Adenosine cyclic phosphate with ultrasonic-assisted pectinase extraction alleviated allergic reactions in RBL-2H3 through inhibiting the inf lux of intracellular Ca2+
Qio Bi, Xioping Feng, Yu Wng, Chunyu Wu, Ye Liu, Jio Sun, Tinli Yue,*, Fngyu Long,*
a College of Food Science and Engineering, Northwest A&F University, Yangling 712100, China
b Beijing Key Laboratory of Plant Resource Research and Development, Beijing Technology and Business University, Beijing 100048, China
c Department of Breast Surgery, The Aff iliated Hospital of Qingdao University, Qingdao 266000, China
Keywords:Ultrasonic-assisted pectinase extraction Cyclic adenosine monophosphate Rat basophilic leukemia cells Ca2+ inf lux Allergy
A B S T R A C T Jujube contains abundant cyclic adenosine monophosphate (cAMP) and the ultrasonic-assisted pectinase extraction (UAPE) conditions for obtaining the maximum cAMP yield from jujube were optimized.Orthogonal array design was applied to evaluate the effects of 4 variables by UAPE on cAMP yield. The results showed that the optimal cAMP yield (783.0 μg/g) was derived at ratio of liquid to solid 5 mL/g,ratio of pectinase to raw material 1.5%, time 60 min and temperature 40 °C. Moreover, the effect of cAMP on the anti-allergic function of action induced by immunoglobulin E (IgE) and its meschanism was investigated through establishing the sensitized cell model in rat basophilic leukemia (RBL-2H3) cells using dinitrophenylated (DNP)-bovine serum albumin (BSA)-IgE. The results showed that cAMP interfered with sensitized cells, effectively inhibited the occurrence of basophil degranulation in dose dependence, and signifi cantly reduced the activity of β-hexosamindase (β-hex), at the optimal concentration of 50 μg/mL. The level of anti-infl ammatory factor interleukin-10 (IL-10) was promoted and the content of pro-infl ammatory factor tumor necrosis factor-α (TNF-α) was suppressed by cAMP. In addition, infl ux of intracellular Ca2+ was repressed effectively. Our results demonstrate that jujube cAMP regulated the cytokine balance in the allergy pathway through blocking the infl ux of extracellular Ca2+, with the prevention of allergy symptoms.© 2023 Beijing Academy of Food Sciences. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd. This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Jujube (Ziziphus jujubaMill), a thorny Rhamnaceous plant, is indigenous to China and widely distributed in the temperate and subtropical areas of the northern hemisphere, especially the arid areas of Northern China [1]. It has also been used as a traditional Chinese medicine for thousands of years with its numerous benef icial effects for human health [2]. Moreover, jujube also has various healthy functions such as antitumor, anti-inflammatory, antioxidant and immune-enhancing effects [3].
Cyclic adenosine monophosphate (cAMP) is one of the most important functional ingredients in jujube [4]. It was found that the content of cAMP in mature jujube was 30-160 µg/g, the highest of that observed in more than 180 natural plants [5]. As the secondary messenger, has the potential effects in maintaining immune system balance and controlling diseases, by releasing enzymes, hormones and other substances. cAMP is a nucleotide derivative involved in regulating biological functions such as metabolism of substances in cells, which is widely distributed in various plants. Additionally,cAMP exhibit various biological activities, such as anti-inf lammatory,anti-tumor, and the protection of the immunity system.
Traditional techniques for the extraction of cAMP from jujube include solvent extraction (SE), reflux extraction (RE), hot extraction (HE) and microwave-assisted extraction (MAE). These extraction methods usually are time consuming and laborious, has low selectivity, and/or low extraction yield [6]. Many new extraction methodologies have been developed to separate cAMP from jujube [7].Enzyme-assisted extraction is a gentle, efficient and environmentally friendly extraction method that has been recently adopted for extracting various kinds of bioactive compounds from plants [8].Pectinase enzyme can be used for extracting bioactive materials from various resources by increasing the solvent’s penetration into the cell walls of the plant through their decomposition [9].Ultrasound-assisted extraction (UAE) is widely used because of its capillary effects and enhancing influence on mass transfer and cell disruption [10]. The mechanical effects of ultrasound and the cavitation phenomenon which is caused by ultrasonic waves increase the solvent permeability into plant cells, increase the mass transfer,and consequently, increase the extraction efficiency [11]. Ultrasonicassisted pectinase extraction (UAPE) combines the two methods and is a highly promising technique [12], which, to date, has been used for obtaining extracts from plants such as orange (Citrus sinensis,var.Marss) processing waste [13], celery (Apium graveolensL.) and Sohiong (Prunus nepalensis) juice [14]. Now, despite that UAPE has been widely applied in the procedure of extraction from different materials, there are very few literature about exploring the optimally extractive factors of cAMP by UAPE.
cAMP, as an important functional ingredient in jujube, is helpful to better understand the healthcare functions of jujube. It is necessary to study the healthcare effect of cAMP. Recent epidemic studies suggest that the estimated North American population prevalence of reported food allergy is 3.9% to 8% in infants and children and 6.6% to 10% in adults, respectively [15]. And the prevalence is much higher than appreciated in the past. Currently, there are no approved treatments for food allergy, and the standard of care for food allergy is still strict dietary avoidance of allergy foods [16].The current pharmaceutical intervention is limited to provide immediate relief of symptoms from accidental exposure. A major focus of current research is the development of disease-modifying treatments that modulate the allergic immune response, protecting against accidental exposure [17] and some other studies [18]found cAMP had a beneficial effect on modulating the allergic immune response. At present, however, the literature on the effects of cAMP on allergies is limited.
