Flavonoids of Rosa rugosa Thunb. inhibit tumor proliferation and metastasis in human hepatocellular carcinoma HepG2 cells
Zhiguo Zhng*, Meilin Wng Shu Xing*, Chen Zhng*
a School of Food Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan 250353, China
b School of Chemistry and Pharmaceutical Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan 250353, China
c School of Science, The Hong Kong University of Science and Technology, Hong Kong, China
Keywords:
Rose flavonoids
Tumor metastasis
Apoptosis
Human liver cancer
A B S T R A C T
Rosa rugosa Thunb. is recognized as both medicine and edible in China. The article investigated the antitumor activity of rose flavonoids. Water-extracted rose flavonoids (RFW) and ethanol-extracted rose flavonoids (RFE)were achieved by extracting with distilled water and 70% ethanol, respectively. The effects of the two extracts on proliferation inhibition, apoptosis inducement and metastasis prevention of human HepG2 hepatocellular carcinoma cell lines were tested, via optical/fluorescence microscopy, MTT detection, Transwell assay,flow cytometry and Western blot, etc. The results indicated that rose flavonoids at low concentration(10-40 μg/mL) had a better inhibitory effect on migration and invasion of He pG2 cells in a dose-dependent manner, while rose flavonoids at high concentration (80-160 μg/mL) could induce apoptosis of HepG2 cells by up-regulating the expression of pro-apoptotic proteins p53 and Bax, and down-regulating the expression of anti-apoptotic proteins Bcl-2, leading to the functioning of caspase-3 and caspase-9. The effect of RFE at the same concentration was significantly better than that of RFW. Conclusion, this study found that rose flavonoids had a certain inhibitory effect on proliferation and metastasis of human liver cancer cells HepG2,indicating the application of rose flavonoids in preventing and treating of liver cancer.
1. Introduction
Malignant tumors have always been a difficult problem in medical research and are also one of the main diseases that severely affect human health and threaten human life. As one of the 6 most common malignant tumors predicted in the world, liver cancer has extremely high mortality and disability rates, which is second only to lung cancer [1,2]. Although clinical treatment and drug therapy can reduce its morbidity and mortality in patients at present, both chemotherapy and anticancer drugs with strong side effects will greatly weaken patients’ own immunomodulatory ability. Fra nco et al. [3]found that rose extract was effective in suppressing leukemia virus, human immunodeficiency virus and T-cell leukemia virus. Therefore, natural products with high-efficiency and low-toxicity anti-tumor activity substances have become a great potential for development.
Flav onoids compounds can prevent some types of cancer in an effective manner [4-6]. Flavonoids are a class of compounds that exist in nature with the structure of diphenyl chromone, which mainly bind to sugar to form glycosides in plants, and only a small part of which exists in the form of free status (aglycone) [7,8]. The existence of these substituents results in more than 9 000 kinds of flavonoids compounds in the world, which has good protection effect on health [9].Most fruits and plants contain flav onoids compounds in their roots,stems, flowers, leaves, peels, and grains [10]. As a common bioactive substance, flav onoids compounds have attracted high attention due to their antioxidant, anti-aging, anti-tumor and other bioactive functions [11].
Rosa rugosaThunb., a kind of deciduous shrub of Rosa from Rosaceae family, is not only an excellent Chinese medicinal material,but also can be used as common food in China. Rose petals are used to produce teas, rose wines, jams and juices. Pingyin country in Shandong province, China is rich in roses due to its special terrain and climate. Pingyin rose is known as the representative of traditional Chinese roses because of its sweet, mellow aroma, rich fragrance and excellent quality. Chinese pharmacopoeia describes that rose can harmonize blood and promote circulation of blood, regulate vital energy, and have therapeutic effect on wandering arthritis, anorectic dysentery, acute mastitis, early onset of pyogenic infections, and stomachache (due to emotional depression and hyperactive liver-qi attacking the stomach) [12]. Dietary herbals also indicates that rose is mainly beneficial to lung and spleen, liver and gallbladder, and can exorcise pathogenic factors and be used as delicious food which makes people feeling refreshed [13]. Hence we can see that rose has extremely high edible and medicinal value.
