Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry(Myrica rubra Sieb. et Zucc.) cultivars
Zhe Liu, Jign Zhng,, Shengmin Lu,,*, Weimin Tng, Yiin Zhou, Siew Young Quek
a Institute of Food Science, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China
b School of Tea and Food Technology, Anhui Agricultural University, Hefei 230036, China
c Food Science, School of Chemical Sciences, The University of Auckland, Auckland 1010, New Zealand
d Riddet Institute, Centre of Research Excellence for Food Research, Palmerston North 4474, New Zealand
Keywords:
Chinese bayberry
Drying
Phenolic components
Antioxidant activities
In vitro hypoglycemic activities
A B S T R A C T
The effects of two different drying methods, namely hot-air drying and freeze drying, on the phenolic profiles,antioxidant and hypoglycemic activities of pulp extracts from two main Chinese bayberry cultivars (‘Biqi’or ‘BQ’ and ‘Dongkui’ or ‘DK’) were evaluated. The results showed that ‘BQ’ bayberry provided higher total phenolic (TPC), total flavonoid (TFC) and total anthocyanin (TAC) contents than ‘DK’ bayberry after the same drying method, while its antioxidant and hypoglycemic activities were highly affected by drying method. Freeze-dried samples possessed significantly higher TPC, TFC and TAC, and exhibited more potent antioxidant and hypoglycemic activities than hot-air dried ones (P < 0.05). Freeze-dried ‘DK’ bayberry exhibited similar antioxidant activities and α-amylase inhibitory activity, but higher α-glucosidase inhibition than freeze-dried ‘BQ’ bayberry (P < 0.05). Compared with hot-air dried samples, freeze-dried samples had significantly higher contents of cyanidin-3-O-glucoside, myricitin-3-O-rhamnoside, quercetin-3-O-galactoside,quercetin-3-O-glucoside and quercetin-3-O-rhamnoside, which had been proved to be the main contributors to the in vitro hypoglycemic activities of bayberry pulp extract. All these results demonstrate that freeze drying is more suitable for the preservation of phenolic components in the pulp extract of Chinese bayberry, and the pulp extract of freeze-dried ‘DK’ could be applied as a potential hypoglycemic food additive.
1. Introduction
The number of adults with diabetes has risen almost 4 fold to 422 million since 1980, and diabetes will become the seventhlargest cause of death in the disease category by 2030 according to the statistics of World Health Organization (WHO) (http://www.who.int/diabetes/global-report/en). Therefore, preventing and controlling diabetes and its complications is becoming increasingly important globally.
Phenolic substances originated from plant had demonstrated hypoglycemic activities in different experimental models. For examples, among the 11 monomeric compounds ofPyrusspp. peel extract, chlorogenic acid, vanillic acid, ferulic acid and rutin have shown the most potentα-glucosidase inhibitory activitiesin vitro[1,2];delphinidin-3-arabinoside and malvidin-3-galactoside in blackberry could effectively inhibited the enzymatic activity of carbohydratesin vitro[3]; myricetin present in black legumes exhibited potent inhibition ofα-amylase,α-glucosidase and lipase (IC50: 0.38, 0.87 and 15 μg/mL, respectively) [4].
Chinese bayberry (also called red bayberry,Myrica rubraSieb. et Zucc.) has been grown in southern China for more than 2 000 years and is a significant resource of beneficial bioactive substances such as phenolic acids, anthocyanins and flavonols [5]. Literature has shown that pulp extracts from three bayberry cultivars (‘Biqi’, ‘Ciji’, and‘Tanmei’) exhibited potent glucose consumption activities, which had a significant correlation with the contents of cyanidin-3-O-glucoside(C3G) and quercetin-3-O-glys [6]. In C57BL/6 mice, the blocking of body weight gain and the lowering of blood glucose were thought to be associated with the presence of myricetin in red bayberry extract [7].Although red bayberry has potential hypoglycemic effect, the fruit can be easily damaged and has an extremely short shelf life of only 3-4 days at ambient temperatures [8]. To conserve the fruit, freezing or drying immediately after harvest is essential. Compared with freezing storage, hot air drying is much popular in practice due to its lower storage space, cost and energy consumption.
Drying removes moisture from agricultural products, thus extending their shelf lives by reducing deterioration caused by microorganism or enzymes [9]. Drying using hot air has been widely applied in the production of dry agricultural products due to its low cost [10]. However, hot-air drying may deteriorate the product quality such as causing degradation of ascorbic acid because of long drying time and high temperature applied during the process [11]. On the other hand, freeze-drying conserves nutrition well but requires high energy consumption and lengthy processing time, hence increasing the processing cost [12]. In this study, we aimed to assess the effect of the above two drying methods, namely hot-air and freeze drying,on the contents of phenolic components in the pulp extracts of two Chinese bayberry cultivars (‘Biqi’ or ‘BQ’, and ‘Dongkui’ or ‘DK’).This was followed by evaluation of thein vitroantioxidant and hypoglycemic activities of the extracts, and determination of the major flavonoids contributed to the activities. The findings from this study will provide a basis for development of hypoglycemic food ingredients from Chinese bayberry pulp extract.
2. Materials and methods
2.1 Chemicals
Standard samples of C3G, myricitin-3-O-rhamnoside (M3R),quercetin-3-O-glucoside (Q3Glu), quercetin-3-O-rhamnoside (Q3R),quercetin-3-O-galactoside (Q3Gal), 2,2-diphenyl-1-picrylhydrazyl(DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)(ABTS),α-amylase andα-glucosidase were purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin-Ciocalteu reagent (1 mol/L)andp-nitrophenyl-α-D-glucopyranoside (pNPG) were acquired from Shanghai Yuanye Biotechnology Company (Shanghai, China). All other solvents used were of analytical grade.