The rat basophilic leukemia (RBL-2H3) cell line, a subline of the rat basophilic leukemia, expresses many of the characteristics and functions of mast cells [19]. Mast cells are the main effector cells of allergic diseases, playing important roles in both immediate allergic reactions and late-phase reactions. Therefore, RBL-2H3 cells are often used as an alternative model for mast cell lines that cannot be subcultured for allergic reactions [20,21]. The present study focused on the extract of cAMP from jujube as well as cAMP itself with RBL-2H3 of allergy, which provides the beneficial role of jujube in regulating allergy response.
2. Materials and methods
2.1 Materials
The dried Chinese jujube (Zizyphus jujubaMill. cv.Goutouzao) was purchased from Qingjian in the Shaanxi Province of China and then immediately transported to the laboratory.The jujube samples without disease or any visible blemishes that were uniform in shape were hand-picked, their seeds were removed, were cut into pieces. And then crushed into a powder(Q-250B, Shanghai Bingdu Co., Ltd., Shanghai, China) at 2 500 r/min for 2 min and sieved through a 100-mesh screen. The obtained sample was stored in a sterile polypropylene pouch at 4 °C in a refrigerator before the experiments. The used pectinase enzyme (20 000 U/g) was purchased from Xi’an Lihe Biological Technology Co., Ltd. (Xi’an, China). RBL-2H3 cells purchased from Procell Life Science & Technology Co., Ltd. (Wuhan,China). The following materials were purchased from the indicated commercial sources: ketotifen fumarate (Sigma Aldrich Technology Co., Ltd., St. Louis, Missouri, USA); Fluo-3-AM,PIPES buffer, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2Htetrazolium bromide (MTT) and Trition X-100 (Beijing Solarbio Technology Co., Ltd., Beijing, China); Pluonic F-127, antidinitrophenylated (DNP)-immunoglobulin E (IgE), DNP-BSA(bovine serum albumin) and 4-methylumbelliferyl-N-acetyl-β-Dglucosaminide (4-NAG) (Sigma Aldrich, USA); cAMP (99.9%purity, Solarbio, China) were dissolved in PIPES buffer with dimethyl sulfoxide at a concentration of 0.1 mol/L and stored at-20 °C before use. The final concentration of dimethyl sulfoxide in the assay system described below was 0.1% (V/V).
2.2 UAPE
The UAPE method was conducted using an ultrasonic bath (KQ-500, Kunshan Ultrasound Instrument Co., Ltd., Kunshan, China)with a fixed power. Briefly, 20 g of dried jujube powdered sample was accurately weighted with an analytical balance (BSA223S,Beijing Sartorius Scientific Instrument Co., Ltd., Beijing, China)and mixed (444730, Guangzhou Aika Instrument Equipment Co., Ltd., Guangzhou, China) with 100 mL distilled water. Then, the desired concentration of pectinase enzyme for different treatments(Table 1) was added to the above mixture of jujube powder. The beaker contents were mixed for 1 min to dissolve the pectinase enzyme uniformly in the liquid phase. The flask was covered with petri film to avoid solvent evaporation before UAPE. Then each mixture was extracted at different operational conditions, including the ratio of liquid to solid, ratio of pectinase to raw material,extraction temperature and extraction time. After extraction, the extracted solutions were centrifuged (HC-3018R, Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd., Anhui, China) at 5 000 r/min for 20 min. The supernatants were separately collected,then passed through 0.45 µm filters (YY8-1-88, Xi’an Lihe Biological Technology Co., Ltd., Xi’an, China) and stored in a refrigerator at 4 °C for subsequent analysis.
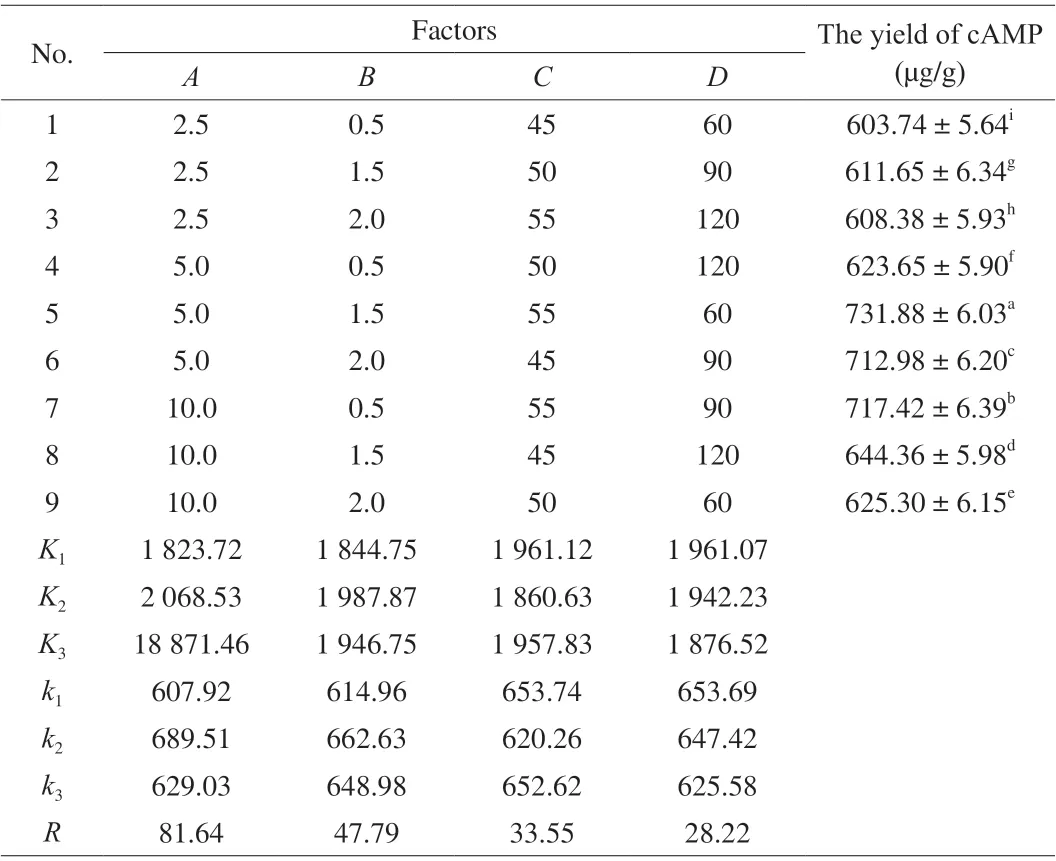
Table 1 The orthogonal experiment design and results of ultrasound-assisted enzymatic extraction.