Franco et al. [3]and Qiong et al. [14]found that flavonoids compounds, accounting for up to 3.30% of rose in content by ultraviolet spectrophotometry, could effectively enhance and improve liver function, reduce the toxic effect of alcohol on liver, remove toxins, and have good effect of liver protection. Olech et al. [15]and Renata et al. [16]used the ethanol extract of rose detected better anti-tumor activity on ovarian cancer (TOV-112D), cervical cancer(HeLa), cancer (T47D) and tumor (A549) cell lines by Brdu test. In addition, many studies have confirmed that rose water extract has many biological activities such as antioxidant, anti-inflammatory, and cytotoxicity [17-19]. Flavonoids compounds in rose are considered to be the main anticancer component on the basis of their extensive biological activity in reducing expression of cancer genes, but few literatures have yet confirmed the effective mechanism of rose flavonoids on human hepatoma carcinoma cells.
In this study, distilled water and 70% ethanol solution were used to extract rose flavonoids respectively, and the contents of the flavonoids were determined. Then, the effects of rose flavonoids on proliferation and metastasis of human liver cancer cell-line HepG2 were studied. We verified the effect of low dose (10-40 µg/mL) on migration,invasion and inhibition of human liver cancer cells HepG2. With methotrexate, a common cell cycle-specific anti-cancer drug, as a positive control, verified the effects of high dose (80-160 µg/mL) on morphology,apoptosis, and protein expression of HepG2 cells. It is hoped that from the perspective of drug and food homology, it can provide a theoretical basis for studies and usage of rose against liver cancer.
2. Materials and methods
2.1 Chemicals and general experimental procedures
Dried roses, selected from Pingyin County, Jinan, China, was used as raw materials for extracting rose flavonoids in this study.The human liver cancer cell lines (HepG2) used in the study was purchased from American type culture collection (ATCC). Dimethyl sulfoxide (DMSO) was purchased from Sinopharm Chemical Reagent Co., Ltd. Fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing bioengineering Materials Co., Ltd. DMEM medium and Annexin V-FITC/PI Apoptosis Detection Kit were purchased from Jiangsu Kaiji biotechnology Co., Ltd. Transwell plate and Matrigel were purchased from Corning Incorporated (USA). All antibodies used for western blotting were purchased from ABclonal Biotech Co.,Ltd. The BCA protein assay kit, Ponceau S, MTT and NC film were purchased from Shanghai solarbio Co., Ltd.
2.2 Extraction of rose flavonoids
Dried roses were used as raw materials for extracting rose flavonoids in this study. Two parts of dried rose (100.00 g in each)were accurately weighed, placed in a beaker, and added with 1 000 mL of distilled water and 1 000 mL of 70% ethanol solution (analytical purity) respectively according to 1:10 material-liquid ratio. After digestion in the water bath at 40 °C for 2 h, the mixed solution was filtered with 4 layers of gauze to obtain the first filter residue and the first filtrate, and the first filter residue was re- filtered after re-digestion for 2 h in the material-liquid ratio of 1:5 to obtain the second filter residue and the second filtrate. The first filtrate and the second filtrate were mixed together and centrifuged in a high speed centrifuge for 10 min under the condition of 4 000 r/min to obtain the supernatant.The supernatant was concentrated in a rotary evaporator to obtain the evaporated liquid, and the collected evaporated liquid was frozen,dried, and stored in a refrigerator at 4 °C [20,21].
2.3 Determination of rose flavonoids content
The rutin solution was prepared with a certain concentration gradient to draw a rutin standard curve, and 1 mg/mL of waterextracted rose flavonoids (RFW) and 1 mg/mL of ethanol-extracted rose flavonoids (RFE) were prepared respectively to calculate the content of flavonoids according to the rutin standard curve [22].