2.2 Bayberry samples preparation
During the fruit seasons of 2017 and 2018, two Chinese bayberry cultivars (BQ and DK), which had been identified by bayberry breeding research Professor Qi Xingjiang in Zhejiang Academy of Agricultural Sciences, were manually picked in an orchard in Xianju County, Zhejiang Province and transferred to the laboratory within 4 h after harvest. Fruits with almost uniform size, shape and color without any visible sign of damage or disease were selected. Fruit samples were dried either through hot air drying in an oven (DHG-9070A, Shanghai Jinghong Experimental Equipment Co., Ltd.,China) at 60 °C for 72 h or freeze drying by freeze dryer (SCIENTZ-18N, Ningbo Xinzhi Biotechnology Co., Ltd., China) under cold trap temperature −50 °C and vacuum pressure 40 Pa for 72 h according to the results of preliminary experiments. The moisture contents of the final dried samples were both around 5% . The pulp of the fruit was manually removed from the kernel and milled into fine powder using a pulverizer (BJ-200, Deqing Baijie Electric Co., Ltd., China),followed by storage at −40 °C.
2.3 Preparation of bayberry pulp extracts
Two grams of powder sample prepared from fruit pulp as mentioned above was mixed with 40 mL of 95% ethanol. Extraction was conducted via ultra-sonication method (40 kHz, 500 W) for 30 min at room temperature ((25 ± 2) °C, R.T.) using a sonicator(KQ-500DB, Kunshan Ultrasonic Instrument Co., Ltd., China). The extract was centrifuged at 4 000 r/min for 20 min at R.T. and the residue was re-extracted twice as described above. The supernatants from each extraction round were collected and concentrated using a rotary evaporator (R1002B, Shanghai Shensheng Technology Co.,Ltd., China). The concentrate was then lyophilized by a freeze dryer(SCIENTZ-18N, Ningbo Xinzhi Biotechnology Co., Ltd., China) and used for analysis.
2.4 Total phenolics, flavonoid and anthocyanin determination
Total phenolics content (TPC) was measured by the Folin-Ciocalteu method as reported by Singleton et al. [13]with minor modifications. The appropriately diluted sample solution (1 mL)and Folin-Ciocalteu reagent (1 mol/L, 2.5 mL) were mixed in a test tube, and 5% sodium carbonate solution (5 mL) was added, followed by adding distilled water to a volume of 25 mL. The mixture was left to react for 1 h at 25 °C in the dark, and the absorbance was measured at 765 nm. The results were calculated as mg gallic acid equivalents (GAE)/g dry weight (DW) of extract. Total flavonoids content (TFC) was evaluated according to the method reported by Alothman et al. [14]with few modifications. The appropriately diluted sample solution (1 mL) and sodium nitrite solution (5% ,1 mL) were mixed, shaken, and stood still for 6 min. Then, 10% aluminum nitrate (1 mL) was added and the mixture solution was shaken and left for another 6 min, followed by adding 4% sodium hydroxide (10 mL) and anhydrous ethanol to 25 mL. Finally, the mixture was kept in the dark for 15 min at R.T. before absorbance read at 509 nm. The results were calculated as mg rutin equivalents (RE)/g DW.Total anthocyanin content (TAC) determination was conducted based on the method of Lee et al. [15]with minor revisions. The absorbance at 510 nm was measured after mixing a 0.5 mL sample with 4.5 mL of KCl-HCl (0.2 mol/L, pH 1.0) in the dark for half an hour. The absorbance at 700 nm was measured after reacting 0.5 mL of the sample with 4.5 mL of CH3COONa-CH3COOH (0.2 mol/L,pH 4.5) in the dark for another half an hour. TAC was calculated as mg C3G equivalents/g DW.
2.5 Analyses of anthocyanins and flavonols by HPLC
The analyses of anthocyanins and flavonols were carried out according to Zhang et al. [16]. Individual anthocyanins and flavonols compounds were characterized using a high performance liquid chromatography (HPLC) system (600 controller, 2 475 multi λ fluorescence detector, Waters). A SunFireTMC18(4.6 mm × 250 mm)column was used for analysis at 25 °C. The mobile phase consisted of 0.1% (V/V) formic acid in water (eluent A) and 0.1% (V/V) formic acid in acetonitrile (eluent B). The following conditions were adopted for the HPLC: mobile phase B increased from 10% up to 38% in the first 40 min, to 48% in the next 20 min and 100% for the last 10 min, then decreased to 10% within 5 min, and kept at 10% for 5 min.Flavonols and anthocyanins were measured at 350 nm and 520 nm,respectively, and their values were expressed as mg/g DW.
2.6 Analysis of DPPH and ABTS radical scavenging activities
The DPPH free radical scavenging activities of samples were measured according to the method reported by Shimada et al. [17]with minor modifications. Different concentrations of the sample(0.2 mL, water solution) and DPPH solution (0.2 mmol/L, 5 mL,ethanol solution) were mixed, shaken and reacted in the dark at 25 °C for 30 min, and the absorbance (Ai) at 517 nm was measured.The blank group contained anhydrous ethanol and DPPH solution,and the control group contained absolute ethanol and sample solution.Their absorbances were recorded asA0andAj, respectively. The DPPH radical scavenging rate (% ) was calculated according to equation (1) and median effect concentration (EC50) values were expressed as mg DW/mL.