2.3 Optimization of UAPE
The Taguchi experimental design approach has been used for optimization of extraction variables. It is a robust methodology against uncontrollable environmental changes (also known as noise factors), as is the case for raw material variability [22]. An orthogonal L9(3)4test design in the UAPE group was used to investigate the optimal extraction condition of cAMP from jujube on the basis of the single-factor test. As seen from Table 1, the extraction experiment was carried out with 4 factors and 3 levels which were set as follows:A, ratio of liquid to solid (2.5:1, 5:1, 10:1,V/m);B, ratio of pectinase to raw material (0.5%, 1.5%, 2.0%);C, extraction temperature (45,50, 55 °C);D, extraction time (60, 90, 120 min).
2.4 Analysis of cAMP standard
To prepare the standard solution, 100 mg cAMP standard was dissolved with 5 mL ultra-pure water (UPHW-1, Chengdu Ultrapure Technology Co., Ltd., Chengdu, China) and then transferred in a 100 mL volumetric flask. The above solutions were diluted with ultra-pure water to final concentrations of 40, 60 and 80 µg/mL,respectively. The cAMP standard solutions of different concentrations were filtered through 0.45 µm filters and each 20 µL sample was injected into the chromatography column. Record the integrated area of cAMP chromatographic peaks and draw a standard curve.
2.5 HPLC analysis
The analysis of the cAMP content in the jujube fruits was determined according to the method of Zhang et al. [23] by high performance liquid chromatograph (HPLC, CBM-20A, Shimadzu,Japan) [5]. The chromatographic column used was XTerra MS-C18(4.6 cm × 250 mm, 5 µm, Waters, Massachusetts, USA), the ratio of mobile phase wasV(methanol) :V(0.05 mol/L potassium dihydrogen phosphate) = 1:9, flow rate was 1.0 mL/min, the temperature was 40 °C, and the ultraviolet detector was UV 259 nm × 0.2 absorbance units full scale. The extraction yield of cAMP (µg/g) was calculated as the amount of the extracted cAMP (µg) per gram of jujube samples.
2.6 Cell culture
RBL-2H3 cells were grown in Eagles’ minimal essential medium(Shanghai BasalMedia Technologies Co. Ltd., Shanghai, China)containing 15% (V/V) heat-inactivated fetal bovine serum (Gemini Biotechnology Co., Ltd., Carlsbad, CA, USA), 1% 100 × MEM nonessential amino acid solution, 100 U/mL penicillin and 100 mg/mL streptomycin (Thermo Fisher Scientific Co., Ltd., Shanghai, China).Cells were incubated for 1-4 h at 37 °C, after which the medium was removed and the culture washed with phosphate buffered saline (PBS).Cells were detached with trypsin-EDTA solution (Solarbio, China).After the cells were washed, they were resuspended in medium and used for subsequent experiments. Cultures were continuously maintained at 5% CO2with 37 °C in dishes. The RBL-2H3 used in this study was the cells that pass the primary cells to the 7thpassage.
2.7 MTT assay for cell viability added with cAMP
MTThave been dissolved at a final concentration of 5 mg/mL in HEPES buffer saline, and filtered through a 0.22 µm filter. Each culture well was delicately washed once with PBS before adding 10 µL (1:10,m/V) of MTT solution. After 4 h incubation (37 °C, 5%CO2), the formed formazan crystals were dissolved in overdose of dimethyl sulfoxide (DMSO) and absorbances were read at test 490 nm wavelengths with a plate reader [24]. Cell survival was expressed as the percentage of formazan absorbance. Results were the mean values and standard deviation (SD) from at least three different experiments in triplicate [25].
2.8 β-Hexosaminidase (β-hex) secretion assay
Cell cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2, and the culture medium was changed daily.Experiments were done during the exponential phase of cell growth.RBL-2H3 cells (2 × 105cells) in 24-well plates were sensitized with 0.5 µg/mL mouse anti-DNP monoclonal IgE for 24 h. The cells were washed with PIPES buffer and incubated in a buffer containing 5.6 mmol/L CaCl2and 0.1% BSA for an additional 10 min. The cells were incubated with 1 mL MEM containing 0, 12.5, 25 and 50 µg/mL of cAMP for 2 h and washed with PBS buffer. The cells were stimulated for 1 h with DNP-BSA (10 µg/mL), which activated RBL-2H3 cells to trigger allergic reactions, and the cells and the culture supernatants were used for analysis byβ-hex release assay or enzyme-linked immunosorbent assay (ELISA).
To determine the degranulation of the mast cells, theβ-hex activity was measured. The supernatant (50 µL) was mixed with an equal volume of the substrate solution (2 mmol/Lp-nitrophenyl-Nacetyl-β-D-glucosaminide in 0.1 mol/L sodium citrate buffer, pH 1.5),and the mixture were incubated for 1 h at 37 °C. The reaction was terminated by adding 500 µL of stopping buffer (0.1 mol/L Na2CO3/NaHCO3, pH 10). The absorbance at 405 nm was measured with a microplate reader. As a control, the release ofβ-hex induced with IgE and DNP-BSA was used (100%). The inhibition of degranulation was calculated as follows:

whereA1is the absorbance from the reaction mixture of IgE (+) +DNP-BSA (+) + cAMP (-) at 405 nm, andA2is IgE (+) + DNP-BSA(+) + cAMP (+).
