Influence of soil physicochemical properties,particle size fractions and mineralogy on the leaching potentials of arsenic and antimony in abandoned mine soils
A.S.M.Fazle BARI ,Dane LAMB ,GeoffR.MACFARLANE and Mohammad Mahmudur RAHMAN
1Global Centre for Environmental Remediation (GCER),College of Engineering,Science and Environment,The University of Newcastle,Callaghan,NSW 2308(Australia)
2Department of Soil Science,Sher-e-Bangla Agricultural University,Dhaka 1207(Bangladesh)
3Chemical and Environmental Engineering,School of Engineering,RMIT University,Melbourne,Victoria 3000(Australia)
4School of Environmental and Life Sciences,The University of Newcastle,Callaghan,NSW 2308(Australia)
ABSTRACT At abandoned mine sites,arsenic(As)-and antimony(Sb)-enriched soils are often disposed of through onsite burial or capping.In highly weathered mine sites,the mobility of As and Sb is typically controlled by iron(Fe)(III)/Fe(II)phases;thus,the suitability of such disposal methods and appropriate testing techniques are questionable.In the present study,leaching potentials of As and Sb were examined using the toxicity characteristic leaching procedure(TCLP),waste extraction test(WET),and WET-extended procedure(WET-EXT)at three abandoned mine site soils in Australia.The leached concentration of As regularly exceeded USEPA criteria(5 mg L-1).The highest leached concentrations of As and Sb were observed in the finest particle size fraction(<0.053 mm)by WET-EXT(1 040 mg L-1 for As and 21.10 mg L-1 for Sb)followed by WET(800 mg L-1 for As and 20.90 mg L-1 for Sb).The TCLP method resulted in the lowest concentrations of leached As(0.000 9 mg L-1)and Sb(0.000 3 mg L-1).Crystalline and amorphous As-bearing Fe oxides were the main phases in the soils studied.However,the best correlations of leached As determined by TCLP(0.832),WET(0.944),and WET-EXT(0.961)were found with the non-specifically sorbed(NS1)As fraction.The mineralogical and sequential extraction data clearly indicate the dominant role of Fe geochemistry in controlling leachability of As and Sb.The TCLP method was unlikely to be suitable for assessing leachability,as it exhibited no relationship with leachable Fe and substantially lower leached As and Sb than the other two methods.Given the high to extremely high leachable As and Sb concentrations,most of the soil samples would not be recommended for placement in capping works,old shafts,or reduction systems(e.g.,collection in drainage basins).
Key Words: particle size fraction,sequential extraction,toxic element,toxicity characteristic leaching procedure,waste extraction test
INTRODUCTION
Arsenic (As) and antimony (Sb) are potentially toxic and carcinogenic metal(loid)s that often coexist in terrestrial environments.Contamination with these toxic elements usually occurs during mining and ore processing(Gálet al.,2007;Bariet al.,2020).Abandoned mine site soils often contain various As and Sb minerals,including arsenopyrite(FeAsS),pyrite(FeS2),scorodite(FeAsO4·2H2O),tooeleite(Fe6(AsO3)4(SO4)(OH)4·4H2O),tripuyite(FeSb2O6),and stibnite(Sb2S3)(Modabberi and Moore,2004;Bariet al.,2020).Oxidative dissolution of sulfide minerals can release As and Sb and seriously contaminate the surrounding soil and groundwater (Haffert and Craw,2008;Filellaet al.,2009).Moreover,these toxic elements can cause adverse health effects to humans through the food chainviathe soilwater-plant system,i.e.,they may accumulate from the soil to crop tissues bound for human consumption(Leeet al.,2007).