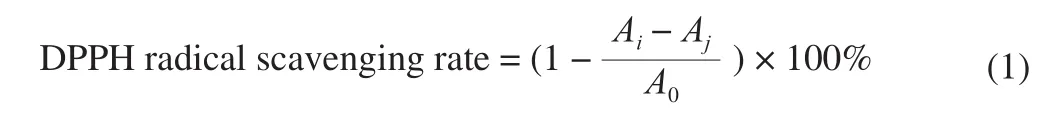
The ABTS cation radical eliminating abilities of the samples were determined by the method described by Zhang et al. [18]with minor modifications. The ABTS solution (7 mmol/L, 5 mL) was mixed with potassium persulfate solution (140 mmol/L, 88 µL) and left in the dark for 16 h as ABTS stock solution. The ABTS stock solution was diluted with anhydrous ethanol to the absorbance of 0.70 ± 0.01 at 734 nm. The sample solution (50 µL) and the diluted ABTS stock solution (4 mL) were mixed well by vortex and left to react in the dark at R.T. for 10 min. The absorbance at 734 nm was measured. The negative control group contained absolute ethanol and diluted ABTS stock solution. EC50values were represented as mg DW/mL.
2.7 α-Amylase inhibition assay
The inhibitory activity ofα-amylase was determined using the method depicted by Johnson et al. [19]. 50 µL of the sample solution or positive control (acarbose) was mixed with 500 µL solution ofα-amylase (13 U/mL) from type VI-B porcine pancreas and dissolved in 0.02 mol/L sodium phosphate buffer (pH 6.9), followed by incubation at 37 °C for 10 min. The reaction was started by adding starch solution (500 µL, 1% , dissolved in boiled sodium phosphate buffer). After kept at 37 °C for 25 min, the reaction was terminated by the addition of dinitrosalicylic acid color reagent (1 mL) and boiling in a water bath for 5 min. The mixture was appropriately diluted before the absorbance (Atest) was measured at 520 nm. Theα-amylase inhibition effect was calculated by the equation (2) and half maximal inhibitory concentration (IC50) values were represented as mg DW/mL.
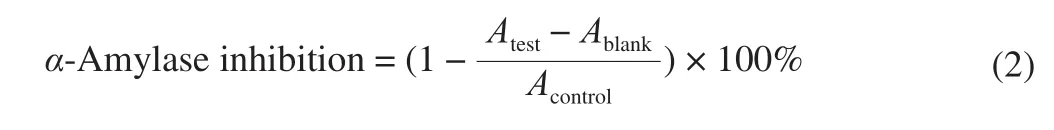
whereAcontrolrepresented the absorbance of control group that the sample solution was replaced by the sodium phosphate buffer andAblankrepresented the absorbance of blank group that the enzyme solution was replaced by the sodium phosphate buffer in the reaction mixture.
2.8 α-Glucosidase inhibition assay
The inhibitory activity against glucosidase was conducted based on the method of Xu et al. [20]with minor revisions. 40 µL of the sample solution and 100 µL ofα-glucosidase solution (0.4 U/mL,dissolved in 0.01 mol/L potassium phosphate buffer, pH 6.8) were added into 560 µL of the buffer solution (0.1 mol/L, pH 6.8), and the mixture was shaken and incubated at 37 °C for 15 min. The reaction was started by adding 100 µL of the PNPG solution (5 mmol/L).After reacted for 30 min at 37 °C, 400 µL of Na2CO3(0.2 mol/L)was added to stop the reaction and the absorbance was measured at 405 nm. The enzyme solution was replaced by an equal volume of potassium phosphate buffer in the blank group, and the sample solution was replaced by an equal volume of potassium phosphate buffer in the control group. Acarbose was used as a positive control.Theα-glucosidase inhibition effect was calculated by the equation (3)andIC50values were represented as mg DW/mL.
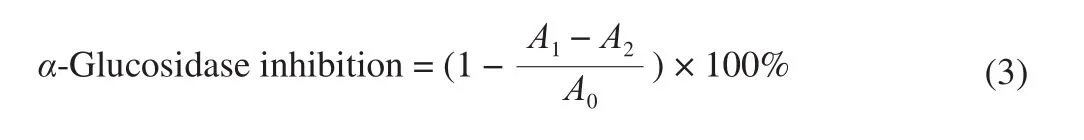
whereA1,A2andA0re flected the absorbance of the sample group,the blank group and the control group, respectively.
2.9 Statistical analysis
All experiments were performed in triplicate and the values were represented as mean ± SD. The correlations among C3G, Q3Gal,M3R, Q3R, Q3Glu, DPPH, ABTS,α-amylase andα-glucosidase inhibitory activities of extracts of two bayberry cultivars dried by hot-air and freezing were analyzed. Statistical significant difference and correlation were analyzed by SPSS Statistics (17.0, SPSS China)andP< 0.05 was considered as significant difference according to Duncan’s new multiple range test. All graphical representations were processed with Office 2016 (Office Professional Plus 2016, Microsoft Office, USA).
3. Results
3.1 TPC, TFC and TAC in pulp extracts
Significant differences of TPC, TFC and TAC in bayberry pulp extracts were overall observed among the two cultivars and two drying methods (Table 1). There were significantly higher TPC, TFC and TAC in the extract of ‘BQ’ bayberry than those in the ‘DK’ cultivar(P< 0.05) respectively, regardless of drying methods. TAC ranged from 1.18 mg C3G equivalents/g DW in ‘DK’ to 42.51 mg C3G equivalents/g DW in ‘BQ’, a wider variation than those of TFC and TPC,2.46 mg RE/g DW to 6.67 mg RE/g DW and 5.84 mg GAE/g DW to 11.97 mg GAE/g DW, respectively. According to Table 1, there were significant differences between two drying methods. TPC, TFC and TAC in the freeze-dried bayberry pulp extract were distinctly higher than those in hot-air dried one (P <0.05). Previous research suggested that the stability of phenolic compounds and anthocyanins would be negatively affected by increased temperature and the presence of oxygen [21]. Compared with freeze drying, the process of hot-air drying requires higher temperature and increased air flow,which are needed to promote water evaporation and reduce the relative humidity, and finally results in the deterioration of phenolic substances and anthocyanins in raw materials.
