopic Mg11B2 superconductor
M.Shahazi,Y.Hao,D.Patel,H.Liang,Y.Yamauchi,M.S.A.Hossain
a QUT Centre for Materials Science and School of Chemistry and Physics,Queensland University of Technology(QUT),Brisbane,QLD 4001,Australia
b School of Mechanical and Mining Engineering,Faculty of Engineering,Architecture and Information Technology(EAIT),The University of Queensland,Brisbane,QLD 4072,Australia
cAustralian Institute for Bioengineering and Nanotechnology(AIBN),The University of Queensland,Brisbane,QLD 4072,Australia
d JST-ERATO Yamauchi Materials Space-Tectonics Project and International Center for Materials Nanoarchitectonics(WPI-MANA),National Institute for Materials Science(NIMS),1-1 Namiki,Tsukuba,Ibaraki 305-0044,Japan
Abstract Low activation isotopic boron(11B)based magnesium diboride(Mg11B2)superconductors doped with biomass-derived activated carbon were synthesized using 11B and magnesium powder via solid-state reaction.The effect of carbon doping on the lattice structure and superconducting properties of Mg11B2 bulks were evaluated using X-ray powder diffraction,high resolution transmission electron microscopy,scanning electron microscopy and magnetization measurements.Precise refinement of structural parameters indicates successful substitution of carbon in Mg11B2 bulks.The critical current density(Jc)of carbon doped Mg11B2 synthesized at 650 °C was enhanced more than two times compared with the pure Mg11B2 bulk.Similar improvement was observed for the Mg11B2 bulks heat-treated at 800 °C.This enhancement is due to successful substitution of biomass-derived carbon with high surface area into Mg11B2 lattice.The flux pinning mechanism of pure and doped Mg11B2 bulks were investigated using the Dew-Hughes model.This study provides information regarding enhancement of the Jc of low activation Mg11B2 superconductors suitable for next-generation fusion magnets.
Keywords:Magnesium diboride;Critical current;Flux pinning mechanism;Fusion reactor applications.
1.Introduction
Magnesium diboride(MgB2)gained immediate attention since its superconducting discovery in 2001 despite the emerging high-temperature superconducting materials[1,2].MgB2is highly attractive for practical applications due to its excellent and unique features including(i)simple stoichiometry and relatively high critical temperature(Tc)for non-oxide superconductors,(ii)high upper critical field(Hc2),(iii)large current transport capability and(iv)possibility of using a simple method to synthesize superconductive materials with good properties[3–7].Also,it is promising superconducting material for liquid-helium free superconductor applications due to its highTc[8–10].
MgB2superconductors are beneficial for fusion reactor applications compared with Nb-based superconductors due to its higherTcof 39 K,low activation energy and shorter decay time[11–13].However,neutron irradiation results in reduction of the amount of MgB2as the reaction10B+n→1Li+He(gas)occurs.This leads to reduction of critical current density(Jc)[14].Therefore,MgB2wire with natural boron is not suitable for fusion applications.It is demonstrated that11B isotope is stable under neutron irradiation and therefore MgB2superconducting material made with11B could be beneficial for fusion applications such as in International Thermonuclear Experimental Reactor(ITER)or other fusion reactors[15].However,replacing natural boron with11B results in decreased superconducting performance including loweringTc[16],irreversibility field(Hirr)andJc[17].The isotope effect onTchas been experimentally determined for boron isotope by Bud’ko et al.and later extended the study to the effect of10B,11B and natural boron(mixture of10B and11B)on the phonon frequency that showed a pronounce isotope effect for the phonon mode[18].
Pinning centres in superconductors provide pinning force to balance the Lorentz force and then prevent the movement of the flux in type-II superconductors.MgB2suffers from weak flux pinning and hence extensive research has been carried out to enhance the flux pinning force of MgB2materials such as ion irradiation and element/compound doping[19–26].Different types of pinning centres can be introduced,e.g.,grain boundaries,point defects,impurities and lattice variations induced by chemical doping[27–31].Chemical doping especially doping with carbon(C)containing materials is very effective for enhancing the flux pinning centres and thereby improvingHirrandJcof MgB2with natural boron as carbon can substitute for boron in the MgB2lattice[32].Either carbon element or C-based compounds can enhance theHc2and flux pinning force to obtain higherJcat high magnetic field for MgB2with natural boron[33].Though,Cheng et al.found that C-based chemical doping cannot create effective pinning centres needed for Mg11B2synthesized using amorphous11B powder[17].They concluded that the substitution of carbon for boron can be easily occurred on natural boron due to higher chemical activity of natural boron than11B[17].Therefore,carbon doping fails to increase theJcvalue of isotope Mg11B2superconductor.