Many metalliferous ore mining sites are in abandoned status worldwide(Mayeset al.,2009;Bolanet al.,2017).However,limited attention has been focused on the impact of mining activities/wastes,which can disperse metal(loid)toxicants,including As,Sb,lead (Pb),and zinc (Zn) into adjacent environments.Due to the lack of knowledge of such potential impacts,massive volumes of highly contaminated mining wastes generated from abandoned metalliferous mines have historically been haphazardly left on mining areas,and in some circumstances,transported into neighboring waterbodies(Bolanet al.,2017).In addition,the major concern associated with abandoned mine sites containing sulfide minerals is the generation of acid mine drainage,which contains significant metal(loid)loads(Nordstrom,2011).Toxic and carcinogenic metal(loid)exposure from abandoned mine sites occurs in various locations in Australia,including in New South Wales(NSW)(Bolanet al.,2017).Moreover,the most common and easiest method of abandoned mine waste disposal is to relocate and isolate the waste in landfills or cover them with clean soil without considering highly engineered structures or the subsequent leaching processes.Although landfilling is a simple and economical method of waste disposal,it can lead to serious environmental pollution owing to the release of contaminants(El-Fadelet al.,1997).Therefore,it is essential to evaluate the leaching potentials of toxicants from contaminated soils to assess their suitability for disposal.
When soil is contaminated with extreme levels of toxic elements,it is necessary to determine the risks posed by contamination.For risk assessment,three routes are typically considered:direct human exposure,leaching,and ecological risk(Janget al.,2002).Most national guidelines to remediate contaminated soils are based on total toxic element content,which is unlikely to represent the relevant portion that is available for leaching to groundwater.Leaching approaches are typically used to estimate the mobility of toxic elements in groundwater(Hartleyet al.,2004).For this reason,it is crucial to identify whether the waste is hazardous or nonhazardous using different leaching methods before disposal of wastes containing As or other metal(loid)s.Different chemical leaching procedures,such as the synthetic precipitation leaching procedure (SPLP) (USEPA,1994),the U.S.Environmental Protection Agency (USEPA) toxicity characteristic leaching procedure(TCLP)(USEPA,1992),and the California waste extraction test (WET) (USEPA,2003),have been used to determine potentially harmful waste materials and possible movement from landfill sites.Among them,TCLP is the most widely used test for characterizing As-containing wastes as either hazardous or non-hazardous,although it underestimates the leaching of As under landfill conditions (Hooperet al.,1998;Ghoshet al.,2004;Jinget al.,2008).Specifically,when the leachate concentration of As is>5 mg L-1,waste is considered hazardous(USEPA,1992).The WET method determines higher leachate As concentrations than the TCLP and is designed to assess leaching from municipal solid waste(Hooperet al.,1998;Jinget al.,2005).Previous studies have considered both TCLP and WET for landfill disposal of As-bearing solid wastes,but the application of these methods to abandoned mine soils is scarce.Generally,TCLP is used to define extracted metal(loid)s from solid wastes,whereas WET is used to determine wastes that can contaminate groundwater in municipal solid waste landfills.On-site disposal or capping of highly As-and Sb-contaminated soils at abandoned mine sites is common.However,As and Sb mobility is controlled by the iron Fe(III)/Fe(II)phases in highly weathered conditions.Redox processes are crucial factors controlling the mobility of metal(loid)s in sulfide tailing deposits.Reductive dissolution of Fe minerals enhances the mobility of As,Fe,and manganese(Mn)within sulfide tailings(Lindsayet al.,2015).Similarly,the mobility of Sb in soils,sediments,and groundwater systems is mainly governed by its interactions with Fe(III)oxide minerals.Under reducing conditions,the reductive dissolution of these minerals enhances the release of both As and Sb(Burtonet al.,2019).
Leaching studies are usually based on ethylenediaminetetraacetic acid(EDTA),TCLP,water extraction,and sequential extraction(Sukandaret al.,2006;Madridet al.,2007;Zonget al.,2016).The leaching potentials of heavy metal(loid)s,including As and Sb,differ for different particle fractions of the soil(Acostaet al.,2011).In addition,minerals in abandoned mine soils play a pivotal role in As and Sb leaching.However,studies on the interactive effects of mineralogy and soil particle size fraction on the leaching of As and Sb in abandoned mine soils are rare.Thus,the objectives of this study were to i)evaluate the effects of particle size fractions and physiochemical properties of abandoned mine soils on the leaching of As and Sb,ii)investigate the role of mineralogy in the leaching of As and Sb,and iii)investigate the influence of As fractionation on the leachability of As and Sb.