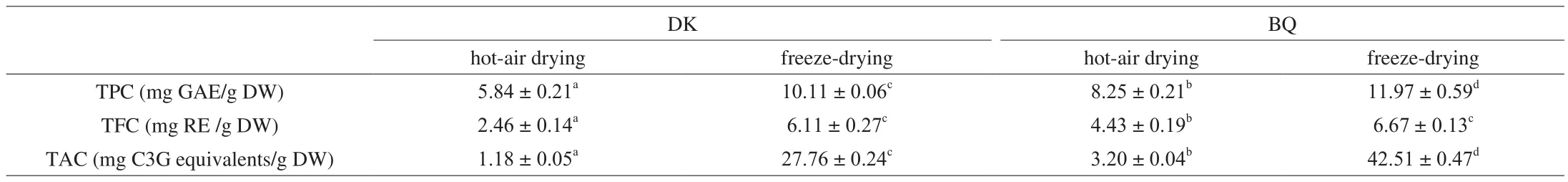
Table 1TPC, TFC and TAC in pulp extracts of two Chinese bayberry cultivars dried by hot-air drying and freeze-drying.
3.2 Quantification of individual flavonoids
Based on the results of the standards and literatures, peak 1 (Fig. 1)was confirmed as C3G after comparing with data from references and peak 2, 3, 4, 5 and 6 were identified as myricetin deoxyhexoside,M3R, Q3Gal, Q3Glu and Q3R, respectively [22-24]. HPLC analysis revealed that C3G and myricetin deoxyhexoside were present in lyophilized bayberry samples but undetected in hot-air dried ones.This result indicated that freeze-drying had a significant advantage in preserving myricetin deoxyhexoside or C3G while hot-air drying might cause the degradation of them [24]. The bayberry fruits are abundant in secondary metabolites of plants such as quercetin and myricetin [24]. As shown in Table 2, the pulp extract of ‘DK’bayberry after freeze-drying (FDDK) had the greatest amount of M3R((0.49 ± 0.04) mg/g DW) in all samples. The pulp extract of ‘BQ’bayberry after freeze-drying (FDBQ) had highest amount of Q3Gal((0.55 ± 0.04) mg/g DW) while Q3Gal in that of ‘DK’ bayberry after hot-air drying (HDDK) was not detected. FDDK and FDBQ had the highest amount of Q3Glu ((0.24 ± 0.02) mg/g DW) and Q3R((0.49 ± 0.01) mg/g DW) respectively in all samples. Based on the determinations of TPC, TFC and TAC, it was suggested that freezedrying is a better method to prepare the pulp extract of bayberry than the hot-air drying. In this study, contents of all the five flavonoids in freeze-dried samples was significantly higher than those in hot-air dried ones (P< 0.05) regardless of bayberry cultivars.