2.9 ELISA
The cell supernatants which reacted with cAMP were obtained after sensitized by DNP-IgE and stimulated by DNP-BSA as the method above. The concentrations of Th1 type cytokine tumor necrosis factor-α (TNF-α) in the cell culture supernatant were measured with ELISA kit after 6 h of stimulation with 10 µg/mL DNP-BSA, and the concentrations of Th2 type cytokine interleukin-10(IL-10) in the cell culture supernatant were measured with ELISA kit after 4 h of stimulation with 10 µg/mL DNP-BSA. The measurement method was used according to the manufacturer’s instructions.
2.10 Ca2+ influx assay
After digesting with trypsin, 2 × 105cells/mL RBL-2H3 cells were collected, inoculated in a 24-well cell culture plate overnight after sensitized to an anti-DNP-IgE 0.5 µg/mL. Cells were washed 2-3 times with Tyrode’s buffer and were subjected to treatments with 5 µmol/L Fluo-3-AM fluorescent probe for 30 min without light,washed the cells again and co-incubated with cAMP of different concentrations (25, 50, 100 µmol/L) for 30 min, at 5% CO2, 37 °C in the incubator. After washing again, stimulated with 40 µL of DNPBSA (30 µg/mL). Cells’ average fluorescence intensities were directly examined under the fluorescence microscope after incubation for 1.5 h and PBS washing.
2.11 Statistical analysis
Results were presented as means ± SD. The data were analyzed using the SPSS version 25.0 (SPSS Inc., Chicago, USA) for analysis of variance (ANOVA). The Duncan’s test was used for multiple comparisons. Different letters indicated significant difference between the two groups (P< 0.05).
3. Results
3.1 Effect of UAPE condition on cAMP yield
3.1.1 Effect of ratio of liquid to solid
To determine the impact factors and their variation, the effects of ratio of liquid to solid, ratio of pectinase to raw material, extraction temperature and extraction time on the yield of cAMP from jujube were investigated by single-factor experiments. As seen in Fig. 1a,with an increasing ratio of liquid to solid from 5 to 25 mL/g, the extraction yield firstly decreased and then stabilized as the ratio of pectinase to raw material, extraction temperature and extraction time were fixed at 1.0%, 40 °C and 60 min, respectively. It was obvious that the ratio of liquid to solid was useful for improving the extraction yield of cAMP. But if the extraction was carried out under a higher liquid to solid ratio, the concentration of cAMP in the extraction solution was low. The ratio of liquid to solid of 5 mL/g was sufficient to reach the high extraction yield, and it was used in further experiments.
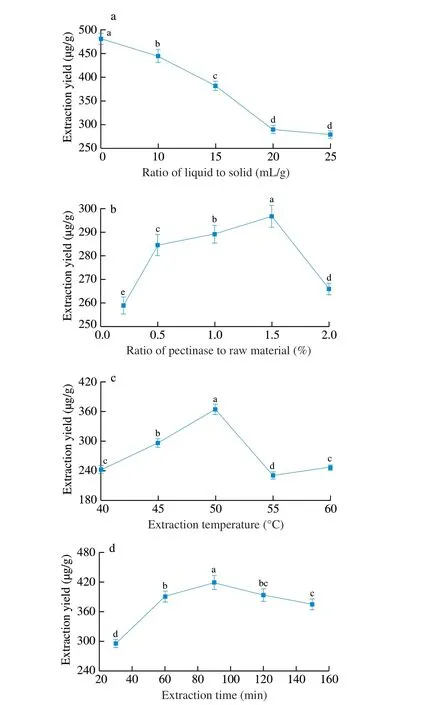
Fig. 1 Effect of (a) ratio of liquid to solid, (b) ratio of pectinase to raw material, (c) extraction temperature, (d) extraction time on ultrasound-assisted enzymatic extraction yield of cAMP from jujube. (a) Ratio of pectinase to raw material 1%; extraction temperature 40 °C; extraction time 60 min. (b) Ratio of liquid to solid 15 mL/g; extraction temperature 40 °C; extraction time 60 min. (c) Ratio of liquid to solid 15 mL/g; ratio of pectinase to raw material 1%; extraction time 60 min. (d) Ratio of liquid to solid 15 mL/g; ratio of pectinase to raw material 1%; extraction temperature 40 °C.
3.1.2 Effect of ratio of pectinase to raw material
Pectinase enzyme concentration should be carefully considered for achieving complete extraction and avoiding enzyme overuse. The effect of enzyme concentrations of 0.5%, 1.0%, 1.5%, 2.0% (m/m)on the cAMP extraction yield was shown in Fig. 1b, while other extraction parameters were given as followings: ratio of liquid to solid 15 mL/g, extraction temperature 40 °C and extraction time 60 min. A prominent increase in the extraction yield was observed with an increase in enzyme concentration from 0.5% to 1.5% and then sharply dropped. Further increase in the enzyme concentration led to a significant decrease in the extraction yield and the result showed that an enzyme concentration of 1.5% could provide sufficient amounts of activity for the hydrolysis of cell walls.
3.1.3 Effect of extraction temperature
As seen in Fig. 1c, various temperatures (40, 45, 50, 55 and 60 °C) were employed during extraction process while maintaining the ratio of liquid to solid 15 mL/g, ratio of pectinase to raw material 1.0% and extraction time 60 min to investigate the effect of temperature on the cAMP yield. It showed that the extraction yield of cAMP rose as the extraction temperature increases from 40 to 50 °C, and started to decrease after extraction temperature exceeded 50 °C. However, when the extraction temperature went beyond a certain threshold (50-60 °C), the extraction yield started to decrease.This might be ascribed to the decreased number of acoustic cavitation bubbles created by the ultrasound.
3.1.4 Effect of extraction time
The effect of extraction time on extraction yield of cAMP from jujube was shown in Fig. 1d. Various extraction times were investigated, namely, 30, 60, 90, 120 and 150 min, while the ratio of liquid to solid, ratio of pectinase to raw material, extraction temperature were fixed at 15 mL/g, 1.0% and 40 °C, respectively.It could be found that with increasing extraction time from 30 to 90 min, the extraction yield of cAMP from jujube increased from low to high till at 90 min to maximum, and then dropped at 90 min. In view of time consumption, extraction time ought not to exceed 120 min.
