Five-year warming does not change soil organic carbon stock but alters its chemical composition in an alpine peatland
Jingcong QIU ,Minghua SONG ,Chunmei WANG,* ,Xiaomin DOU ,Fangfang LIU ,Jiaxin WANG ,Chenying ZHU and Shiqi WANG
1College of Environmental Science and Engineering,Beijing Forestry University,Beijing 100083(China)
2Key Laboratory of Ecosystem Network Observation and Modeling,Institute of Geographic Sciences andNatural Resources Research,Chinese Academy of Sciences,Beijing 100101(China)
ABSTRACT Climate warming may promote soil organic carbon(SOC)decomposition and alter SOC stocks in terrestrial ecosystems,which would in turn affect climate warming.We manipulated a warming experiment using open-top chambers to investigate the effect of warming on SOC stock and chemical composition in an alpine peatland in Zoigê on the eastern Tibetan Plateau,China.Results showed that 5 years of warming soil temperatures enhanced ecosystem respiration during the growing season,promoted above-and belowground plant biomass,but did not alter the SOC stock.However,labile O-alkyl C and relatively recalcitrant aromatic C contents decreased,and alkyl C content increased.Warming also increased the amount of SOC stored in the silt-clay fraction(<0.053 mm),but this was offset by warming-induced decreases in the SOC stored within micro-and macroaggregates(0.053—0.25 and >0.25 mm,respectively).These changes in labile and recalcitrant C were largely associated with warming-induced increases in soil microbial biomass C,fungal diversity,enzyme activity,and functional gene abundance related to the decomposition of labile and recalcitrant C compounds.The warming-induced accumulation of SOC stored in the silt-clay fraction could increase SOC persistence in alpine peatland ecosystems.Our findings suggest that mechanisms mediated by soil microbes account for the changes in SOC chemical composition and SOC in different aggregate size fractions,which is of great significance when evaluating SOC stability under climate warming conditions.
Key Words: functional gene,nuclear magnetic resonance,simulated global warming,soil carbon components,soil enzymes,soil microbial diversity
INTRODUCTION
Global warming may promote ecosystem carbon (C)cycle and thus alter the soil organic carbon(SOC)stock in terrestrial ecosystems.These changes may potentially affect climate warming.Over the past century,northern ecosystems have warmed twice as fast as the global average and this has induced changes in the C cycle and SOC stock within peatland ecosystems(Helbiget al.,2017).Despite covering only 3%of the global land area,peatlands have acted as C sinks for thousands of years,store over 500 Gt of C,and play an important role in global C cycle(Yu,2012).The effect of warming on the SOC stock is complex and results vary depending on the ecosystem and the warming conditions.Warming can generally promote plant growth and increase photosynthetic C input into the soil,both of which could lead to increases in the SOC stock.In contrast,warming and increased litter input can also enhance soil microbial activity.These processes induce the decomposition of SOC and release of C from the soil pool,which will reduce the SOC stock.The different balances among these multiple effects may be the reason why warming has been found to have a positive,neutral,or negative effect on the SOC stock in different ecosystems (Griffiset al.,2004;Grogan and Jonasson,2005).
The functional composition and activity of soil microbial communities play important roles in controlling the processes associated with SOC decomposition(Jiaet al.,2017).Soil microbial communities and many related chemical and biochemical reactions can also be strongly affected by climate warming(Sunet al.,2019).Previous studies have found that warming can stimulate the activity of particular microbial communities with consequences for soil biogeochemical processes(Liet al.,2018).In Arctic tundra regions,climate warming has led to changes in soil microbial community and increased the abundances of functional genes related to the degradation of labile and recalcitrant C,consequently resulting in a C source(Xueet al.,2015).In addition,climate warming could promote soil microbial activity,leading to the production of more extracellular enzymes,which would further enhance SOC decomposition and result in the C loss from soil.In contrast,a study in which warming was only manipulated in the winter months found that winter warming enhanced soil microbial metabolism and resulted in a rapid turnover and decline in soil microbial abundance,which reduced SOC decomposition(Tianet al.,2021).The functionality of microbial communities is a key driver of SOC decomposition and is one of the important factors governing the ecosystem C balance(Campbellet al.,2022).Therefore,knowledge about the effects of climate warming on bacterial and fungal diversity,extracellular enzyme activity,and the abundance of functional genes related to the degradation of labile and recalcitrant C compounds is critical if understanding about the dynamics of SOC stock under global warming is to improve.
Besides the effects of soil microbes on the SOC stock,climate warming can also induce changes in the quality and quantity of leaf litter and root exudates,which could further alter the structure of soil microbial communities and their associated enzyme and functional gene abundances(Qinet al.,2021).Moreover,warming can promote atmospheric C fixationviaincreased plant photosynthesis and productivity.Increases in plant-litter C input provide more labile C for soil microbes,which can be used to enhance soil microbial abundance and activity and then increase original organic matter decomposition(priming effect)(Kuzyakov,2010).However,increased plant growth under warming demands more soil nutrients,especially soil available N.Microbial N mining driven by labile plant C input could induce the decomposition of old SOC in order to meet plant N requirements(Kuzyakov and Mason-Jones,2018).Therefore,the decomposition of old SOC is accompanied by new SOC formation,which causes changes in SOC chemical composition,especially shifts in labile and recalcitrant C compounds,even if the SOC stock remains stable over a certain period.