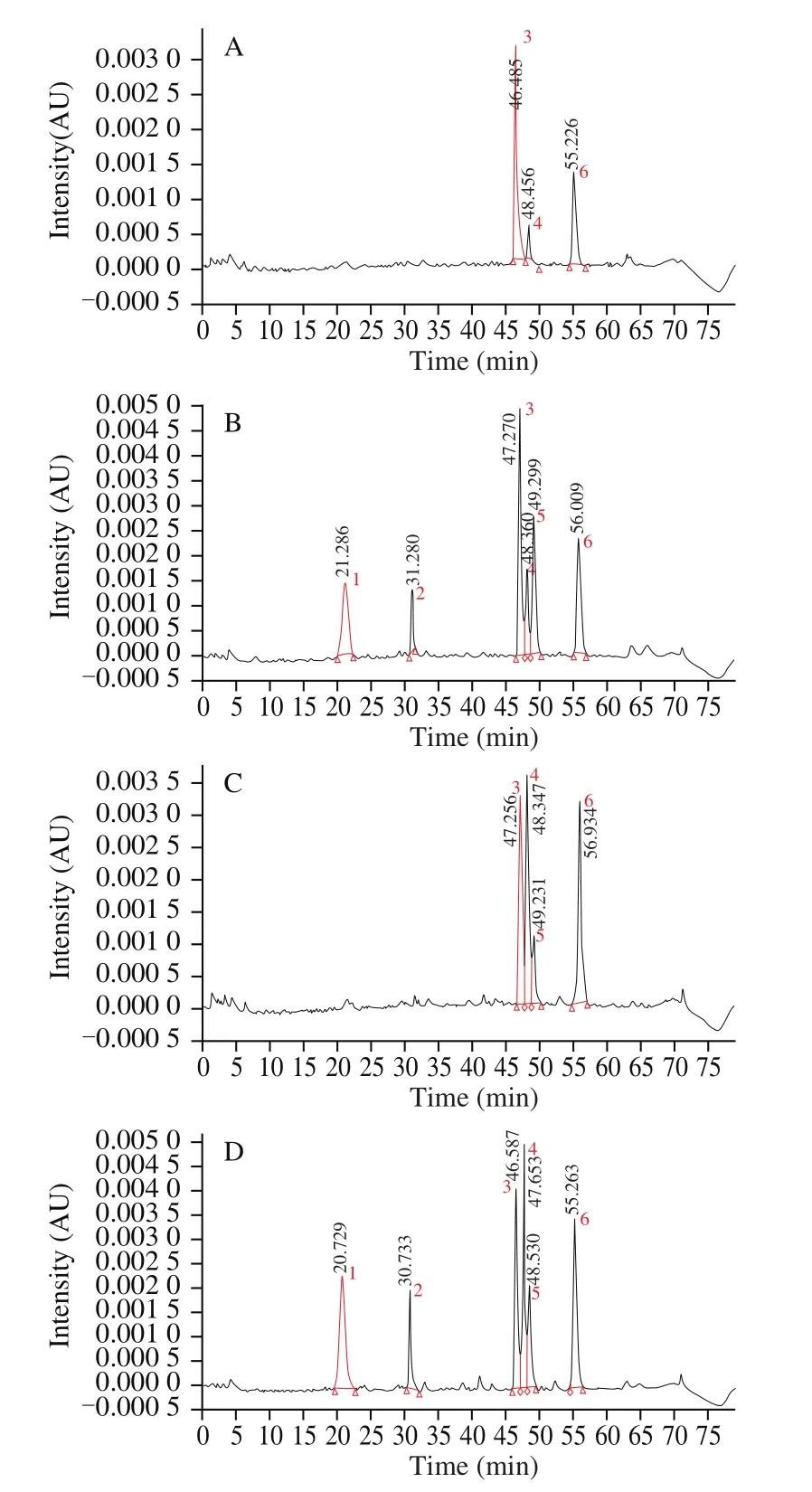
Fig. 1 HPLC profiles of pulp extracts of two bayberry cultivars dried by two methods. (A) HDDK, (B) FDDK, (C) HDBQ (cultivar ‘BQ’ after hot-air drying), (D) FDBQ. λ = 350 nm.
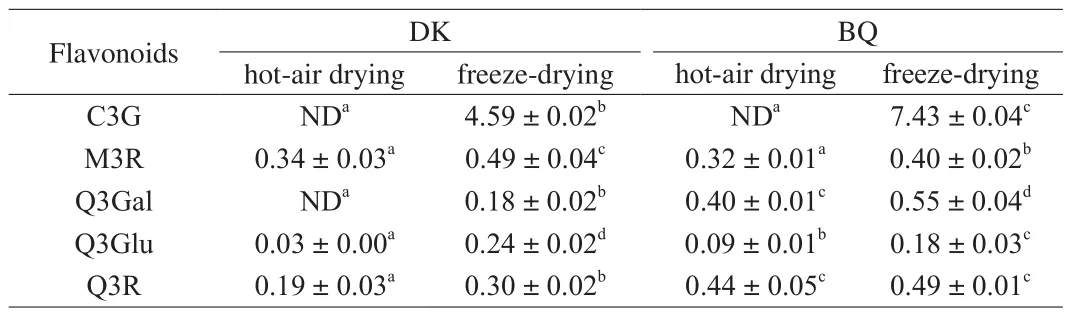
Table 2Contents of 5 flavonoids in the pulp extracts of two Chinese bayberry cultivars dried by two methods.
3.3 Antioxidant capacity
Previous reports had showed that the antioxidant capacity of fruits was related to the presence of phenolic acids and flavonoids[25-27]. EC50value means concentration for 50% of maximal effect in radical scavenging and the lower the value, the higher the scavenging capacity. The results showed that significantly higher DPPH scavenging capacity (Fig. 2A) and ABTS eliminating capacity(Fig. 2B) in the extracts of FDBQ (EC50values were (5.42 ± 0.13) and(3.53 ± 0.05) mg DW/mL, respectively) and FDDK (EC50values were (6.24 ± 0.40) and (4.27 ± 0.05) mg DW/mL, respectively)than those in HDBQ (EC50values were (13.82 ± 0.47) and(9.22 ± 0.35) mg DW/mL, respectively) and HDDK (EC50values were(18.65 ± 0.14) and (16.35 ± 0.81) mg DW/mL, respectively),while still lower than those of vitamin C ((0.06 ± 0.01) and(0.13 ± 0.01) mg DW/mL) (P< 0.05). In addition, slightly higher DPPH scavenging capacity (Fig. 2A) and ABTS eliminating capacity(Fig. 2B) were observed in the extracts of HDBQ and FDBQ than those in HDDK and FDDK, respectively.