In this work,we studied the microstructure,superconducting properties and flux pinning mechanism of Mg11B2bulks synthesized using magnesium(Mg)powder,crystalline isotope boron(11B)powder and biomass-derived carbon.Cdoped Mg11B2showed improved high fieldJcandHirrcompared to undoped samples.It is demonstrated that the activated porous carbon with high surface area is beneficial as dopant compared to other carbon sources reported in the literature[17].Furthermore,the flux pinning mechanism of Mg11B2and C-doped Mg11B2bulks investigated in this study for the first time.
2.Materials and methods
2.1.Syntheses of carbon from biomass sources
Plant biomass powders were pre-carbonised at 400 °C for 6 h.Pre-carbonised samples were mixed with potassium hydroxide(KOH)separately at a mixing ratio of 1:1(by wt.%)and ground and mixed in an agate mortar until a paste-like homogeneous mixture was formed.The mixture was transferred on a heating alumina boat and stored for 24 h before carbonisation at higher temperatures.During carbonisation,the heating rate was 2 °C/min and the hold time was set to 3 h.After carbonisation,the as-prepared C materials were treated with dilute hydrochloric(HCl)solution(0.1 M)to remove the excess KOH and washed with distilled water several times.Finally,the product was dried in a vacuum oven at 80 °C for 12 h[34].
2.2.Magnesium diboride(Mg11B2)and biomass C-doped Mg11B2 syntheses
All preparations and pre-processing of materials were carried out in a glove box containing 99.99% purity Argon(Ar)with<5 ppm oxygen(O).Molar ratios of Mg powder(99.9%purity,-325 mesh)supplied by Alfa Aesar and isotope crystalline boron powder(98.5% purity)supplied by Pavezyum are weighted,grounded in an agate mortar and pressed into a pellet of 0.5 g.For carbon doped samples,molar ratios of isotope boron powder and biomass carbon are weighted and mixed in agate mortar.Then magnesium powder added and mixed well.Similar to the pure samples,the powder presses into a pellet of 0.5 g.The pellets were wrapped in zirconium foil.The tightly sealed pellet was removed from the glove box,placed into alumina crucible inside a tube furnace with Ar gas flow.Table 1 summarises key parameters for reactions and the products of reaction presented in this work.A consistent heating rate of 2 °C/min was used for all reactions and at different maximum reaction temperatures of 650 °C and 800 °C.The heating rate is held constant for 30 min for all the reactions.
2.3.Characterization
Polycrystalline samples were characterized by X-ray powder diffraction(XRD)using Co Kαradiation in Bragg Brentano geometry with 0.02° 2θsteps and a counting time of 10 s per step using a PANalytical X-ray diffractometer.Precise refinements of structural parameters were undertaken using a corundum internal standard.Diffraction patterns were refined and indexed using the software program Topas[35].Quantitative estimates of phase abundance in each product were determined by Rietveld refinements using Topas.In general,phase abundances determined by this technique are within<5% relative error[36].The XRD patterns indicated that many synthesized products contain Mg11B2as a dominant phase and secondary phases such as MgO and unreacted Mg in variable proportions depending on the synthesis conditions listed in Table 1.

Table 1Selected starting conditions and products for Mg11B2 syntheses.
Morphological and elemental assessment of all samples were undertaken with a Zeiss SigmaTMField Emission Gemini scanning electron microscope(FE-SEM)equipped with an Oxford Instruments SDD XMax 50 mm2detector for energydispersive X-ray spectroscopy(EDS)analysis.These samples are prepared for FE-SEM/EDS by placing a thin layer of powder onto aluminium stubs with double-sided C tape.Samples were not coated with a conductive coating to avoid analytical interference(s).Elemental analysis was carried out at an accelerating voltage of 15 kV at an 8.5 mm working distance.Excessively charging samples were imaged at lower accelerating voltages of 5 kV.