Soil organic C is mostly derived from plant leaf litter and root detritus,which are inputted into the soil and decomposed by soil organisms to varying degrees.Furthermore,soil C exists as different chemical compounds with different properties and turnover rates(Luoet al.,2019).The chemical structure and composition of SOC could determine the decomposition rate at which C is transferred from the soil to the atmosphere in response to warming.The SOC composition revealed by chemical functional group analyses can indicate the extent of degradation during SOC decomposition(Kgel-Knabner,1997).Moreover,the alkyl C and O-alkyl C fractions can indicate the relative stability of the SOC (Pautleret al.,2010).Although chemical compounds can signify the relative stability of SOC,the actual stability of SOC is closely associated with the biodegradability of the compounds and their availability to soil microbes.Thus,the amounts of soil particulates with physical,chemical,and biological protection will determine SOC decomposition rates.In general,macro-and microaggregates are mostly derived from plant litter and root exudates and largely consist of particulate organic residues.Silt-clay is mostly composed of minerals associated with organic matter(Witzgallet al.,2021).Soil particles can be classified into labile or recalcitrant fractions,and these fractions may have different sensitivities and responses to warming(Tianet al.,2021).Therefore,integrating signals associated with the chemical and physical compositions of SOC could improve understanding about SOC responses and sensitivities to warming.
The Zoigê Alpine wetland is situated in the eastern part of the Tibetan Plateau,China.It is the largest wetland in China and peatland,swamp,and marsh are widely distributed across the area.The average depth of the peat layer is about 1 m and a large amount of C is stored in the soil (Boagaet al.,2020).However,the Tibetan Plateau is experiencing climate warming at a rapidly increasing rate (Liet al.,2020)and the temperature has increased by more than 0.25°C per decade over the past 50 years (Duan and Xiao,2015).This rapid warming could stimulate a considerable release of SOC and any consequent increase in carbon dioxide(CO2)concentration in the atmosphere would further accelerate climate warming.To date,little is known about how climate warming alters the balance between C input and output.Furthermore,there is little information on the main mechanisms underlying warming-induced changes in soil labile and recalcitrant C fractions,which could have important effects on SOC stocks.Here,we hypothesized that warming could increase plant photosynthetic C input to a soil and simultaneously promote the decomposition of SOC already stored in the soil.The magnitudes of the C input and output could vary over different rates of warming.Balancing the C input and output could ultimately leave SOC stock unchanged regardless of warming rates.In addition,different rates of warming could induce shifts in soil microbe composition and enzyme activity,leading to further changes in the chemical components associated with SOC.
Therefore,the aims of this study were to i)test ecosystem respiration and plant biomass responses to preset warming conditions and measure their contributions to the balance between C input and output,ii)identify the effect of warming on soil microbial composition,enzyme activity,functional gene abundance,and SOC chemical composition,and iii)reveal the mechanisms underlying warming-induced changes in labile and recalcitrant SOC and SOC stock.We manipulated a gradient soil warming experiment that uses open-top chambers(OTC)to explore the effect of 5-year experimental warming on SOC chemical composition,dynamics,and stock in the Zoigê Alpine peatland on the Tibetan Plateau.Ecosystem respiration and plant biomass were measured and the SOC chemical components in the topsoil were characterized using13C nuclear magnetic resonance(NMR)after 5 years of warming.Soil microbial composition,soil enzyme activity,and functional gene abundance were also quantified.
MATERIALS AND METHODS
Site description andexperimental design
The manipulated warming experiment was carried out in the Zoigê National Nature Reserve Administration area(33°58′N,102°95′E;3 452 m above sea level)on the margin of the eastern Tibetan Plateau(Fig.S1a).It has a subalpine,continental,and monsoonal climate.The Zoigê wetland is a typical alpine wetland ecosystem and is the largest highaltitude peatland in the world.The annual mean temperature is 1.4°C and the average annual precipitation is 654 mm with concentrated precipitation from April to October(Lianget al.,2021).The growing season is from May to September,and the soil in the wetland is classified as humus swamp soil,which corresponds to histosols according to the FAO soil taxonomy(Gaoet al.,2016).The total vegetation cover is 93.38%and the dominant species areCarexmuliensisHand-Mazz.andCarex lasiocarpaEhrh.with a total coverage of 87%.Other abundant companion species arePotentilla anserinaL.andKobresia tibeticaMaxim.
The warming experiment started in May 2016.There were four treatments,which were no warming (control,CK)and three warming treatments(low-temperature(LT),medium-temperature(MT),and high-temperature(HT)warming).The experimental plots were arranged in a randomized complete block design with six replicates.Open-top chambers were used as the warming devices,and they had diameters of 120.4,131.9,and 143.5 cm at the base(Fig.S1,see Supplementary Material for Fig.S1).The OTCs conformed to the experimental scheme used in the International Tundra Experiment(Henry and Molau,1997).The OTCs were set up in the plots throughout the year to imitate climate warming.After installation of the OTCs,air temperatures at 5 cm above and below the ground and the soil moisture in each sample plot were recorded every 30 min using full-automatic multipoint soil temperature recorders(Onset,USA).
Measurement of ecosystem respiration andplant biomass
Ecosystem respiration,the only CO2flux data collected,was measured using static opaque chambers,which were made of stainless steel and consisted of removable covers(50 cm length × 50 cm width × 50 cm height) without bottoms and with fixed bases(50 cm length×50 cm width×20 cm height).The base was fixed in the wetland soil in advance and remained intact throughout the sampling periods to reduce any disturbance when the chamber was installed.A removable cover box was inserted into the fixed base when collecting gases.Two fans(10 cm in diameter for each one)and a digital thermometer were installed inside to ensure uniformity of the air and to monitor air temperature.The chambers were closed for half an hour and then gases were collected using plastic syringes every 10 min between 9:00 a.m.and 11:00 a.m.once a week in the growing season(May to September)from 2016to 2020.The CO2concentration was determined by a 4890D gas chamber chromatograph(Hewlett Packard,Palo Alto,USA)and soil CO2flux(F,mg CO2m-2h-1)was calculated according to Eq.1(Wanget al.,2018),
where dc/dtis the quotient for CO2concentrationc vs.timet,which is the variation rate for CO2,Mcorresponds to the mole mass of CO2with a value of 44 g mol-1,PandTindicate the ambient pressure and Kelvin temperature,respectively,V0,P0,andT0are the gas volume,atmosphere pressure,and temperature values,respectively,at standard conditions,andHis the height of the closed chamber.A coefficient of 12/44 was used as a multiplier to measure the flux by C rather than CO2(mg C m-2h-1).