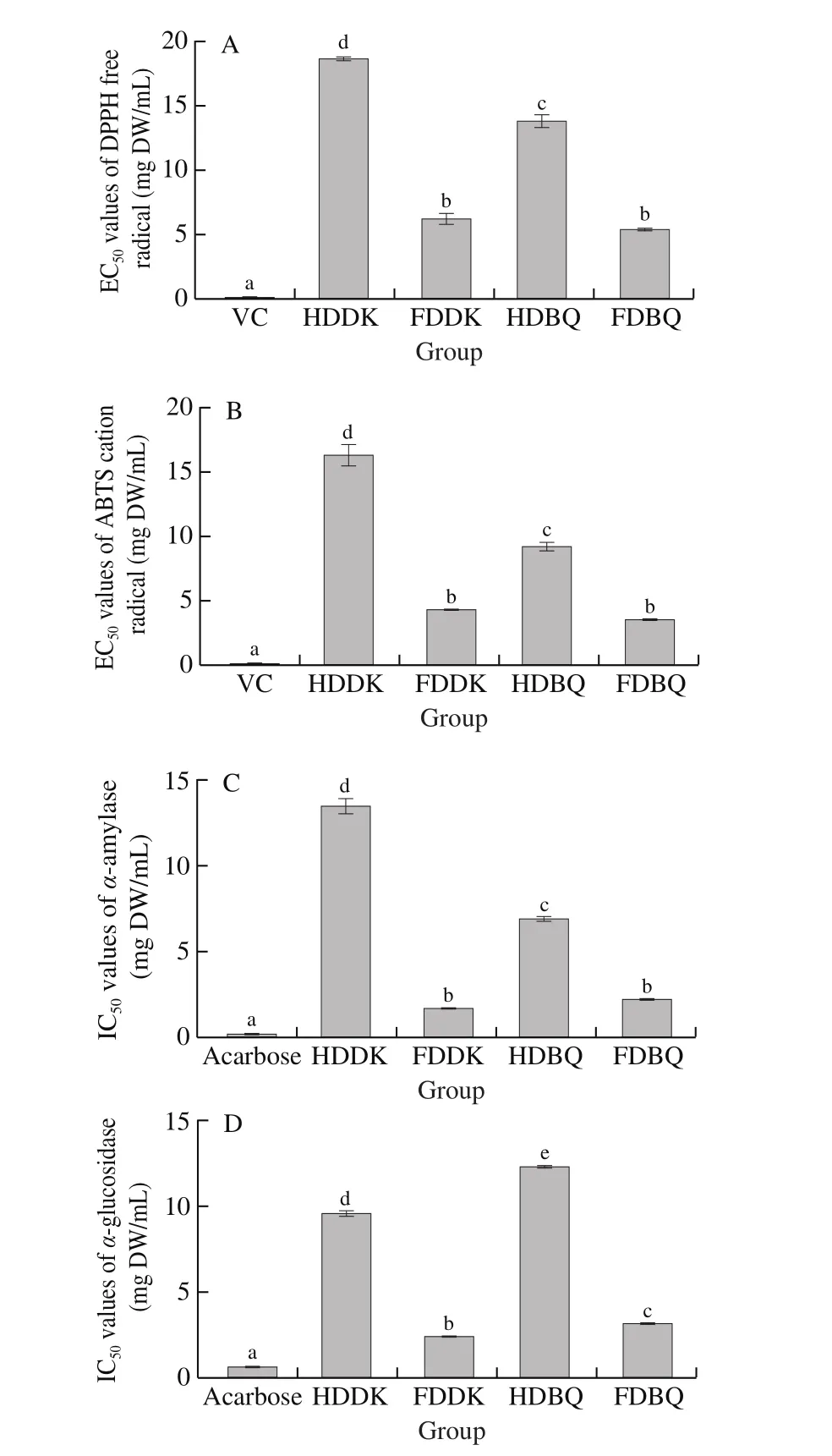
Fig. 2 In vitro antioxidant and hypoglycemic activities in pulp extracts of two Chinese bayberry cultivars dried by two methods. EC50 values of(A) DPPH scavenging capacity and (B) ABTS radical eliminating activity. IC50 values of (C) α-amylase and (D) α-glucosidase inhibitory activity. Different letters on the top of bars denoted the significant difference at P < 0.05.
3.4 α-Amylase and α-glucosidase inhibitory activities
In vitroα-amylase andα-glucosidase inhibitory bioassays are common in a preliminary study on hypoglycemic activity of a candidate drug or bioactive component and usually expressed as IC50value of inhibitory activity [28]. The lower the IC50value was, the higher inhibitory activity to glycometabolic enzymes showed. The extracts of FDBQ and FDDK showed significantly higherα-amylase andα-glucosidase inhibitory activities, expressed as lower IC50values in Fig. 2C ((2.19 ± 0.06) and (1.71 ± 0.03) mg DW/mL) and Fig. 2D((0.62 ± 0.02) and (0.47 ± 0.01) mg DW/mL), than those of HDBQ((6.95 ± 0.13) and (2.46 ± 0.02) mg DW/mL) and HDDK ((13.54 ± 0.44)and (1.92 ± 0.03) mg DW/mL), while they were still lower (P< 0.05)than those of acarbose ((0.16 ± 0.01) and (0.00 ± 0.01) mg DW/mL),respectively. This indicated that hot-air drying had a significant lowering effect on thein vitrohypoglycemic activity of bayberry pulp extract. Plant extracts possessα-amylase andα-glucosidase inhibitory activities might be related with the presence of phenolic compounds,and the potency of enzymatic inhibition would be affected by the sample preparation [29]. A previous study reported that freezedriedPhyllanthus niruriextracted by 80% ethanol solution showed significantly higherα-glucosidase inhibitory activity than that of ovendried sample [30], which is consistent with our results. In addition,the inhibitory activities of FDDK extract againstα-amylase andα-glucosidase were slightly higher than those of FDBQ, respectively.However, the HDDK extract showed a significantly higher inhibitory activity againstα-glucosidase than that of HDBQ (P< 0.05). These results suggested ‘DK’ bayberry pulp extract had betterin vitrohypoglycemic activities than ‘BQ’ extract.