3.1.5 Optimization of UAPE conditions and validation of the model
An orthogonal array design (OAD) of UAPE of cAMP was designed based on the previous results from single factor experiments in order to optimize the combination of parameters. Four factors, the ratio of liquid to solid, ratio of pectinase to raw material, extraction temperature and extraction time, were selected for optimization. The total evaluation index was used to analysis by statistical method. The analysis results of OAD and extreme difference (Rvalue) analysis are presented in Table 1. The cAMP obtained from each test was pretreated and quantitatively analyzed according to the method as above discussed in Section 2.10. Although the maximum yield of cAMP was 731.88 µg/g, we couldn’t select the best extraction conditions only based on these outcomes in Table 1, and a further orthogonal analysis was wanted. The analysis of extreme difference indicated that the influential order of the 4 factors on the extraction yield of cAMP wasA(ratio of liquid to solid) >B(ratio of pectinase to raw material) >D(extraction time) >C(extraction temperature)(Table 1). According to variance analysis, the contributions of ratio of liquid to solid, ratio of pectinase to raw material and extraction time for the extraction yield of cAMP were significant, whereas extraction temperature was not significant factor. The order was in agreement with the order based on the values ofFin variance analysis (data not shown). Based on this analysis, and considering the cAMP extraction efficiency, the cost of energy and the feasibility of experiment, the optimum conditions of extraction were therefore determined as follows:A2B1D1C1(ratio of liquid to solid 5 mL/g, ratio of pectinase to raw material 1.5%, extraction time 60 min, extraction temperature 40 °C). Through confirmatory test, we got the highest yield of cAMP(783.0 µg/g).
3.2 Cytotoxicity of cAMP
The cytotoxicity of cAMP on the RBL-2H3 cells was evaluated quantitatively by the MTT assay after the 24 h treatment of cells with different concentrations of cAMP. As shown in Fig. 2, the appropriate cAMP concentration work on the RBL-2H3 cells was determined to be 0-100 µg/mL. The concentration of the cAMP down to 100 µg/mL had no detectable effect on the cell viability of the RBL-2H3 cells compared with the control, which were used for subsequent experiments. The cell viability decreased significantly when the concentration of cAMP up to 600 µg/mL.

Fig. 2 Cytotoxicity of cAMP on RBL-2H3 cells. The cells were incubated with different concentrations (0, 50, 100, 300, 600, 1 000 µg/mL) of cAMP for 24 h. The cytotoxicity of the samples was determined by MTT assay. The results were expressed as the mean ± SD (n = 5-8). The cell viability results were not significantly different treated with cAMP ranged from 0-100 µg/mL(P > 0.05). The control was cells untreated with cAMP.
3.3 Observation of degranulation of RBL-2H3 cells
The morphology of the cells was observed under an inverted microscope after being treated with cAMP at different final concentration of 25, 50, 100 µg/mL to RBL-2H3 cell allergy model.Simultaneously, blank group, negative control group, allergy group(without any treatment) and positive control group (ketotifen fumarate 50 µg/mL) were used for comparison. The results were shown in Fig. 3.
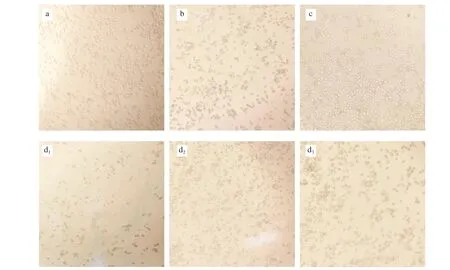
Fig. 3 Effect of different concentrations of cAMP on degranulation of RBL-2H3 cells. The degranulated images of the sensitized cells mediated by IgE were collected after being stimulated. Different groups were set as follows. (a) Blank group, with no IgE sensitization, no DNP-BSA stimulation, no cAMP intervention.(b) Allergy group, RBL-2H3 cells were sensitized with IgE and stimulated with DNP-BSA, no cAMP intervention. (c) Positive control, sensitized cells been stimulated with DNP-BSA after pretreatment with ketotifen fumarate at 50 µg/mL. (d1, d2, d3) Test group, sensitized cells been stimulated with DNP-BSA after pretreatment with different concentrations of cAMP. were separately shown on from left to right Sequential fluorescence images of antigen-stimulated cells treated with cAMP of 25, 50 and 100 µg/mL.
High density cells in the blank group were distributed evenly with good growth, fiber shape, smooth and shiny edges, large and clear morphology. Compared with the blank control group, the allergy group showed obvious degranulation reaction. The cells were shrunk to granulate or oval shape and showing deeper color, as well, the particulate matter can be seen inside the cells of the allergy group. It can be seen from the Fig. 3 that different concentrations of cAMP had mitigation effect on cell degranulation in a certain degree,although it was not as good as the positive control group, which was used allergy treatment drug ketotifen fumarate 50 µg/mL, showing significant alleviated effect on degranulation. The degree of swelling and deformation of fraction cells applied by 50 and 100 µg/mL cAMP were weakened and surrounded by fewer particles. While sensitized cells treated with 25 µg/mL cAMP had a less apparent effect.
3.4 Effects of cAMP on degranulation in RBL-2H3 cells stimulated with antigen
β-Hex activity is a key indicator for evaluating cellular allergy,whether it has the potential to inhibit the activation and degranulation of mast cells were determined by measuring the activity ofβ-hex [26].The results ofβ-hex activity in different treatment of cAMP groups were shown in Fig. 4. In the allergy group, the degranulation rate of cells was high to 55.69% by being sensitized with anti-DNP and stimulated with DNP-BSA, was significantly different from the control group withβ-hex activity at 29.47% (P< 0.01). Compared with the control group, 100 µg/mL cAMP in the test group had no inhibitory effect onβ-hex release (P> 0.05). Theβ-hex activity after treated with 25 and 50 µg/mL cAMP were 38.13% and 38.68%,respectively (P< 0.01), and the value of the positive control group reached the normal level. The present studies have found that cAMP significantly reduced the release ofβ-hex in sensitized RBL-2H3 cells, and markedly reduced the secretion ofβ-hex enzymes by about 20%, reaching the same normal level as ketotifen fumarate. The positive control group reached normal level.