Transmission Electron Microscopy(TEM)and highresolution transmission electron microscopy(HRTEM)imagesof the samples were obtained with a JEOL 2100 TEM,operated at an accelerating voltage of 200 kV.
2.4.Superconducting properties measurements
Zero field cooled(ZFC)and field cooled(FC)magnetisation curves as a function of temperature were acquired at 100 Oe field using a Cryogenic Ltd Mini Cryogen-free System(Cryogenic Ltd.,UK)with a 5 T magnet.Tcwas determined as the intersection of the linearly extrapolatedM(T)with theM=constant line.
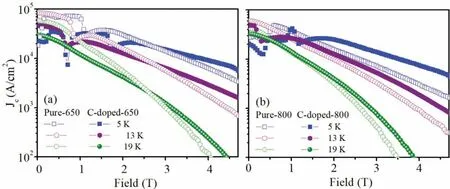
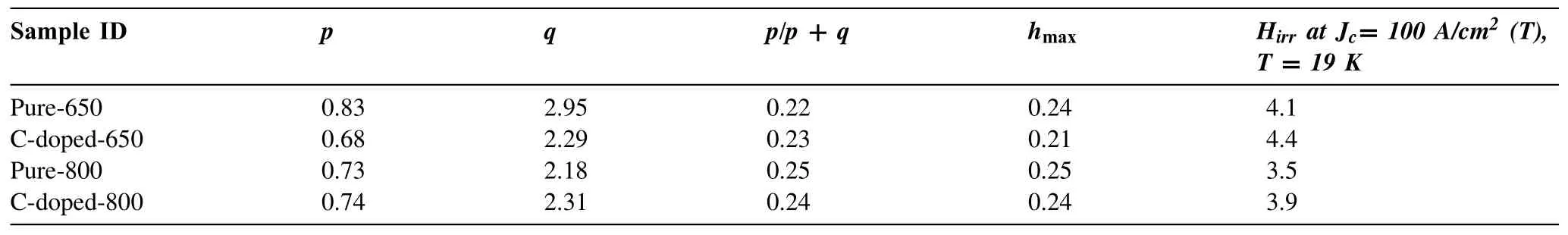
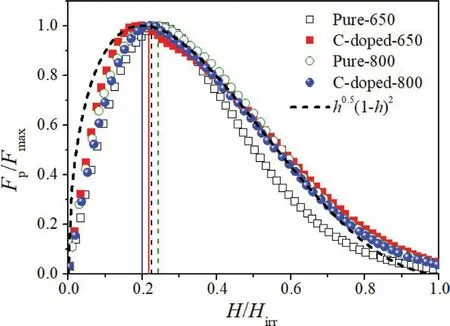
Magnetic measurements were performed on the bar with the dimension of 1×2×4 mm3cut from the Mg11B2pellets with the field applied parallel with the length of the sample.Magnetic hysteresis loops of the samples were measured over a temperature range of 5–30 K for all the samples.A magneticJcwas calculated using the Bean model:Jc=20ΔM/[Va(1-a/3b)](a We present a selection of experimental data to demonstrate the range of conditions for which a high yield of C-doped and pure Mg11B2may form.Table 1 presents data for reactions that result in the formation of C-doped and pure Mg11B2as a major phase as determined by XRD Rietveld refinement.Below is the description of starting materials and final products from different reactions. Table 2Lattice parameters(a-& c-axis),cell volume and Tc of selected Mg11B2 bulks. Fig.1(a)and(c)show the low and high-resolution TEM(HR-TEM)images of biomass-derived carbon.The inset in the left bottom corner is the corresponding selected area electron diffraction(SAED)image.The disordered lattice observed by HR-TEM and the diffraction ring in SAED indicates the amorphous structure of biomass carbon.Fig.1(c)and(e)show the low and HR-TEM images of isotope11B powder.The inset in the left bottom corner is the corresponding SAED image.A HR-TEM image and the corresponding SAED patterns of11B powder confirm its crystalline nature.There is an inhomogeneous distribution of particle size of boron powder as indicates by HR-TEM image(Fig.1(c)). Fig.1.TEM and HR-TEM images for(a and c)biomass-derived carbon and(b and d)isotope boron.Inset:SAED pattern of same area.The scale bars in(a)and(b)are 500 nm,while the scale bars in(c)and(d)are 20 nm. Fig.2.XRD patterns for the pure and carbon doped Mg11B2 bulks heat treated at 650 °C and 800 °C. Fig.2 shows the XRD patterns for the pure and carbon doped Mg11B2bulks heat treated at 650 °C and 800 °C.According to XRD results,Mg11B2is the main phase(>83.5%)after heating at 650 °C and 800 °C while a small number of secondary phases was observed which was magnesium oxide(MgO)and unreacted Mg.An increase in temperature for the same starting ratio and hold time for undoped samples,improved the yield of Mg11B2,presumably due to the conversion of excess Mg. Fig.3.SEM images of(a)pure Mg11B2 bulk synthesized at 650 °C,(b)10% C-doped Mg11B2 bulk synthesized at 650 °C,(c)pure Mg11B2 bulk synthesized at 800 °C and(d)10% C-doped Mg11B2 bulk synthesized at 800 °C.The scale bar is 1 μm.The yellow arrows indicate the MgO particles. Table 1 summaries variables that affect the relative proportion of C-doped and pure Mg11B2products.It provides outcomes for(i)variation in heat treatment temperature(Tmax)for pure and C-doped Mg11B2and(ii)variation in starting ratios of C as a dopant.A maximum yield of 92.3%Mg11B2was obtained for Pure-800 withTmax=800 °C andthold=30 min.No unreacted Mg phase is found for this sample.However,for C-doped samples,Mg11B2yield slightly enhanced from 83.6% to 86.9% with increasing temperature from 650 °C to 800 °C for C-doped-650 and C-doped-800,respectively. Table 2 describes variation in lattice parameter and cell volume of the pure and C-doped Mg11B2bulk samples obtained using Rietveld