METERIALS AND METHODS
Sampling and characterization of soils studied
The geology and other characteristics of the study sites have been reported previously(Bariet al.,2020)and are described in the Supplementary Material.Soils were collected from three abandoned mine sites: Webbs Consols (WC1 and WC2 soils),Halls Peak(HP1 and HP2 soils),and Mole River(MR1—MR3 soils).After collection,the soil samples were air-dried,homogenized,and sieved using an automatic shaker to separate the soil into the following particle fraction groups for further characterization:i)4—2 mm,ii)2—1 mm,iii)1—0.25 mm,iv)0.25—0.125 mm,v)0.125—0.053 mm,and vi)<0.053 mm.The micropipette method was used to determine the textural class of bulk soil(<2 mm)(Miller and Miller,1987).Both water(ultrapure,Milli-Q)and calcium chloride(CaCl2,0.01 mol L-1)solution with a soil:solution ratio of 1:5 were used to determine the soil pH(Rayment and Higginson,1992).The total organic carbon(TOC)content was determined using a carbon,nitrogen,and sulfur(CNS)analyzer(LECO,USA).The cation exchange capacity(CEC)of the soil was estimated as previously described(Gillman and Sumpter,1986).Soil samples were digested in a microwave using aqua regia,following the USEPA 3051 protocol.The samples were then filtered and analyzed by either inductively coupled plasma(ICP)-mass spectrometry(MS)or ICP-optical emission spectrometry(OES)to determine total major and trace elements.Air-dried and finely ground soil samples were used for the mineralogical study.The crystalline phases of the minerals were studied for both soil samples(pre-and post-leaching)by X-ray diffraction(XRD)(PANalytical Empyrean diffractometer,PANalytical B.V.,The Netherlands)under the following operating conditions:copper(Cu)-Kαmonochromatic radiation,0.02°for 2θstep size with 1.25 s counting(5°—70°).The morphology and elemental composition of the selected soils were analyzed using scanning electron microscopy with energy dispersive spectroscopy(SEM-EDS,ZEISS Sigma VP FESEM,Germany).Transmission electron microscopy with energy dispersive spectroscopy (TEM-EDS,JEOL LaB62100,Japan) was used to identify the diffraction patterns of the As minerals,morphology,and composition of the selected soils.
Fractionation of As
Arsenic fractionation for each soil sample was determined as previously described (Wenzelet al.,2001)and it included five sequential steps.The detailed procedure is presented in Table I.At the end of each extraction step,the suspension was filtered(0.45 μm)using mixed cellulose ester filters after centrifugation(4 000 r min-1for 15 min)for further analysis by ICP-OES.
Leaching tests
Three leaching tests were conducted to assess the leaching potentials of As,Sb,and other co-contaminant elements in the soil samples from the abandoned mine sites.
TCLP.The TCLP test was conducted according to the EPA method 1311 (USEPA,1992).To prepare the TCLP solution,5.7 mL of glacial acetic acid and 64.3 mL of 1 mol L-1sodium hydroxide(NaOH)were added to 500 mL of ultrapure water (18.2 MΩ cm) and diluted to 1 L.The solution pH was adjusted to 4.93±0.05.Each soil sample(1 g)and 20 mL of the TCLP solution were in a falcon tube and placed on an end-over-end shaker for 18 h;the solution was collected through filtration after centrifugation.