Fig. 4 Determination of different concentrations of cAMP effected on β-hex release in IgE-antigen-complex-stimulated RBL-2H3 cells. The IgE-sensitized RBL-2H3 cells were pretreated with cAMP for 1 h, followed by treatment with antigen (DNP-BSA 10 µg/mL) for 30 min. The absorbance was measured on a microplate reader at 450 nm. Blank group, without IgE + DNP-BSA + cAMP.Allergy group, IgE + DNP-BSA; Positive control, IgE + DNP-BSA + ketotifen fumarate (50 µg/mL). Test group, IgE + DNP-BSA + cAMP. The values were mean ± SD (n = 6). Different letters indicated significant differences between groups (P < 0.05).
3.5 Effects of cAMP on IL-10 and TNF-α in RBL-2H3 cells stimulated with antigen
The supernatant of RBL-2H3 cells was collected, and the levels of pro-inflammatory factor TNF-α and anti-inflammatory factor IL-10 released by the cells were measured by ELISA kits. The results were shown in Fig. 5. Compared with the blank group, the content of two different cytokines in RBL-2H3 cells after DNP-BSA stimulation and sensitization was significantly increased (P< 0.01). The concentration of IL-10 after different treatments was shown in Fig. 5a. Compared with the allergy group, the concentration of anti-inflammatory factor IL-10 co-incubated with cAMP increased significantly, with 25, 50 and 100 µg/mL cAMP increasing by 28%, 31% and 40%,respectively. The manner of cAMP work on the concentration of IL-10 showed a cAMP concentration-dependent increase. After treatment with different concentrations of cAMP, the concentration of TNF-α was shown in Fig. 5b. It can be seen that compared with the allergy group, the concentration of pro-inflammatory factor TNF-α in the cells was shown a downward trend, and cAMP at 25 and 50 μg/mL were reduced by 30% and 48%, respectively(P< 0.01). The effect on reducing IL-10 concentration of 50 µg/mL cAMP was equivalent to that of ketotifen fumarate, which was as a positive control group.
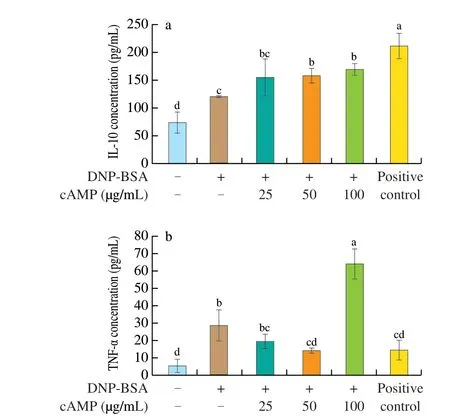
Fig. 5 Effect of cAMP on secrection of cytokines in RBL-2H3 cells.Detection of (a) IL-10 and (b) TNF-α in the supernatant of IgE-mediated RBL-2H3 cells treated with cAMP after being stimulated by DNP-BSA.Each value represented the mean ± SD (n = 5-8). Different letters indicated significant differences between groups (P < 0.05). Where positive control was the concentration from the reaction mixture of IgE + DNP-BSA + ketotifen fumarate (50 µg/mL).
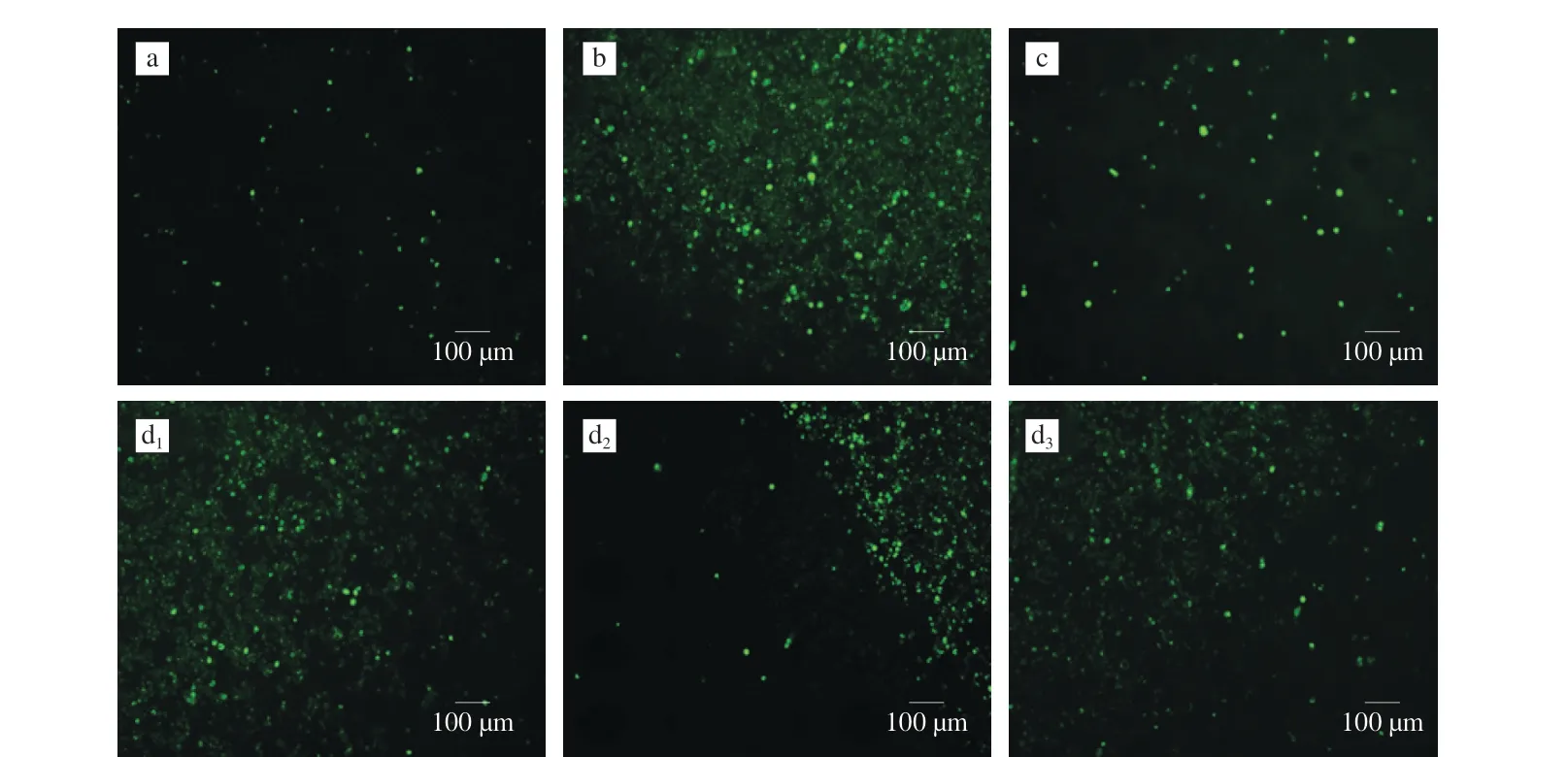
Fig. 6 Effects of cAMP on Ca2+ influx induced by DNP-BSA in RBL-2H3 cells. Fluorescence images collected at 5 s intervals (P < 0.01) in the test group and bank group were significantly different from the allergy group. (a) Blank group; (b) Allergy group; (c) Positive control; (d1, d2, d3) Test group.
3.6 Measurement of intracellular Ca2+
In the immunological mechanism of allergic reaction, Ca2+in the extracellular fluid served as a second messenger, which had the function of promoting the proliferation and differentiation of allergyrelated immune cells. Therefore, calcium was closely related to food allergies, and the intracellular calcium ions influxes were monitored using fluorescent probe Fluo-3-AM, which can specifically bind to calcium ions and reflect green light after being emited by fluorescence excitation. This method can be used to judge changes in calcium ion concentration.
After cAMP acted on the RBL-2H3 cell allergy model, the intracellular free calcium ion concentration was observed by inverted fluorescence microscope. The results were shown in Fig. 6. It can be seen from Fig. 6 that the concentration of Ca2+in the blank group was low, and the density and intensity of the fluorescent dots in the allergy group were significantly enhanced, which indicated that the calcium ion concentration was significantly increased. In the cAMP concentration range of 25-100 µg/mL, the decrease of calcium ion content was opposite to the increase of cAMP concentration.