refinement.It was found that both lattice parameters and cell volume decreased with increasing carbon doping.The lattice parameters and cell volume were also decreased asTmaxenhanced from 650 °C to 800 °C for the same doping level.The level of carbon substitution,x,in the formula Mg(B1-xCx)2,can be estimated as x=7.5Δ(c/a),where c and a are the lattice parameters determined from XRD patterns andΔ(c/a)is the change in c/a compared to a pure sample.The substituted carbon amount is 0.6 for both C-doped-650 and C-doped-800 samples. The morphology of Mg11B2powders obtained from samples pure-650,C-doped-650,pure-800 and C-doped-800 is shown in Fig.3.The pure and C-doped samples showed similar microstructure having clear and sharp grain boundaries with randomly orientated hexagonal grains that were connected with the surrounding particles.The particle size of pure Mg11B2was several hundred nanometres which increased with C-doping and temperature.Mg11B2bulk with 10% C-doping synthesized at 800 °C showed a larger particle size.Pore structures were also observed due to the volume shrinkage during the formation of Mg11B2as the density of Mg11B2is higher than the density of the mixture of unreacted magnesium and11B[37].The particle size difference in Mg11B2samples is related to inhomogeneous distribution of particle size of starting boron powder as shown by TEM image(Fig.1(c)).Similar particle size difference is reported by Bateni et al.[38,39]which indicates the role of starting boron particle size in the size of synthesized MgB2. Fig.4.TEM and HR-TEM images for two samples:(a and c)Pure Mg11B2 synthesized at 650 °C and(b and d)C-doped Mg11B2 synthesized at 800 °C.Inset:SAED pattern of the same area.The scale bars in(a)and(b)are 500 nm,while the scale bars in(c)and(d)are 10 nm. Fig.4 shows TEM and HR-TEM images of selected Mg11B2bulks for undoped and C-doped samples.A high density of wave fringes was observed in pure and C doped Mg11B2as shown by red arrows in the Fig.4(b)and 4(d).Beside the large Mg11B2grains with the size 50–200 nm,there are many grains with size<10 nm which give rise to diffraction rings in the SAED.A line scan EDS spectrum of C-doped-650 and C-doped-800 are shown in Fig.S1.EDS spectra confirm the presence of magnesium,boron and carbon elements in the carbon doped samples. Fig.5(a)shows the normalized magnetic susceptibility of all Mg11B2bulks under 100 Oe magnetic field.All samples showed a clear superconducting transition between 33 and 36 K.TheTcvalues are summarized in Table 2.TheTcof 35.5 K and 35.9 K were recorded for the pure samples synthesized at 650 °C and 800 °C,respectively.TheTcvalues decreased to 33.9 K and 34.4 K with addition of 10% C-doping for C-doped-650 and C-doped-800,respectively.Fig.5(b)shows the field dependence of critical current density for pure Mg11B2and C-doped samples synthesized at 650 °C and 800 °C at 13 K.It is found that the high fieldJcvalues of all doped samples enhanced compared to pure Mg11B2samples.Moreover,theJcperformance of both pure and C-doped Mg11B2samples decreased for the samples synthesized at higher temperature of 800 °C.This is due to increased crystallinity of Mg11B2samples synthesized at 800 °C. Fig.6(a)presents the in-fieldJcperformance of the pure and C-doped Mg11B2bulks synthesized at 650 °C at several operating temperatures belowTc.The zero-fieldJcof pure and C-doped Mg11B2are in the range of 0.2–0.9×105A/cm2for pure and C-doped samples atT<20 K.The high fieldJchave been enhanced for a C-doped sample atT=5 K,13 K and 18.6 K and field greater than 3 T.Similar behaviour observed for pure and C-doped samples synthesized at 800°C as shown in Fig.6(b).The zero-fieldJcof pure and C-doped Mg11B2bulks are in the range of 0.2–0.6×105A/cm2.Flux jumping was observed for both pure and doped Mg11B2bulks at 5 K.No flux jumping was observed for pure and C-doped samples atT=19 K.TheHirrvalues of 3.5 T,3.9 T,4.1 T and 4.4 T obtained at 19 K andJc=100 A/cm2for Pure-800,Cdoped-800,Pure-650 and C-doped-650,respectively.TheHirrenhanced for both C-doped Mg11B2bulks synthesized at 650°C and 800 °C.This is due to effective carbon substitution on the boron site within Mg11B2which resulted in an increasedHirr. Fig.5.