3.5 Pearson correlation coefficients
Flavonoids had shown significant correlations with antioxidant activity and starch digestive enzyme inhibition activity in previous studies [4,31]. The Pearson correlation coefficients among chemical compounds, antioxidant activity and enzyme inhibition activity in Chinese bayberry pulp were displayed in Table 3. Three significant trends were observed. Firstly, a significant correlation was found between the contents of flavonols and antioxidant activities(P< 0.05). Extremely negative correlation was present between C3G and EC50of DPPH (−0.905), or ABTS (−0.834) scavenging activity(P< 0.01). In addition, the correlation coefficient also indicated a strong negative correlation between M3R and EC50of DPPH scavenging ability (−0.761), and Q3Glu and ABTS scavenging ability(−0.923) (P< 0.01), respectively. Secondly, there were significantly negative correlations between most of the five flavonoid monomers and IC50ofα-amylase orα-glucosidase inhibitory activity (P< 0.01).The IC50ofα-amylase inhibitory activity was negatively correlated with the content of Q3Gal (−0.759), Q3Glu (−0.758) or Q3R (−0.796)(P< 0.01). Similarly, the IC50ofα-glucosidase inhibitory activity was also found to show negative correlations with C3G (−0.901), M3R(−0.883) and Q3Glu (−0.852) (P< 0.01). Finally, an exceedingly positive correlation was observed to exist between IC50values ofα-amylase andα-glucosidase inhibitory activities and EC50values of antioxidant activities (P< 0.01). The correlation analysis further confirmed that flavonols present in pulp extracts of Chinese bayberry fruits contributed to their antioxidant and hypoglycemic activities.Furthermore, the bioactivities of Chinese bayberry extract were correlated with various flavonoids, and this might be the reason for the differences in bioactivities caused by drying methods and cultivars.
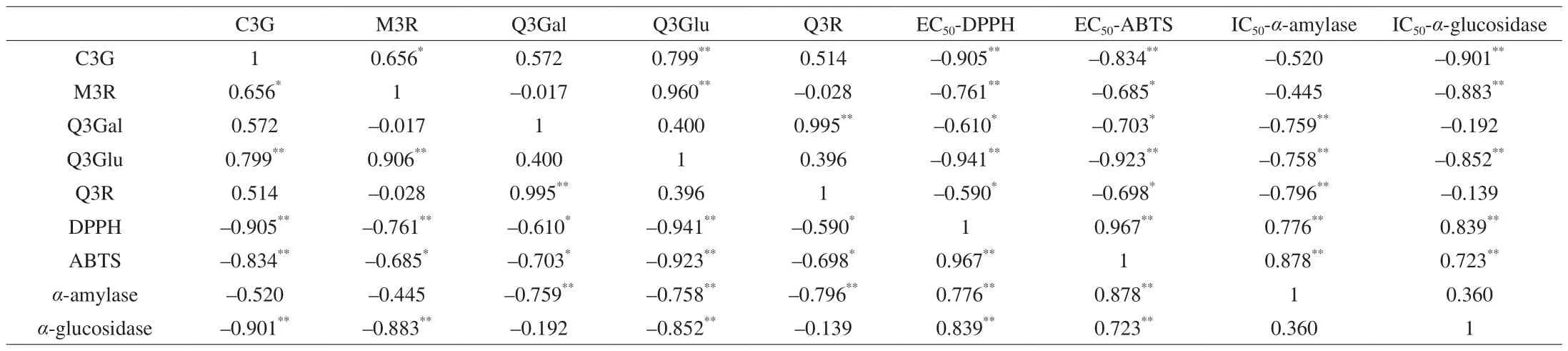
Table 3Pearson’s correlation coefficients between contents of 5 flavonoid monomers, antioxidant activities and hypoglycemic enzyme activities in pulp extracts of two Chinese bayberry cultivars dried with two methods.
4. Discussion
Drying is a traditional and useful method to preserve fruits and vegetables which has been used since a very ancient time. Hot air drying is the most commonly used drying method in the practice due to its low cost and convenient operation [32]. However, its high temperature and long treatment time can lead to several undesirable effects, including bioactive compounds degradation. Hot air drying caused more loss of phenolics inRhodomyrtus tomentosaberries than combined microwave-hot-air-drying [33]. A similar result was reported that hot air drying persimmon showed a dramatically loss in TPC and TFC [34]. In our study, TPC, TFC, TAC and the contents of certain flavonoids (C3G, M3R, Q3Gal, Q3Glu and Q3R) in the hot air-dried bayberry pulp extract were significantly lower than those in freeze-dried samples (P< 0.05) (Tables 1 and 2). It is well known that phenolic compounds are susceptible to oxidation during heating.The decrease in TPC, TFC, and TAC of hot-air dried samples might be that hot air accelerated the oxidation of phenolic and flavonoids by polyphenol oxidase in fruit, and the degradation of anthocyanins might be caused by adverse impacts of high temperature on their structures,such as cleavage, polymerization and derivatization [24,35,36].Although drying is a commonly used method for preserving fruits,different drying methods may cause the loss of nutrients, bioactive compounds and phenolic profiles in fruits to different degrees. Freeze drying, which is conducted at low temperature and pressure, is widely known as an effective drying technique for better preservation of nutrients and bioactive components in fruits. Freeze-dried cacao pod husks possessed significantly higher contents of TPC and TFC than that of hot-air dried samples [37]. A similar research reported that ginger rhizome after freeze drying showed obviously higher TPC and total carotenoids than that of oven-dried samples [38]. The reason might be that the low temperature and oxygen partial pressure applied by freeze drying could inhibit the activity of polyphenol oxidase and reduce the occurrence of oxidation [39,40]. Thus, it is suggested that lyophilization is a more suitable drying technique for the preservation of high bioactive and high-value fruits including Chinese bayberry.