WET and WET-extended procedure(WET-EXT).The WET extraction was performed according to the California Code of Regulations(USEPA,2003).We hypothesized that WET extraction using citrate would target a considerable amount of non-specifically sorbed(NS1),specifically sorbed(SS2),and amorphous Fe and Al oxides-bound(AF3)fractions.The WET extraction solution contained 0.2 mol L-1citrate;the solution was prepared with monohydrate citric acid and titrated to pH 5 with 4 mol L-1NaOH.The solution was purged with nitrogen gas for 30 min to simulate an anoxia environment.Briefly,1 g of soil sample and 10 mL of WET solution were added to each falcon tube,and the tubes were placed on a rotary shaker for 48 h.The WET test was extended(WET-EXT)by increasing the extraction time from 48 to 96h.
Statistical analysis
Results are presented as the mean of duplicate data.SPSSS 25 (IBM,USA) was used for statistical analysis.Spearman’s correlation coefficient was used to define the relationships between variables.
RESULTS AND DISCUSSION
Soil characteristics
The detailed physicochemical characteristics of abandoned mine soils at each particle size fraction were discussed in our previous study(Bariet al.,2020)and are presented in Table SI(see Supplementary Material for Table SI).The studied soil samples were acidic in nature,with a pH range of 3.92 to 5.89.The acidic pH of the soils may be attributed to the oxidation of sulfide minerals or acidic wash from elsewhere on site.The TOC content of the soils ranged from 6.7 to 50.6g kg-1.The lowest pH(3.92)was found in soils from WC1 and the highest pH(5.98)was found in MR2.The highest TOC was determined in HP1,whereas the lowest was determined in MR2.The highest total As concentration(107 800 mg kg-1) was recorded in MR3 and the lowest(15 mg kg-1)in MR2,similar to that found for Sb(highest,1 010 mg kg-1;lowest,0.13 mg kg-1).

TABLEISequential extraction procedure for As in abandoned mine soils
Leaching potentials of As and Sb
The different leaching test results for the bulk soil(<4 mm)and particle size fractions are presented in Table II.The bulk soil released less As and Sb than the fine particle size fractions(0.25—0.125,0.125—0.053,and<0.053 mm)during the leaching tests.The ranges of leached As concentrations in the various soil particle fractions were 0.000 9—55.00,0.31—800.00,and 0.46—1 040.00 mg L-1for TCLP,WET,and WET-EXT,respectively.The ranges of leached Sb were 0.0003—2.13,0.001—20.90,and 0.001—21.10 mg L-1for TCLP,WET and WET-EXT,respectively.The data showedthat leached As concentrations in abandoned mine soils from MR3 exceeded the regulatory limit(5 mg L-1by USEPA)according to TCLP results,whereas the WET-EXT and WET tests showed that those in WC1,WC2,MR1,and MR3 exceeded the regulatory limit for soils.However,the regulatory limit for TCLP used in Australia is 0.7 mg L-1As(Clancyet al.,2013),and most soil samples exceeded it.Leached Sb concentrations in MR3 exceeded the regulatory limit by WET-EXT and WET.

TABLEIILeached concentrations of major and trace elements from abandoned mine soils obtained in different leaching testsa) in each particle size fraction of the studied soils
Of the three tested methods,TCLP leached the lowest concentrations of As and Sb(0.000 9 and 0.000 3 mg L-1,respectively).In addition,it showed a similar leaching pattern for both As and Sb.The highest As (1 040 mg L-1) was leached by WET-EXT,followed by WET (798 mg L-1).Similarly,WET-EXT leached the highest Sb(21.10 mg L-1),followed by WET(20.90 mg L-1).
The variation in the leached concentrations of As and Sb among the leaching methods was due to the use of different chemicals,duration of extraction,and air headspace volume,which influenced the leaching of As and Sb.Citric acid was used in WET,whereas acetic acid was used in TCLP.The higher As and Sb leaching by WET was due to the chelation of Fe by citrate following the dissolution of minerals and sorbents and subsequent mobilization of these elements(Hooperet al.,1998;Jinget al.,2005).Leaching tests are designed to estimate the leaching potential of waste for short periods compared with actual disposal or landfills.Eighteen hours were used for the TCLP procedure,whereas WET took 48 h.Higher As concentrations were observed in TCLP with increasing extraction duration (Hooperet al.,1998;Stuckmanet al.,2011).The use of air as the headspace gas in TCLP resulted in a lower As concentration in the leachate compared to that in the leachate of WET(Ghoshet al.,2004).