Live aboveground plant organs in each plot (25 cm× 25 cm) were harvested and determined at the aboveground biomass in August 2020 and this represented plant productivity during the year.To determine the belowground biomass,18 soil cores(3.5 cm in diameter)up to 15 cm depth in each plot were collected.The depth was based on the fact that most of the roots were distributed within the 0—15 cm soil layer(Zhanget al.,2019).Every three soil cores were mixed,which left six mixed samples for each experimental treatment.The roots were carefully removed from the soil samples.Then,the roots and aboveground samples were carefully rinsed under a tap,air-dried,and finally oven dried at 65°C to a constant mass.
Soil sampling andphysicochemical analyses
Soil sampling followed the same procedure as that for underground plant biomass.The roots were first removed and then the soil samples were collected,passed through a 2-mm sieve,and mixed.Part of the sample was air-dried to measure the physicochemical properties and the rest was used as a fresh sample for analyses of soil microbial composition,abundance of functional genes,and soil enzyme activity.
A wet sieving method was used to separate the soil aggregate fractions(Fenget al.,2018).First,100 g(equivalent dry weight)of fresh soil was gently broken apart and separately passed through sieves with mesh sizes of 2,0.25,and 0.053 mm in that order.The soil aggregates on the upper 2-mm sieve were submerged in water for 5 min before conducting the wet-sieving process.Then,the sieve nest was vertically vibrated(in and out of water)in the bucket at 50 repetitions min-1with an amplitude of 3 cm for 2 min.The soil aggregates remaining on each sieve were transferred to glass beakers.Then,the soil suspension(<0.053 mm)was repeatedly centrifuged,and the soil aggregates were finally separated into two size ranges(0.053—0.25 and>0.25 mm).All samples were dried at 40°C and the C and nitrogen(N)contents were measured using the combustion method.The SOC and total nitrogen(TN)contents were determined by a Vario MACRO cube elemental analyzer (Elementar,Germany)and the microbial biomass carbon(MBC)content was determined using the chloroform fumigation-extraction method(Liet al.,2018).
Solid-state 13C NMR analyses
The chemical structures of the SOC were characterized using solid-state13C NMR spectroscopy with crosspolarization and magic angle spinning(CPMAS-NMR).The soil was washed with 2%(weight:weight)hydrofluoric acid and rinsed twice with deionized water.Then,the treated samples were freeze-dried and ground to pass through a 150-μm sieve(Liet al.,2020).Approximately 100 mg of the treated sample was analyzed using an Avance III™HD 500 MHz NMR spectroscope(Bruker,Germany).The spectra were classified into four main chemical shift regions:alkyl C(0—50 ppm),O-alkyl C(50—110 ppm),aromatic C(110—160 ppm),and carboxylic C(160—210 ppm)(Liet al.2020,Tianet al.,2021).
Soil microbial composition andabundances of function genes
Microbial DNA was extracted from 0.5 g of soil using an Omega Bio-tek DNA isolation kit(Norcross,USA)according to the manufacturer’s instructions.The bacterial and fungal abundances were measured by qPCR using a BioMark™HD system (Fluidigm,USA).The V3/V4 hypervariable region of the bacterial 16S rRNA was amplified using the primers 338F(5′-ACTCCTACGGGAGGCAGCA-3′)and 806R (5′-GGACTACHVGGGTWTCTAAT-3′),and the fungal ITS gene was amplified using the primers ITS1F(5′-CTTGGTCATTTAGAGGAAGTAA-3′)and ITS2R(5′-GCTGCGTTCTTCATCGATGC-3′).The PCR protocol was as follows: 95°C for 5 min,followed by 25 cycles of 95°C for 30 s,50°C for 30 s,and 72°C for 40 s,with a final extension at 72°C for 7 min.Then,the amplicons were extracted,purified,and quantified(Kanget al.,2022).Functional genes related to the decomposition of natural C sources (e.g.,starch,cellulose,chitin,and lignin) were determined using GeoChip 5.0.Briefly,DNA samples were labeled with Cy-5 fluorescent dye using a random priming method and purified by a QIA quick purification kit(Qiagen,Germany).GeoChip hybridization was carried out at 42°C for 16h on a MAUI®hybridization station(BioMicro,USA).After hybridization,the GeoChips were scanned at 633 nm with a NimbleGenMS200 scanner (Roche,Switzerland),using laser power and a photomultiplier tube gain of 100%and 75%,respectively.Raw data from the Agilent feature extraction process were submitted to the microarray data manager system(http://ieg.ou.edu/microarray/)and analyzed according to Liet al.(2022).