(a)FC and ZFC magnetization of pure and C-doped Mg11B2 bulks synthesized at 650 °C and 800 °C as a function of temperature in an applied field of 100 Oe showing the superconducting transition temperature.(b)Measured critical current density as a function of applied magnetic field for undoped Mg11B2 and doped samples at 13 K. Fig.6.(a)Field dependence Jc of pure and biomass-derived C-doped Mg11B2 bulks synthesized at 650 °C at T=5 K,12.6 K and 19.1 K.(b)Field dependence Jc of pure Mg11B2(open symbol)and biomass-derived C-doped(close symbol)synthesized at 800 °C at T=5 K,13 K and 19 K. Flux pinning force densities(Fp=μ0JcH)can be useful in analysing the mechanisms that controlJcin superconducting materials.The pinning force of superconductors is a function of temperature and magnetic field and is determined by the micro and nanostructure of the sample[40,41].The field dependence of normalized flux pinning force can indicate the pinning mechanism operating in the specific sample[41–43]. To investigate the flux pinning mechanism in detail,we calculated the pinning force density(Fp)from theJcand the applied field usingFp=μ0JcHat various temperatures belowTc.As proposed by Dew-Hughes,the pinning mechanism does not change with temperature,if the normalized pinning forceFp(h)=as a function of reduced fieldh=H/Hirrdemonstrates a scaling relation,Fp(h)α hp(1-h)q,whereis the maximum pinning force,pandqare the exponents[44].The values ofpandqdepend on the type of defects,their dimensionality(e.g.,point,two dimensional or bulk),the type of interaction and the nature of pinning centres.The deviation from the scaling law with temperature and magnetic field indicates the change in vortex-lattice period and various sizes of pinning centres. Fig.7 presents the normalized pinning force as a function of the reduced field for Mg11B2bulks for Pure-650,C-doped-650,Pure-800 and C-doped-800 at different temperatures.TheHirrwas estimated using the criteria ofJc=100 A/cm2.It should be noted the scaling of the normalized pinning force is done based on the reduced field byHirrwithh=H/Hirrinstead ofHc2withh=H/Hc2because the difference betweenHc2andHirris sizable,and is more significant at the low-temperature regime in the case of MgB2[45,46].All thecurves forT Fig.7.Field dependence of reduced pinning force with the fitting results obtained using hp(1-h)q at several temperatures for pure and biomass-derived C-doped Mg11B2 bulks.(a)Pure-650,(b)C-doped-650,(c)Pure-800 and(d)C-doped-800. Table 3Summary of fitting parameters and hmax position for all Mg11B2 bulks. Fig.8 compares the normalized flux pinning force with the standard normal surface pinning model and normal point pinning.In general,all Mg11B2bulks possess theFpvshcurves that relate to normal surface pinning rather than point pinning.Flux pinning due to the point pinning centres would shift the peak of theFpvs.hcurve to the higherhvalues compare with the pure sample with a small amount of nanoparticle doping.However,for Mg11B2bulks synthesized in this study,the C-doped Mg11B2bulks show a slight decrease in peak position toward the lower magnetic field.This indicates the modest role of point pinning centres compared with the two-dimensional pinning centres for all Mg11B2bulks.Similar behaviour observed for MgB2with g-C3N4content where two-dimensional pinning centres were responsible for the improved pinning properties[22]. Fig.8.Field dependence of reduced pinning force with the fitting results obtained using