Based on TCLP,the finest particle fraction(<0.053 mm)of WC1 released 1.27 mg L-1of As,which may be considered a non-hazardous waste classification,as the regulatory limit of As is 5 mg L-1by USEPA.However,WET and WET-EXT released 270 and 385 mg L-1of As,respectively.Similarly,TCLP released only 2.13 mg L-1of Sb from the finest particle fraction of MR3 while WET and WET-EXT released 20.90 and 21.10 mg L-1of Sb,respectively.The WET showed a stronger correlation(0.974)between the total and leached As concentrations compared with TCLP(0.776)(Table SII,see Supplementary Material for Table SII).A similar pattern was found for all the soil samples examined in this study.Consistent with our findings,previous studies also reported that TCLP may underestimate the leaching of As and Sb from landfills.Therefore,the leaching test by TCLP for landfills containing elements that form oxyanions(e.g.,As,Sb,molybdenum(Mo))may not be ideal(Hooperet al.,1998;Ghoshet al.,2004;Jinget al.,2008).However,higher As leachability by WET compared to TCLP has been reported(Ghoshet al.,2004);the nitrogen gas used to maintain the anoxic environment,which is more reducing,influences As mobilization in WET.
Leaching potentials of As and Sb based on soil particle size
The leached As and Sb concentrations increased with decreasing particle size for all the leaching methods examined (Figs.1 and 2).The lowest leached As (0.001 mg L-1)and Sb(0.000 3 mg L-1)concentrations were observed in the coarse(4—2 mm)particle size fraction of soil samples obtained by TCLP from HP2 and MR2,respectively.The highest As(55.0 mg L-1)and Sb(2.10 mg L-1)concentrations were found in the finest particle size fraction of soil from MR3.In addition,WET and WET-EXT displayed higher concentrations of As and Sb in the fine particle size fractions and the lowest concentrations in the coarse particle size fractions.The highest As(800 mg L-1)and Sb(20.90 mg L-1) concentrations of leachates were found in the finest particle size fraction by WET and the lowest(As,0.40 mg L-1;Sb,0.001 mg L-1)in the coarse(4—2 mm)particle size fraction.The WET-EXT released the highest amounts of As(1 040 mg L-1)and Sb(21.10 mg L-1)from the finest particle size fraction of soil among the three examined leaching methods.Inuiet al.(2016)reported that fine particles have a greater leaching potential for As and other metal(loid)s.Higher leaching potentials of As,Sb,Pb,and cadmium(Cd)were also observed in the fine particle size fractions due to the higher concentrations of these elements and larger surface area available in the fractions to interact with the extraction solution(Zhou and Hursthouse,2019).
Strong correlations were observed between the total and leached concentrations of As and Sb using all the leaching methods.The WET-EXT showed the highest correlations for As(0.960)and Sb(0.716)(Table SII).Zonget al.(2016)also reported that the leaching of heavy metals was higher in fine particle fractions than in the other soil fractions.The key factors that influence the leaching potentials of As and Sb in mine tailings are ore treatment,geogenic material oxidation,and weathering.In addition,mineralization,As distribution,oxidation states,and crystallinity of As are more favorable for the fine particles of mine tailings than for coarse particles.Sparingly soluble As minerals are present in coarse particle fractions,whereas fine particle size fractions may be associated with a more or less soluble pentavalent amorphous phase of As(Meunieret al.,2011).In addition,fine particles usually contain high concentrations of As and Sb,and this fraction provides a high specific surface area for reaction with the extraction solution,resulting in higher levels of extraction(Zhou and Hursthouse,2019).