4. Discussion
4.1 UAPE treatments on cAMP yield and the optimization extraction condition with OAD
The ratio of liquid to solid, ratio of pectinase to raw material,extraction temperature and time were considered as major factors that could affect the output of cAMP to determine the optimal condition of UAPE by single factor experiments. The ratio of liquid to solid of 5 mL/g was sufficient to reach the high extraction yield with the treatment of UAPE, as the ratio of pectinase to raw material,extraction temperature and extraction time were fixed at 1.0%, 40 °C and 60 min, respectively.
Similarly, with the different enzyme concentrations of 0.5%,1.0%, 1.5%, 2.0% (m/m), the enzyme concentration of 1.5% showed the highest cAMP extraction yield. Further increase in the enzyme concentration led to a significant decrease in the extraction yield, which might be that the viscous enzyme solution with a high concentration of enzyme is not conducive to the enzymatic reaction [8].
The extraction temperature could markedly influence the recovery of bioactive ingredients during liquid-solid extraction [27].Increasing the temperature of the extraction medium could increase the diffusivity of the solvent into the cells; which could also enhance desorption and solubility of the target compounds of the cells,resulting in the dissolution of the components [28]. However, when the extraction temperature went beyond a certain threshold (50 °C),the extraction yield started to decrease. This might be ascribed to the decreased number of acoustic cavitation bubbles created by the ultrasound.
With increasing extraction time from 30 to 90 min, the extraction yield of cAMP showed the tendency of improving till at 90 min to maximum, and then dropped at 90 min. This phenomenon might be due to the active ingredients will not be dissolved when the solubility of dissolving-out substances became saturated with the increase of extraction time, while the loss of cAMP was increased with the viscosity of extracts increased when extraction time increased [29].
After the single factor experiments, orthogonal tests were followed up to optimize the UAPE extraction conditions. And the best treatments including ratio of liquid to solid 5 mL/g, ratio of pectinase to raw material 1.5%, extraction time 60 min, extraction temperature 40 °C were observed, with the highest yield of 783.0 µg/g cAMP.Meanwhile, the present study indicated that the UAPE should be a potential technology to obtain cAMP in jujube.
4.2 The effect of cAMP on different stages of type I hypersensitivity
The main type of food allergy, type I hypersensitivity, was an IgEmediated immune response [30], which reactions were distinguished biphasically [31], and were divided into early and late hypersensitivity reactions [32]. The early reaction of allergies occurred within a few minutes of exposure to the allergen [33], while the late reaction occurred several hours later, involving the secretion of cytokines,such as TNF-α, IL-4, IL-10, etc. [34].
The cell allergy model induced by DNP-IgE/DNP-BSA mechanism was successfully established with appropriate concentration of cAMP for the allergy of RBL-2H3. The concentration range of cAMP effected on RBL-2H3 cells was determined to be 0-100 µg/mL, and the CC50(cell line half-toxic concentration) was between 300 and 600 µg/mL, which provided a feasible method for evaluating the anti-allergic effectin vitro.