hp(1-h)q for pure and biomass-derived C-doped Mg11B2 bulks at 21 K.The black dash line represents the reference curve for two-dimensional flux pinning with h0.5(1-h)2. Absence of effective flux pinning centres in pure Mg11B2bulks result in decrease ofJcat high magnetic field as the Lorentz force will lead moving of vortices in MgB2matrix once its higher than the flux pinning force.To improve theJc,we need to induce effective pinning centres to prevent moving of vortices and deterioration of supercurrent.The effect of flux pinning centres on theJcof superconducting bulks depend on the type of dopants,their size and distribution within the superconductor matrix.In addition,it is crucial to optimize the amount of pinning centres without destroying the connectivity of superconducting bulks.Chemical doping especially doping with carbon and carbon containing materials is very effective for enhancing the flux pinning centres and thereby improvingHirrandJcof MgB2with natural boron as carbon can substitute boron in the MgB2lattice[22,32,52–56].However,Cheng et al.found that C-based chemical doping cannot create effective pinning centres needed for Mg11B2synthesized using amorphous isotope11B powder and could not be effective to improve theHirrandJcperformance of Mg11B2materials[17].They concluded that the substitution of carbon for boron can easily occur for natural boron due to higher chemical activity of natural boron than11B[17].In this work,we demonstrate biomass-derived carbon can improve high fieldJcandHirrof Mg11B2.The high flux pinning efficiency of carbon doped samples in this study is related to successful substitution of carbon in Mg11B2structure due to high surface area of biomass-derived carbon. All pure and C-doped samples showed a clear superconducting transition between 33 and 36 K.The biomassderived C-doped Mg11B2bulks(C-doped-650 and C-doped-800)showed lowerTcdue to successful carbon doping and enhanced crystalline defects.The reduction ofTcwas in agreement with the expectation that electron doping moves the Fermi level to a lower density of state region[32].The transition width was broadened for Mg11B2bulk(C-doped-650)because of a lower amount of superconducting phase compare with Mg11B2bulks(Pure-650,Pure-800,C-doped-800).However,C-doped Mg11B2bulk(C-doped-800)with a higher sintering temperature of 800 °C showed higherTcand sharper transition compare with biomass-derived C-doped Mg11B2bulk synthesized at 650 °C. The zero-fieldJcis determined by the quality of connectivity of Mg11B2bulk in whichJcreduces slowly with an increasing magnetic field while the high fieldJcis controlled by flux pinning as the result of stronger Lorenz force on the flux lines.TheJc(0)values of pure Mg11B2and C-doped Mg11B2from sample Pure-650,C-doped-650,Pure-800 and C-doped-800 were 0.9×105,0.5×105,0.6×105and 0.5×105at 12.6 K,respectively.HigherJc(0)of pure Mg11B2synthesized at 650 °C and 800 °C indicated better connectivity and smaller impurity scattering of pure Mg11B2bulks compared with C-doped Mg11B2bulks.However,the high fieldJcof both C-doped Mg11B2bulks synthesized at 650 °C and 800 °C enhanced due to improved pinning centres from effective carbon doping.The lattice parameters of pure Mg11B2(Pure-650)werea=b=3.085˚A andc=3.522˚A.The substitution resulted in a significant reduction in the in-plane lattice parameter and a small change in the interplanar distance.The refinement results of the XRD data indicated that the lattice parametera-axis decreased with C-doping and thec-axis stayed almost constant.This was because when carbon substitutes boron in the lattice,the lattice parameter shrinks,as carbon atoms are smaller than boron atoms.The lattice shrinkage has been reported in many C-based doped MgB2,such as carbon nanotube,nanocarbon and amorphous carbon[20,22,23,48–50].Substitution of carbon in the