Soil extracellular enzyme activityassay
Six extracellular enzyme activities related to organic C degradation were investigated in this study.There were four hydrolytic enzymes:β-1,4-xylosidase (BX),βglucosidase(BG),β-D-cellobiohydrolase(CBH),andNacetylglucosaminidase(NAG),and two oxidases,peroxidase(PER) and phenol oxidase (POX).The activities of the hydrolytic enzymes were determined by a Fluoroskan™microplate fluorometer(Thermo,USA)and a Multiskan FC microplate reader(Thermo,USA)in fluorescence mode(Liet al.,2020).Briefly,1 g of fresh soil(<2 mm)was added to 125 mL of 50 mmol L-1(pH 5.0)sodium acetate buffer and mixed for 1 min.Then,200 μL of the suspension and 50 μL of the fluorescent substrate were placed in separate 96-well plates for each target enzyme(substrates shown in Table SI,see Supplementary Material for Table SI).The solutions were then incubated in dark at 20°C for 4 h.The reaction was terminated by adding 10 μL of 1 mol L-1NaOH into each well.Finally,the fluorescence intensity of the hydrolytic enzymes was measured at an absorbance of 460 nm using a Multiskan FC microplate reader with the excitation and emission wavenumbers set at 365 and 450 nm,respectively.The activities of the two oxidases,POX and PER,were measured using a Labserv K3(Thermo,USA)in UV mode.Similar procedures to the other enzymes were employed,except thatL-3,4-dihydroxyphenylalanine(L-DOPA)was used as the substrate solution and the mixture was cultured at 20°C for 18 h.
Statistical analysis
One-way analysis of variance(ANOVA)and the least significant difference(LSD)test were used to test the differences in soil properties,SOC chemical composition,microbe composition,abundance of functional genes,soil enzyme activities,ecosystem respiration,and above-and belowground plant biomass among the warming treatments.The datasets were checked for normality using the Levene’s and Mauchly’s tests prior to the ANOVA and LSD test.Non-normal data were transformed before analysis.Linear regression analyses were used to examine the ecosystem respiration relationships with above-and belowground plant biomass and soil enzyme activities.Spearman correlation analyses were performed to detect the correlations between soil enzyme activities and SOC chemical composition.The significance levels wereP <0.05.All analyses were performed using SPSS 25.0(IBM,USA).
RESULTS
Effect of warming on plant biomass andsoil properties in the alpine peatland
The soil temperature increased in the LT,MT,and HT treatments by 1.32,1.63,and 1.96°C on average in the OTCs from May to September,2016—2020,respectively(Fig.S2a,see Supplementary Material for Fig.S2).Warming tended to decrease soil moisture compared to the ambient control soil,but the difference was not significant(P >0.05)(Fig.S2b).The warming treatments did not alter pH,SOC or TN compared to CK(P >0.05)(Fig.1a,b,Table SII,see Supplementary Material for Table SII).Mean seasonal ecosystem respiration in LT,MT,and HT increased by 20.42%,52.65%,and 79.21%,compared to CK,respectively(P <0.05).Moreover,ecosystem respiration was significantly different among the three warming treatments(P <0.05)(Fig.1c).Soil MBC was significantly higher in LT,MT,and HT than in CK(P <0.05)(Fig.1d).Warming significantly increased above-and belowground plant biomass compared to CK and the greater the temperature increase,the greater the amount of plant biomass produced(P <0.05)(Fig.1e and f).

Fig.1 Soil organic C(SOC,a),total N(TN,b),mean seasonal ecosystem respiration(Re,c),and microbial biomass C(MBC,d),as well as above-and belowground plant biomass(e and f)under warming and control treatments in an alpine peatland on the Tibetan Plateau.Error bars indicate standard errors of means(n=6for a,b,d,e and f and 3 for c).Bars with different letters indicate significant differences(P <0.05).CK=control,no warming treatment;LT=low-temperature warming;MT=middle-temperature warming;HT=high-temperature warming.
Warming had a distinct effect on the SOC stored in the different soil particle size fractions,although warming did not alter the total SOC in soils.It induced a significant decrease in the SOC stored in both the macro-and microaggregate fractions(>0.25 and 0.25—0.053 mm,respectively)(P <0.05),but the SOC stored in the silt-clay fraction(<0.053 mm)significantly increased(P <0.05)(Fig.2).