Influence of physicochemical properties on the leaching of As and Sb

Fig.1 Leached concentrations of As from particle size fractions of abandoned mine soils in different leaching tests:toxicity characteristic leaching procedure(TCLP),waste extraction test(WET),and waste extraction test-extended procedure(WET-EXT).WC1 and WC2=soils from Webbs Consols;HP1 and HP2=soils from Halls Peak;MR1,MR2,and MR3=soils from Mole River.
The effects of soil physicochemical properties on As and Sb leachability were determined using Spearman’s correlation analysis and are presented in Table SII.The leaching of As and Sb,as measured by the three leaching methods,showed strong correlations with soil electrical conductivity(EC),total As,total Sb,and total Fe.In contrast,soil pH,Mn,and Al were negatively correlated with leached As and Sb.The positive correlation with total As,Sb,and Fe may be attributed to the higher elemental concentration and dissolution of Fe-As minerals.The negative correlation of leached As and Sb with Mn and Al can be attributed to the As transformation enabled by Mn and Al oxide complexation (Daset al.,2013).Soil pH is the most important factor controlling the mobility of metal(loid)s in abandoned mine areas,either by influencing the soil properties(e.g.,CEC,point of zero charge,etc.) or by direct proton (H+)competition on colloidal surfaces(Felletet al.,2011).Low pH has been shown to increase the leachability of As and Sb(Cappuyns and Swennen,2008).
Mineralogyand leaching potential of As
Tooeleite,arsenopyrite,arsenolite,and scorodite were the main As minerals identified using XRD (Fig.3).The detailed mineralogical characteristics are discussed in our previous study(Bariet al.,2020).Briefly,SEM-EDS was used to determine the morphologies of scorodite and tooeleite(Fig.S1,see Supplementary Material for Fig.S1).A platylayered,poorly crystalline structure was observed in the Mole River soil(MR3,particle size<0.053 mm)by TEM(Fig.S2a,see Supplementary Material for Fig.S2)with identical d-spacing(0.26nm)of tooeleite(Fig.S2b).In addition,a homogenous distribution of As and Fe was observed by EDS for the same sample(Fig.S2c).

Fig.2 Leached concentrations of Sb from particle size fractions of abandoned mine soils in different leaching tests:toxicity characteristic leaching procedure(TCLP),waste extraction test(WET),and waste extraction test-extended procedure(WET-EXT).WC1 and WC2=soils from Webbs Consols;HP1 and HP2=soils from Halls Peak;MR1,MR2,and MR3=soils from Mole River.
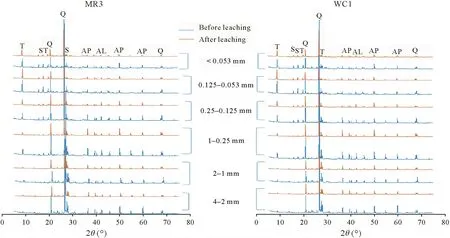
Fig.3 X-ray diffraction(XRD)patterns of two contrasting abandoned mine soils before and after leaching by the waste extraction test-extended procedure.T=tooeleite;S=scorodite;Q=quartz;AP=arsenopyrite;AL=arsenolite;MR3=soil from Mole River;WC1=soil from Webbs Consols.
We observed a wide range of As concentrations,from low(0.000 9 mg L-1)to extremely high(1 040 mg L-1),in the leachate of the different methods(Table II).Limet al.(2009)reported low leaching of As(0.43 mg L-1)from abandoned metal mine tailings using TCLP.The low concentrations of As and Sb in the leachate indicate that readily soluble As and Sb fractions may be released during the oxidation of sulfide minerals for long periods in abandoned mine soils.However,the high leachability of As can be attributed to the presence of less stable As minerals (tooeleite) in the tested mine soil(Herbel and Fendorf,2006).The presence of highly insoluble minerals,including arsenopyrite,scorodite,and stibnite,in the soil resulted in lower leaching of As and Sb.However,the oxidative dissolution of these minerals can be a source of As and Sb in aqueous environments(Fosteret al.,2011;Majzlanet al.,2016).The XRD analysis of the post-leached soil showed that the peak intensities of minerals were reduced owing to leaching by different methods,which indicated the contribution of these minerals to As leaching(Fig.3).
Leaching potentials of As and Sb in relation toFe leaching
Mineralogical investigations confirmed that soil samples were dominated by Fe-As minerals,indicating the coexistence of Fe and As in the studied soil samples.This was expected because As originates from Fe-sulfide ores(Bolanet al.,2017).The present study revealed that the leaching potentials of As and Sb increased with increasing leached Fe for most soil samples(Table II).For instance,leached As(47 mg L-1)and Fe(123 mg L-1)concentrations obtained by WET were the lowest in the coarse(4—2 mm)particle size fraction,whereas the leached As concentration(266mg L-1)increased with increasing leached Fe concentration(780 mg L-1) in the finest (<0.053 mm) particle size fraction of WC1(Table II).The As leaching potential increases with increasing Fe leachability from As-bearing solid residuals(Ghoshet al.,2006).
In addition,the TCLP-leached Fe showed no correlation with leached As(-0.141)and Sb(0.297).In contrast,WET leached more Fe,resulting in a strong correlation with the leached As(0.926)and Sb(0.649).Iron leached by WETEXT also showed a strong correlation with both leached As(0.929)and Sb(0.648)(Table SIII,see Supplementary Material for Table SIII)(correlations were statistically significant,P <0.01).The strong correlations of leached Fe with As and Sb by both the WET and WET-EXT methods indicate that the mobility of As and Sb may be controlled by Fe geochemistry.Lindsayet al.(2015)also have reported that the reductive dissolution of Fe minerals under highly weathered conditions increases the mobility of As,Fe,and Mn within sulfide tailings.Similarly,Burtonet al.(2019)have observed that Sb mobility in soils,sediments,and groundwater systems is mainly influenced by interactions with Fe(III)oxide minerals,and the reductive dissolution of these minerals may release Sb to the environment.
Relationship between leaching potential of As and sequentiallyextracted As
Sequential fractionation patterns of As influence the concentration of As and the physicochemical characteristics of soil samples(Baiget al.,2009).The detailed results from As fractionation have been described previously(Bariet al.,2020) and are presented in Table SI.Most As was bound to AF3 and crystalline Fe and Al oxides-bound fraction(CF4),intermediate amounts of As were found in SS2 and residual fraction(RS5),and the lowest As was found in NS1.In brief,there was no significant effect of the particle size fraction on the sequential fractionation of As(Fig.S3,see Supplementary Material for Fig.S3).
Arsenic in the first two fractions (NS1 and SS2) is typically more mobile than that in the other fractions,as the binding power of As with minerals usually increases from fraction 1(NS1)to fraction 5(RS5)(Kimet al.,2014).The leached concentration of As by TCLP was similar to that of fraction 1 (NS1) and lower than that of fraction 2(SS2),as measured by sequential fractionation(Table SIV,see Supplementary Material for Table SIV).For instance,the leached concentration of As(8.00 mg kg-1)by TCLP was similar to that of fraction 1(8.30 mg kg-1)but lower than that of fraction 2 (83.00 mg kg-1) in the 4—2 mm particle size fraction of WC1.The leached concentration of As by TCLP was lower than that of fraction 1 or 2(Leeet al.,2007).However,leached As concentrations by the WET and WET-EXT methods were higher than those of both sequential fractions(NS1 and SS2)(Table SIV).For example,the<0.053 mm particle size fraction of MR3 showed higher leached As concentrations by both leaching methods(7 987 mg kg-1for WET and 10 431 mg kg-1for WET-EXT)than the two sequential fractions(1 257 mg kg-1for fraction 1 and 966mg kg-1for fraction 2).However,a lower leached As concentration resulted from fraction 3(AF3)in all methods.Because the binding strength of As with AF3,CF4,and RS5 is higher compared to that in the first two fractions (NS1 and SS2 (Kimet al.,2014),it is likely that the extracting solution was unable to extract As from AF3,CF4,and RS5.Correlation analysis between As in the different sequential fractions and leached As by TCLP,WET,and WET-EXT was conducted to explore how the various As sequential fractions contribute to the leached As load(Table SV,see Supplementary Material for Table SV).Strong positive correlation coefficients (P <0.01,n=42) were observed between As fractions (NS1,NS1+SS2,NS1+SS2+AF3,and NS1+SS2+AF3 +CF4)and As leachability by all methods(TCLP,WET,and WET-EXT).The best correlations of leached As obtained by TCLP(0.832)and WET-EXT(0.961)were found with the NS1 As fraction.Notably,NS1 is the smallest fraction,and it might be primarily controlled by the total degree of contamination observed in the high correlation between extracted fractions and total As.The WET exhibited the best correlation of leached As with the first three As fractions(NS1,SS2,and AF3).The correlations of leached As with sequentially extracted As indicated that the main contributor of As leaching was the NS1 As fraction.Iron and Fe-As minerals also play vital roles in the mobility of As.The dissolution of less resistant minerals or sorption-desorption mechanisms are mainly attributed to the release of As and Fe from the wastes.The similar leaching potentials of As and Fe from wastes indicates that the release of As is related to the dissolution of Fe at low pH.Iron geochemistry plays a vital role in controlling the leaching potentials of As and Sb,as indicated by our sequential extraction data,which is also supported by previous findings(Lindsayet al.,2015;Burtonet al.,2019).
CONCLUSIONS
The abandoned mine soils were found to be extremely polluted with As and Sb.These inorganic pollutants can be released into the surrounding environmentvialeaching into the groundwater and surface water.Less As and Sb were leached by TCLP than by WET and WET-EXT.The leaching potentials of As and Sb increased with decreasing particle size.The best correlations of leached As obtained by TCLP,WET,and WET-EXT were found with the NS1 As fraction,which indicated that this fraction was the main contributor to As leaching.The present study suggests that TCLP substantially underestimates the leaching potentials of As and Sb from abandoned mine soils.We demonstrated that the leached concentrations of As often exceeded the USEPA criteria (5 mg L-1),while the leached Sb concentration exceeded this limit only for MR3.In addition,the leaching potentials of contaminants should be studied before disposing As-contaminated soils using appropriate methods.Given the high to extremely high leachable As and Sb concentrations,most of the soils would not be recommended for placement in capping works,old shafts,or reduction systems (e.g.,collection in drainage basins).Thus,TCLP was unlikely to be suitable for assessing leachability for these purposes.The leachable concentrations of As and Sb determined by TCLP were not related to the leached Fe.The mineralogical and sequential extraction data clearly indicate the dominant role of Fe geochemistry in controlling the leachability of As and Sb.The lack of a relationship between leachable Fe and substantially less leached As and Sb indicates that TCLP is not a suitable method for assessing As and Sb leaching at the examined abandoned mine sites.
ACKNOWLEDGEMENTS
We thank Prof.Nanthi BOLAN for his suggestions for improving the manuscript and EMX unit staff.We also acknowledge the University of Newcastle,Australia,for providing tuition fees and stipends to the first author.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found in the online version.
- Pedosphere的其它文章
- Carbon farming by recarbonization of agroecosystems
- Root exclusion methods for partitioning of soil respiration:Review and methodological considerations
- Utilization of lignocellulosic plant residues for compost formation and its role in improving soil fertility
- A critical review of microbially induced carbonate precipitation for soil stabilization:The global experiences and future prospective
- Hand-feel soil texture observations to evaluate the accuracy of digital soil maps for local prediction of soil particle size distribution:A casestudy in Central France
- Viscoelasticity and shear resistance at the aggregate scale of structured and organic carbon-free Chernozems