4.2.1 Observation and assay of β-hex secretion during the degranulation in the early-phase reaction of type I hypersensitivity
Previous experiments reported that cAMP, as a key component of regulatory T cell-mediated suppression, had anti-inflammatory and immune suppressive effects [35,36]. In the early stage of type I hypersensitivity, cell granules were found due to the destruction of the immune system within a few minutes after stimulating sensitization.The alleviation of the degranulation reaction explained that cAMP had the effect of resisting the occurrence of a series of reactions in the early stage of the type I hypersensitivity [32].
By measuring the activity ofβ-hex to determine whether it has the potential to inhibit the activation and degranulation of mast cells.The inhibitory effects onβ-hex release from RBL-2H3 cells revealed that cAMP inhibited the degranulation of basophils and reduced the secretion of intracellular substances by inhibiting the release ofβ-hex.The obvious resistance toβ-hex release in the experiment indicated that cAMP can be developed and utilized as a promising new antiinflammatory agent.
4.2.2 Inhibitory effects of cAMP on IL-10 and TNF-α in the late-phase reaction of type I hypersensitivity
As an important pro-inflammatory cytokine, TNF-α played an important role in late hypersensitivity [37]. cAMP of 25 and 50 µg/mL exhibited against TNF-α, explaining that cAMP had the effect of resisting a series of reactions in the late stage of type I hypersensitivity. Decreased secretion of pro-inflammatory cytokines may also be attributed to cAMP regulating the progress of phosphorylation of some proteins [38]. The change of IL-10 was the opposite. Surprisingly, the effect of cAMP on IL-4 did not obvious,and the effect was not significant (the results in the experiment were not shown).
The signal transduction molecule PI3K mediated the Akt signaling pathway and caused the release of various inflammatory factors [39]. In the experiment, cAMP promoted the release of the anti-inflammatory factor IL-10 and inhibited the release of the inflammatory factor TNF-α, possibly regulated the body’s immune mechanism by affecting the activity of Akt. Allergy and inflammation were associated with enhanced production of Th2 cytokines(IL-10) and decreased production of Th1 cytokines (TNF-α). The effect of cAMP on Th1/Th2 cytokines of RBL-2H3 cells was reflected in the manner that up-regulated the level of IL-10 and down-regulated the TNF-α to exert the Th1/Th2 balance [40]. The levels of the two cytokines also had a certain regulatory effect on the downstream effector cells of allergic reactions [41].
4.3 Inhibitory effects of cAMP on extracellular Ca2+ influx
Intracellular Ca2+was derived from the release of Ca2+in the endoplasmic reticulum calcium pool and the influx of cellular Ca2+,which played a role in cell signal transaction [34]. The intracellular Ca2+concentration was involved in the immune regulation of the human body and had a certain effect on the allergic reaction. After basophils were sensitized by DNP-BSA stimulation, FcεRI, which was a high-affinity receptor for the Fc fragment of IgE, bounds to immune globulin’s cross-linking on the cell membrane, accumulated a large number of tyrosine kinase Syk, and induced phospholipase Cγ phosphorylation accompanied by releasing from intracellular stores of calcium ions from Ca2+release-activated Ca2+channels. cAMP induced plasminogen activator inhibitor-1 expression in mast cells [42].Ca2+served as the junction of many classic signaling pathways, and converted extracellular signals received by cells into intracellular signals. Increased intracellular Ca2+concentration triggered a conformational change by binding to CaM, which affected the Ca2+/CaM signal transaction pathway associated with degranulation, and further activated protein kinases to participate in the transmission of vesicles and the fusion of particles and membranes, which was beneficial to cell degranulation and cytokines production [43].The production of cytokines and inflammatory mediators were also promoted through transcription factor and protein kinase pathways [44]. In addition, some inflammatory mediators were also involved, such as prostaglandins and arachidonic acid [45].cAMP intervention of 25-100 µg/mL dose-dependently significantly inhibited the intracellular flow of Ca2+in DNP-BSA-stimulated sensitized RBL-2H3 cells was found, with the same trend of inhibiting the release ofβ-hex from the RBL-2H3 cells. These findings indicated that cAMP affected degranulation through a Ca2+dependent pathway, and indirect indicated that cAMP suppressed RBL-2H3 cell degranulation and regulated the release of cytokines, which may via impacting the Ca2+influx [46]. Therefore, cAMP can effectively inhibit the absorption of extracellular Ca2+, thereby inhibiting the degranulation of RBL-2H3 cells, affecting the level of cytokines,regulating the immune system, and enhancing immunity.
5. Conclusions
In this study, the extraction of cAMP from jujube by UAPE was optimized by OAD, and the yield of the extracts was evaluated.The optimal conditions to obtain the highest cAMP yield of jujube by UAPE were determined to be at ratio of liquid to solid 5 mL/g,ratio of pectinase to raw material 1.5%, extraction time 60 min and extraction temperature 40 °C. Based on the results, we concluded that UAPE was a promising technique for extracting cAMP from jujube,which might play an important role in improving the extraction yield of cAMP and sustainably using jujube resources. While, our results suggested that cAMP had an anti-allergic effect that reduced levels of degranulation and regulated secretion of cytokine release cytokines by the inhibition ofβ-hex and the blocking of intracellular Ca2+influx production. As a conclusion, all results showed that, although cAMP’s function on the inhibition of protein phosphorylation and the expression of related gene were not determined. cAMP were significantly effective in reducing allergic responses through antiinflammatory action.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgments
This research was supported by grant from the National Key Research and Development Program of China (2018YFC1602201),the Open Research Fund Program of Beijing Key Lab of Plant Resource Research and Development, Beijing Technology and Business University (PRRD-2021-YB8), the National Natural Science Fund (31601395) and the Key Program for Shaanxi Science and Technology (2020NY-146).
- 食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard