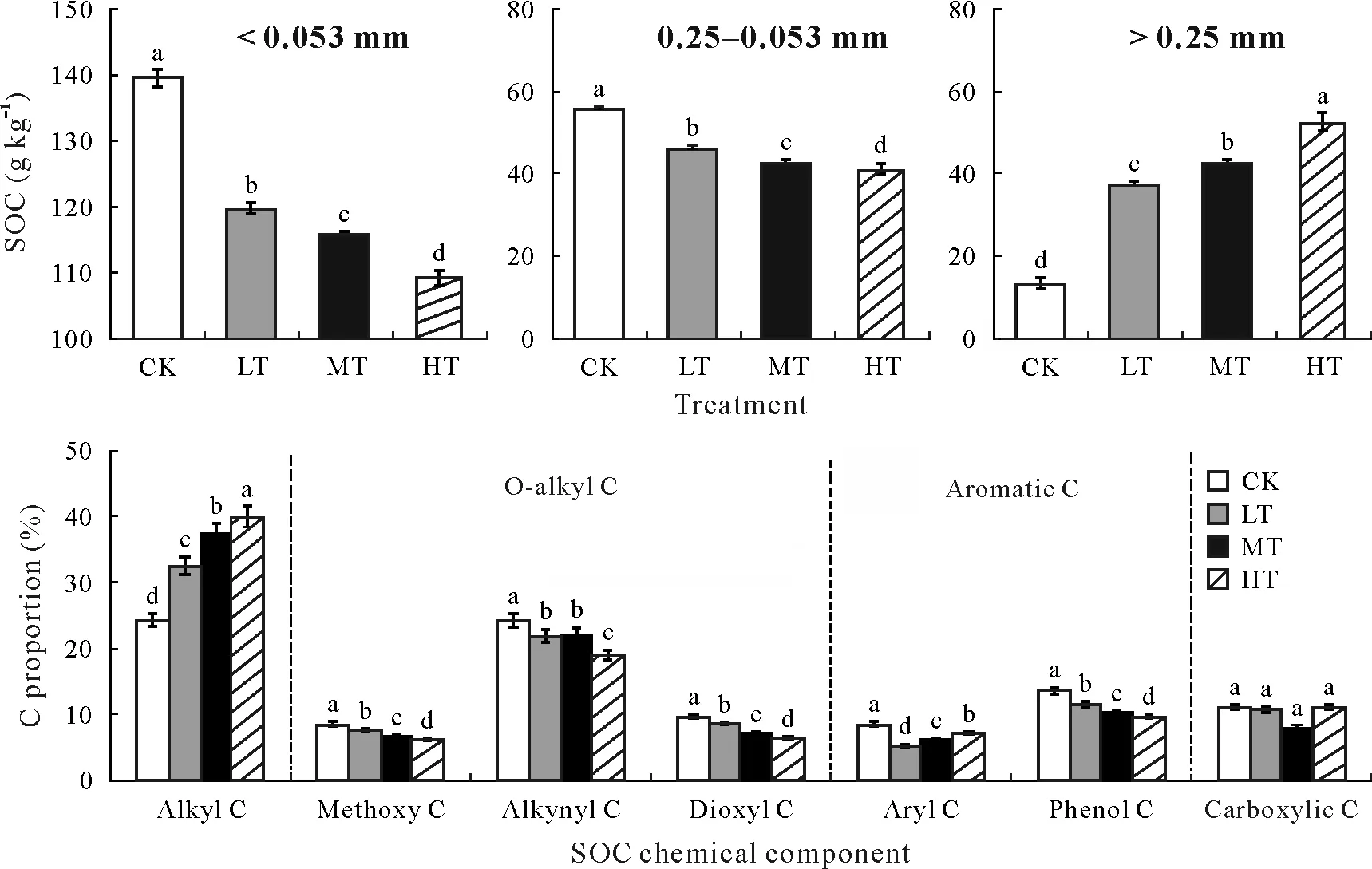
Fig.2 Soil organic C(SOC)stored in different particle size fractions and the distribution of SOC chemical components(using solid-state 13C nuclear magnetic resonance analyses)under warming and control treatments in an alpine peatland on the Tibetan Plateau.Error bars indicate standard errors of means(n=6).Bars with different letters indicate significant differences(P <0.05).CK=control,no warming treatment;LT=low-temperature warming;MT=middle-temperature warming;HT=high-temperature warming.
There was also a significant effect of warming on the different chemical components of SOC(P <0.05).Specifically,alkyl C content increased by 8.13%,12.95%,and 15.56%for LT,MT,and HT compared to CK,respectively,whereas O-alkyl C and aromatic C contents decreased by 4.13%,4.39%,and 10.8%and 3.61%,5.71%,and 4.99%,respectively,for the three warming treatments(LT,MT,and HT) relative to CK (P <0.05) (Fig.2).Specifically,the highest,medium,and lowest decreases in alkyl C were in the order LT,MT,and HT,respectively,compared to CK,whereas the highest,medium,and lowest decreases in phenol C were in the order HT,MT,and LT,respectively(Fig.2).Warming did not alter carboxylic C(P >0.05)(Fig.2).
Effect of warming on soil microbial composition
Warming had no significant effect on bacterial community composition (P >0.05),although some specific variations were observed.For instance,Proteobacteria and Acidobacteria were the prevailing bacterial groups and warming increased the relative abundances of these two groups compared to CK(Fig.3a).However,warming did have a significant effect on fungal community composition(P <0.05).Ascomycota accounted for the largest portion of the fungal community,followed by Basidiomycota (Fig.3a and b).Compared to CK,the relative abundance of Proteobacteria slightly increased by 1.99%,2.51%and 3.09%(P=0.029),respectively,for the LT,MT,and HT treatments,in that order,when the soil temperature rose from 1.32 to 1.96°C on average.The Ascomycota relative abundance increased by 1.86%,9.96%,and 21.41%for LT,MT,and HT relative to CK,respectively.However,the Basidiomycota relative abundance decreased by 14.22%,12.76%,and 14.07%for LT,MT,and HT,respectively(P <0.05).There were no significant differences in bacterial diversity(Simpson,Chao 1,and Shannon indices)among the warming treatments(P >0.05).However,significant increases in fungal diversity occurred after all three warming treatments compared to CK(P <0.05)(Fig.3c—e).

Fig.3 Relative abundances of bacterial(a)and fungal(b)communities and diversity indices of bacteria and fungi(c—e)at 0—15 cm soil depth under warming and control treatments in an alpine peatland on the Tibetan Plateau.In c-e,error bars indicate standard errors of means(n=3),and bars with different letters within bacterial or fungal diversity index indicate significant differences(P <0.05).CK=control,no warming treatment;LT=low-temperature warming;MT=middle-temperature warming;HT=high-temperature warming.
Effect of warming on soil extracellular enzyme activityand the abundance of functional genes
Warming had significant effects on the enzyme activities of three of the four hydrolytic enzymes(P <0.05).Warming did not alter BG activity (P >0.05) (Fig.4a).However,the BX activity was significantly higher for LT,MT,and HT compared to CK,but there was no significant difference between MT and HT (P <0.05) (Fig.4b).The CBH activity was significantly higher for MT and HT than LT and CK(Fig.4c),and a significantly higher PPO activity was observed for LT,MT,and HT than for CK.There were also significant differences in PPO activity among the three warming treatments (P <0.05) (Fig.4d).For the two oxidases,the PER activity was significantly higher in LT,MT,and HT than in CK.In addition,a significantly higher PER activity was observed for HT than LT and MT(Fig.4e).However,warming did not alter the NAG activity(Fig.4f).