boron sites increases the inner band scattering as well as decreasing the mean free path and coherence length.Carbon has one electron more than boron;it has been suggested that the extra electron significantly affects theσ-band and create scattering centres that attribute to the change in superconducting properties of MgB2[57]. The volume friction of MgO for pure and carbon substituted samples are very similar(between 7.7 and 9%).The amount of MgO is slightly higher for the pure sample synthesised at 650 °C(9% for pure Mg11B2compared to 8.1% for carbon substituted one),therefore theJcenhancement of carbon substituted Mg11B2is not due to the presence of MgO.So,it’s unlikely that MgO functioned as effective pinning centres in our case and defects caused by carbon substitution play the major role to enhance the high fieldJc. The value ofJcdepends on a balance between the Lorentz force and pinning forces on vortices;thus there is interest in understanding the mechanism of pinning to design higher performance superconductors.Effective flux pinning centres at higher in-fieldJcshould include both the grain connectivity and the flux pinning performance.The specific type of pinning centres,microstructure and the distribution of the pinning centres determine the flux pinning force for the specific superconductors due to the geometric nature of the interaction between the flux line and the pinning centres based on Dew-Hughes theory.It is known from the Dew-Hughes model,whenp=1/2 andq=2 andp=1 andq=2 describe the surface pinning and point pinning,respectively[58].For example,grain boundaries act as proper pinning centres in MgB2without damaging the connectivity. Further artificial grain boundaries with proper thickness showed a much stronger pinning force compare with the agglomerate dopants[22].The experimental data and fitted results are shown in Fig.6 indicated a different value ofpandq(withp=0.83 andq=2.95)for Mg11B2bulk for Pure-650 compared to the other three samples with 0.67 Mg11B2superconducting bulks using biomass-derived carbon showed enhanced flux pinning centres and supercurrent at high fields.Systematic investigation of microstructure and flux pinning mechanism showed that the effective flux pinning centres have been induced into Mg11B2bulks synthesized using amorphous biomass-derived carbon,crystalline11B powder and Mg.Biomass-derived carbon within Mg11B2generated carbon substitution on the boron sites which resulted in increasedHirrand high field critical current density.It is likely that the activated porous carbon with high surface area is beneficial as dopant compared to other C-sources reported in the literature.The flux pinning mechanism was investigated and understood with a mixture of point pinning and grain boundary pinning for Mg11B2bulks. Acknowledgments We are grateful to the Central Analytical Research Facility within the Queensland University of Technology for access to the characterization facilities.MS is grateful to the Queensland Government for Advance Queensland Research Fellowship in partnership with Siemens Energy(Aust)Pty Ltd and QUT which partially supported this work and by the Australian Research Council,Australia(Grant No.LP160101784).We appreciate sample preparation skills of Minjun Kim and Rebecca Fieth for assistance with TEM data collection.This work was performed in part at the Queensland node of the Australian National Fabrication Facility,a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s researchers. Supplementary materials Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.01.010.3.Results

3.1.Biomass-derived carbon


3.2.Mg11B2 syntheses

3.3.Phases and microstructure of Mg11B2 bulks

3.4.Superconducting properties
3.5.Flux pinning mechanism


4.Discussion
5.Conclusion
 Journal of Magnesium and Alloys2022年7期
Journal of Magnesium and Alloys2022年7期