Chinese bayberries are rich sources of phenolic compounds,such as phenolic acids, anthocyanins and flavonoids, which are identified to play a pivotal role in the regulation of health conditions,including antioxidant activity, anti-inflammation, anti-cancer and anti-diabetes. ‘BQ’ and ‘DK’ are two of the main Chinese bayberry cultivars in Zhejiang Province, China. It is known that the contents of each bioactive compound vary widely among different cultivars.According to Chen et al. [5], total phenolics and flavonoids in fresh ‘BQ’bayberry were 2.07 g GAE/kg fresh weight (FW) and 1.72 g RE/kg FW,respectively, which were markedly higher than the contents in fresh‘DK’ bayberry (1.03 g GAE/kg FW and 0.73 g RE/kg FW, respectively).Similarly, Zhang et al. [16]found that fresh ‘BQ’ bayberry not only showed higher TPC and TFC ((2 531.18 ± 72.20) μg GAE/g FW and (1 911.35 ± 26.42) μg RE/g FW) than fresh ‘DK’ bayberry((1 471.05 ± 20.71) μg GAE/g FW and (1 051.65 ± 44.26) μg RE/g FW),but also possessed higher contents of several individual phenolic compounds, such as C3G, M3R, Q3Gal. In the present study, we found that ‘BQ’ bayberry possessed higher TPC, TAC and TFC than‘DK’ bayberry after hot air or freeze drying (Table 1). Therefore,the contents of phenolic components in dried Chinese bayberries are not merely related with drying methods, but also concerned with the cultivars used in the production.
Phenolic compounds which can be structurally divided into phenolic acids and flavonoids, are natural antioxidant substances found in plants [27,28,31]. Previous studies have shown that phenolic compounds were the major contributors to the antioxidant activity of Chinese bayberry [24]. In addition, several reports have revealed that the antioxidant activities of fruits are generally positively correlated with their phenolic contents. ‘BQ’ bayberry with higher phenolic contents exerted a more potent antioxidant activity than that of ‘DK’bayberry [5]. As mentioned above, drying method is one of the key influence factors on the phenolic contents in fruits. In this study,freeze-dried Chinese bayberries possessed not only significantly higher content of total phenolics but also stronger antioxidant activity than those of the hot-air dried samples (Figs. 2A, 2B). Similar results showed that more significant losses were observed in the contents of total phenolic content and antioxidant properties in tomato and ginger when thermal drying applied compared with freeze drying used [41].Our results suggested that hot-air drying has a significant lowering effect on the antioxidant capacity of the bayberry extract while freezedrying is beneficial to maintain higher antioxidant capacities for both‘BQ’ and ‘DK’ bayberry.
Numerous studies suggested a beneficial effect of flavonoid-rich Chinese bayberries on diabetes. C3G-rich bayberry fruit extract was found to exert a protective effect on pancreatic β cells, significantly reduce the blood glucose level and improve the glucose tolerance in streptozotocin-induced diabetic mice [42]. It has been revealed that the glucose consumption activities of bayberries varied dramatically among different cultivars, and they were highly correlated with their phenolic contents, DPPH scavenging activities, and certain flavonoids including C3G, Q3Gal, Q3Glu, and Q3R in human HepG2 cells [6].Inhibition of the activities of critical enzymes in starch digestion likeα-amylase andα-glucosidase is found to be effective to decrease the postprandial hyperglycemia, and it is regarded as an important treatment strategy in type II diabetes. Chinese bayberry extract possessed potentα-amylase andα-glucosidase inhibitory activities,and the inhibition potency was highly relevant with bayberry cultivars and the drying method applied (Figs. 2C, 2D). A previous research found that C3G, M3R, Q3Gal, and Q3R, the contributors to theα-glucosidase inhibitory activity of Chinese bayberry, exhibited differentα-glucosidase inhibitory activity [43]. It was indicated that the differences in the hypoglycemic activities of bayberry extracts might partially be attributed to their phenolic profiles and contents.Our Pearson correlation coefficients result (Table 3) indicated that the contents of Q3Glu, Q3R and Q3Gal in pulp extracts showed significant correlation withα-amylase inhibitory activity, while the contents of C3G, M3R and Q3Glu had significant correlation withα-glucosidase inhibitory activity (P< 0.01). This result further confirmed that flavonoids present in pulp extracts of Chinese bayberry fruits were contributed to their antioxidant and hypoglycemic activities.
5. Conclusions
Hot-air drying and freeze-drying are two commonly used drying methods in food industry. It depends on the processing purpose to make a choice. Compared with hot-air drying, the pulp extracts prepared by freeze-drying have significantly higher TPC, TFC, TAC,flavonoids andin vitroanti-oxidative and hypoglycemic activities(P< 0.05) for both bayberry cultivars. ‘BQ’ and ‘DK’ bayberries are prevalent cultivars in Zhejiang Province. TPC, TFC, TAC and distinctive flavonoids are hallmarks for constituent analysis of bayberry. Our findings suggested that TPC, TFC, TAC and five major flavonoid monomers contents in the ‘BQ’ bayberry pulp extracts are generally higher than those of ‘DK’ bayberry. In addition,the contents of Q3Glu, Q3R and Q3Gal in pulp extracts showed significant correlation withα-amylase inhibitory activity, while the contents of C3G, M3R and Q3Glu had significant correlation withα-glucosidase inhibitory activity (P< 0.01). This study revealed that those compounds were responsible for different bioactive effects.These results suggested that flavonoids are the main contributors to thein vitrohypoglycemic activity of bayberry pulp extract and those from freeze-dried ‘DK’ bayberry have a great potential to serve as hypoglycemic food ingredients which could be used in functional food and pharmaceutical industry.
Declaration of competing interest
The authors declare no conflicts of interests.
Acknowledgements
This work was financially supported by the Science and Technology Department of Zhejiang Province under Key Research and Development Project (2017C02004).
- 食品科学与人类健康(英文)的其它文章
- Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
- Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
- Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria