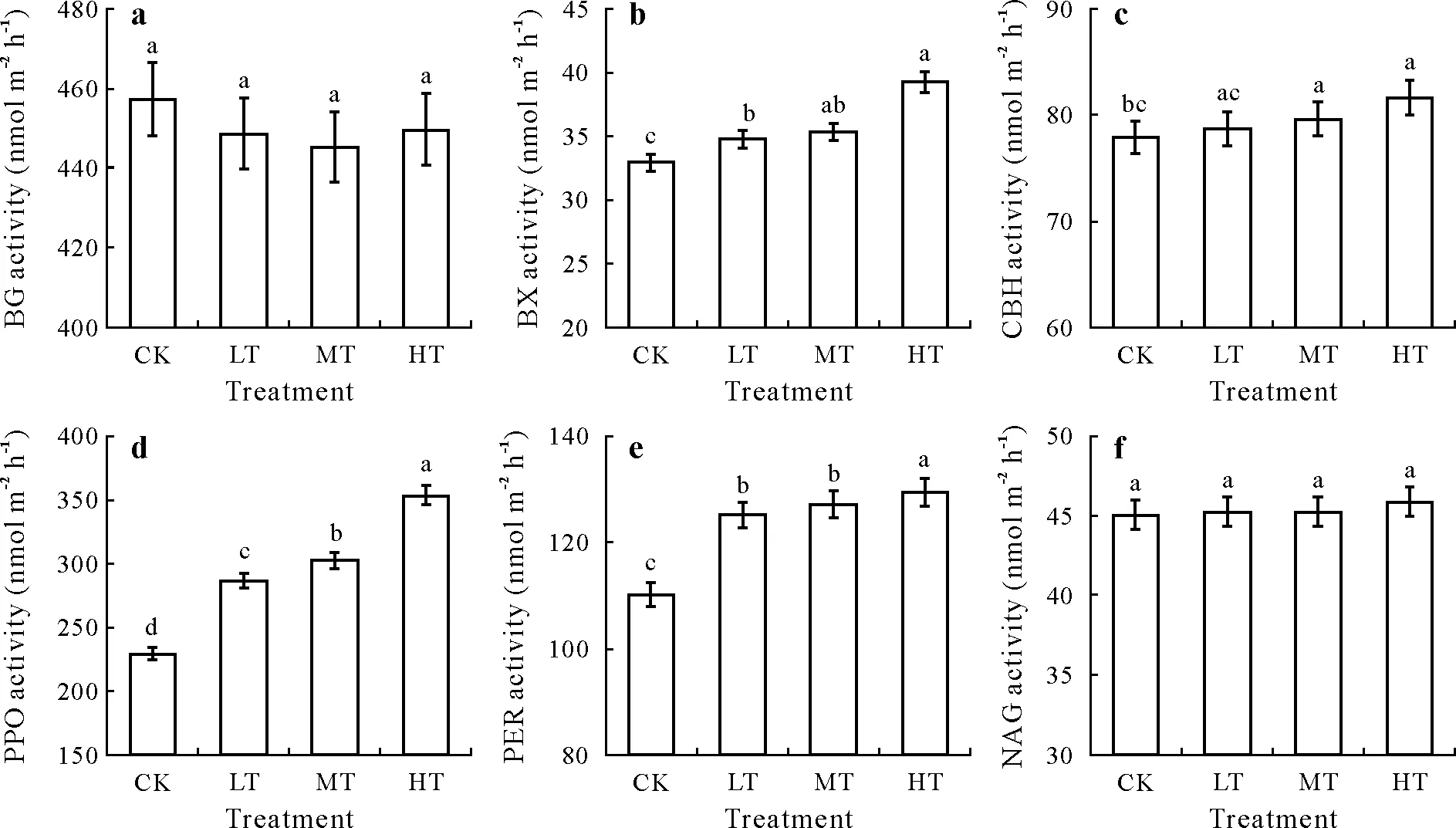
Fig.4 Soil extracellular enzyme activities under warming and control treatments in an alpine peatland on the Tibetan Plateau.Error bars indicate standard errors of means(n=6),and bars with different letters indicate significant differences(P <0.05).CK=control,no warming treatment;LT=low-temperature warming;MT=middle-temperature warming;HT=high-temperature warming;BG=β-1,4-glucosidase;BX=β-1,4-xylosidase;CBH=β-D-cellobiosidase;NAG=N-acetylglucosaminidase;PER=peroxidase;PPO=polyphenol oxidase.
Warming had significantly positive effects on the relative abundances of functional genes related to the degradation of starch and lignin(Fig.5).In contrast,warming had negative effects on the relative abundances of functional genes related to the degradation of chitin and cellulose.There was also a nonsignificant effect on the relative abundances of functional genes related to pectin degradation(P >0.05)(Fig.5).

Fig.5 log2(fold change)of relative abundance of genes in warming versus control treatments for the selected organic matter degradation pathways in an alpine peatland on the Tibetan Plateau.Pathways labeled as+and-indicate significant increases and decreases of relative gene abundance in the warming treatments relative to the control,respectively(P <0.05).CK=control,no warming treatment;LT=low-temperature warming;MT=middle-temperature warming;HT=high-temperature warming.
Ecosystem respiration relationships withplant biomass and soil extracellular enzyme activity
There were significantly positive linear relationships between ecosystem respiration and above-and belowground plant biomass (Fig.6a,b).There were also positive linear relationships between ecosystem respiration and the activities of three hydrolytic enzymes,CBH,BX,and PPO(Fig.6c,e,g).However,there was no significant relationship between ecosystem respiration and BG activity (Fig.6d).For the two oxidases,ecosystem respiration was positively correlated with PER activity and negatively correlated with NAG activity(Fig.6f,h).
DISCUSSION
Our 5-year warming experiment supported our hypothesis that warming increased the input of plant photosynthetic C to soil and simultaneously promoted the decomposition of the old SOC stored in the soil.The magnitudes of the SOC input and output varied among the different warming treatments.The balance between the C input and output left the SOC stock unchanged ultimately,regardless of the rates of warming.In addition,different rates of warming induced shifts in the compositions and activities of soil microbes,which led to changes in the chemical compounds that make up SOC.Specifically,our results revealed that warming significantly promoted both above-and belowground plant biomass and selectively promoted hydrolytic and oxidase enzyme activities,which then contributed to the enhancement of ecosystem respiration.In addition,warming also increased soil MBC and fungal diversity.It also induced a selective increase in the relative abundances of functional genes related to the decomposition of both labile and recalcitrant C compounds.The magnitudes of the C input and output corresponded well with the different rates of warming and they were able to maintain a relatively stable SOC stock in the alpine peatland.
Warming-inducedC input andoutput
Ecosystem respiration is associated with the biotic conversion of organic C to CO2in an ecosystem and is the sum of aboveground autotrophic respiration(e.g.,plants)and belowground heterotrophic respiration(e.g.,soil microorganisms).In this study,a gradual increase in ecosystem respiration with increasing temperature was observed for the different rates of warming.Furthermore,there were gradual increases in above-and belowground plant biomass as the warming rates increased.The increases in plant productivity induced by warming could promote ecosystem respirationviathe enhancement of both canopy respiration(Wanget al.,2017)and root respiration(Linet al.,2011).However,warminginduced increases in plant growth require more soil available N.The labile C input from plant litter and root exudation promoted microbial activity,which further induced decomposition of the originally stored SOC in the soil.This would release more available N (N mining) (Kuzyakov,2010).Our assumption was supported by the gradual increases in soil microbial biomass and fungal diversity,suggesting that different rates of warming could promote soil microbial activities,which then stimulated SOC decomposition(Tianet al.,2021).In addition,warming increased soil extracellular enzyme activities and the relative abundances of functional genes related to the degradation of relatively labile and recalcitrant C compounds.These results consistently supported our original hypothesis.Furthermore,the variations in SOC structure and the correlations between ecosystem respiration and enzyme activities suggested that warming-induced changes in the soil microbial community could enhance soil respiration(Bengtson and Bengtsson,2007)and ultimately contribute to increases in ecosystem respiration(Liskiet al.,2003;Fenner and Freeman,2011).Although the results from this study suggested that both autotrophic respiration by plants and heterotrophic respiration by microorganisms contributed to ecosystem respiration,accurately quantifying either of them was impossible due to a lack of soil respiration measurements in the field.
Warming-inducedalterations to soil microbes,enzyme activities,andfunctional genes affectedC output
The results showed that the effect of warming on the composition of bacterial and fungal groups were different and a significant effect of warming on microbial diversity was only observed in the fungal groups.However,some specific changes were detected in the bacterial groups.For instance,the abundances of the dominant clades,Proteobacteria and Acidobacteria,increased in the warming treatments(Fig.3).For fungal groups,warming increased the relative abundance of Ascomycetes,but decreased that of Basidiomycetes.Ascomycetes react rapidly with labile C substrates,but are very resistant to recalcitrant C,whereas Basidiomycetes efficiently degrade recalcitrant C by producing a range of enzymes(Morrisseyet al.,2016).The variations in fungal composition could contribute to the decrease in O-alkyl C and the increase in alkyl C(Fig.2).Fungi are involved in the decomposition of plant litter and old soil organic matter(Tresederet al.,2016).Furthermore,they were also involved in the occlusion and formation of the organic matter derived from mineral-associated litter.This meant that they were involved in the regulation of SOC persistence(Tianet al.,2021).Therefore,warming-induced microbially mediated mechanisms were likely responsible for SOC dynamics.
Microbes may respond to the warming-induced increases in plant litter C input by regulating the abundances of functional genes and soil enzyme activity.In this study,warming promoted the relative abundances of functional genes related to the decomposition of both labile and recalcitrant C.Furthermore,positive warming effects on the activities of four of the six enzymes were observed.According to the Michaelis-Menten relationship,the quality of the substrate largely determines the enzyme activity(Davidson and Janssens,2006).Soil microbes may release more hydrolytic and oxidative enzymes in response to the increase in plant-litter-derived C caused by warming(Talbotet al.,2012;Pisaniet al.,2015).The increase in plant N requirements under warming conditions may lead microorganisms to increase the abundances of functional genes involved in decomposing old stable C,which leads to an increase in available N(Fenget al.,2017).In this study,warming did not alter soil pH(Table SII).Although warming tended to decrease soil moisture (P >0.05,Fig.S2b),soil water content was not a limiting factor in this peatland ecosystem.Therefore,warming-induced changes to the soil environment may not be the main factor mediating the alterations in functional genes.The interaction between plants,microbes,and substrate properties could be the primary factor driving the alterations in functional genes.
Effect of warming on soil C pools
Although ecosystem respiration and plant biomass were significantly and gradually influenced by the different warming treatments,the total SOC contents remained unchanged(Fig.7).Interestingly,warming had distinct effects on the different SOC chemical components in the different soil particle size fractions.O-alkyl C content decreased by 4.13%—10.8%from the high to low warming treatments,while alkyl C content increased by 8.13%—15.56%,compared to CK(Fig.2).O-alkyl C mainly comes from carbohydrates and peptides and is more readily available for microbial uptake because it is an unstable substrate.O-alkyl C is metabolized by microorganisms to produce alkyl C,which may lead to an increase in alkyl C.In addition,warming did not affect NAG activity(Fig.4),which was closely correlated with alkyl C degradation.Therefore,the constant enzyme activity and increased input of fresh organic C from plant litter may also contribute to the increase in alkyl C content over the short term.In addition,aromatic C decreased by 3.61%—4.99%across the low to high warming treatments compared to CK.Aromatic C in SOC is mostly from lignin,which is not considered to be readily decomposable in soil(Liet al.,2020)but is sensitive to priming effects due to the increases in plant C input and N demand under experimental warming(Pisaniet al.,2015;Jiaet al.,2017).
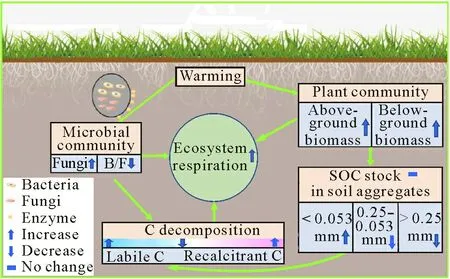
Fig.7 A conceptual diagram illustrating the effect of warming on plant biomass,soil microbial community and enzyme activity,the activity of soil microbial functional genes that control the decomposition of soil organic carbon(SOC)chemical components,and SOC stock in the warming experiment in an alpine peatland on the Tibetan Plateau.B/F=bacteria to fungi ratio.
Warming treatments promoted the decomposition of SOC stored in both the macro-and microaggregate fractions(>0.053 mm).However,warming treatments induced SOC accumulation in the silt-clay fraction(adherence to the mineral surface,<0.053 mm).The balance between the different soil particle size fractions affected the stability of SOC stock after 5 years of warming at different rates.However,we did not subdivide the macro-and microaggregate C into particulate and mineral C.Therefore,we were not sure whether the enhancement of recalcitrant C decomposition was due to the mineral C stored in macro-and microaggregates.
The soil C pool is sustained by a continuous input of plant-derived C.Microbial decomposition plays a crucial role in transforming plant-derived organic matter into soil organic matter.Therefore,the composition and activity of soil microbial community determine the degradation pathway for plant litter residues and thus the persistence of soil organic matter(Tianet al.,2021).The results from this study showed that there were significant increases in soil MBC and fungal diversity in the soil warming treatments.Moreover,the SOC stored in the<0.053 mm particle fraction significantly increased after 5 years of warming.The finding suggested that both plant and microbial necromass could contribute to SOC formation.Therefore,soil microbes played a dual role in the dynamic changes to organic matter.Soil microbes are responsible for the decomposition of organic matter and they also participate in the formation and storage of organic matter.In other words,the increase in C output across the alpine peatland could be offset by the rise in C input.However,our results showed that the SOC stock did not change in the peatland,which might be due to the relatively short duration of the warming treatments.Changet al(2021)found that 3 years of warming had little effect on permafrost SOC concentration and stock,but 6years of warming significantly increased the SOC concentration and stock.Therefore,long-term experiments are needed to accurately evaluate SOC stock in alpine peatland ecosystems under climate warming.
The results showed that the direction of the variable responses was similar across the warming rates and that the response magnitude was well correlated with the warming rates.Although the overall increase in soil temperature was only about 0.3°C between MT and LT and between HT and MT during growth season,the variable responses were statistically significant among the warming treatments.The results suggest that the plants and soil microbes in the alpine peatland subjected to the low temperature treatment are sensitive to warming,even when the increase rate is small.Therefore,more attention should be paid to C dynamics in alpine peatlands when they face climate warming.
CONCLUSIONS
Different rates of warming did not alter the SOC stock in the alpine peatland on the Tibetan Plateau after 5 years of soil warming,although warming increased ecosystem respiration and above-and belowground plant biomass.However,the structure of the SOC did alter after the warming treatments.There were significant decreases in unstable O-alkyl C and stable aromatic C,but a significant increase in stable alkyl C following warming-induced increases in soil fungal diversity,enzyme activity,and functional gene abundance.The increases in C input from plant litter and C outputviaecosystem respiration offset each other,which resulted in a relatively stable SOC stock at the different rates of warming.Furthermore,the warming-induced SOC increase in the siltclay fraction could promote SOC persistence in the alpine peatland ecosystem under climate warming.
ACKNOWLEDGEMENT
This research is supported by the National Natural Science Foundation of China(Nos.41971024 and 41373069).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
CONTRIBUTION OFAUTHORS
Jingcong QIU and Minghua SONG contribute equally to this work.
- Pedosphere的其它文章
- Carbon farming by recarbonization of agroecosystems
- Root exclusion methods for partitioning of soil respiration:Review and methodological considerations
- Utilization of lignocellulosic plant residues for compost formation and its role in improving soil fertility
- A critical review of microbially induced carbonate precipitation for soil stabilization:The global experiences and future prospective
- Hand-feel soil texture observations to evaluate the accuracy of digital soil maps for local prediction of soil particle size distribution:A casestudy in Central France
- Influence of soil physicochemical properties,particle size fractions and mineralogy on the leaching potentials of arsenic and antimony in abandoned mine soils