2.4 Passage cultivation of HepG2 cells
The cells were cultured in a DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin and then cultured in a CO2incubator, containing 5% CO2, at 37 °C. The status of cell growth was observed regularly, the inoculum was replaced according to the cell growth condition, and the cell passage was performed when the cell density reached 90% [23,24]. All experiments were completed independently and three sets of parallel tests were set up.
2.5 MTT assay
MTT test was conducted in this study to assess the availability and cellular activity. HepG2 cells were seeded into 96-well microplates(2 × 105cells/well) and cultured at 37 °C and 5% CO2for 24 h. A total of 100 µL of medium containing different concentration gradients of RFW and RFE (0, 10, 20, 40, 80 and 160 µg/mL) was added to the corresponding well after 24 h of culture (growing cells covered 80% of the bottom) and continued culture for 24 h with equal concentration of methotrexate as a positive control. All samples were dissolved with 0.05% DMSO which did not cause damage to cells [25]. After that, a total of 100 µL of MTT solution (0.5 mg/mL) was then added to each well and continued culture for 4 h. The medium containing MTT was discarded and 100 µL of DMSO was added to each well to fully dissolve them to form crystal formazan. The light absorption value of each well was measured by enzyme-linked immunosorbent assay (ELISA) at wavelength of 570 nm after 30 min of incubation at 37 °C. The inhibition rate was expressed as the absorbance percentage of treated cells to control cells [26,27].
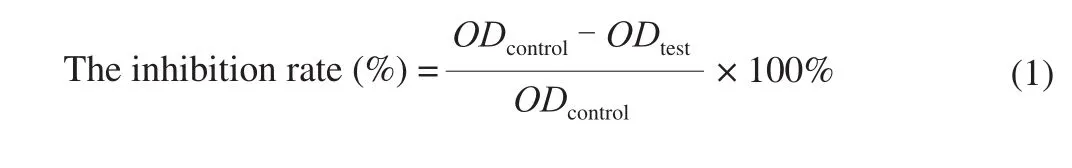
2.6 Detection of HepG2 cells migration by wound healing assay
A wound healing assay was used in this study to investigate the effect of rose flavonoids on migration of HepG2 cells. 2 × 105cells were evenly seeded into one well of a 6-well plate and cultured until cells reatch 90% confluence. A straight line was drawn along the diameter in the center of well by a 10 µL pipette tip. PBS was used to clean the line, and a fresh medium containing different concentration gradients of RFW, RFE, and methotrexate (0, 10, 20, 40 µg/mL)was then added to continue culture. After 24 h of cultivation, the cell migration in scratches was observed by an inverted microscope (× 200 magnification). The width of damaged lines in three sets of parallel tests was measured under microscope and the percentage of width at 0 h was plotted [28,29].
2.7 Detection of HepG2 cells invasion by Transwell test
Transwell system was used in this study to analyze the effect of rose flavonoids on invasion of HepG2 cells. Transwell plate and Matrigel used in the experiment were purchased from Corning Incorporated (USA). Transwell plate was prepared, a layer of Matrigel was placed on the bottom of the upper chamber of each well, and the chamber was cultured in the incubator for 4 h and taken out after gelatine solidified. The lower side of the filter was coated with DMEM medium containing 20% bovine serum albumin (600 µL/well).100 µL cell suspensions containing RFW or RFE (0, 10, 20, 40 µg/mL)were seeded into the upper side (105cells/well) and then cultured in an incubator with 5% CO2, at 37 °C. After 24 h, the cells on the upper surface of the filter were completely wiped off with a cotton swab, fixed with methanol, and cleaned it twice with PBS. Precooled methanol was added from the lower and immersed in the upper,which was fixed at room temperature for 10 min and discarded. After washing in PBS, the bottom of the upper chamber was stained with DAPI and observed under microscope after 15 min to calculate the number of invading cells [30,31]. Three sets of parallel tests were set up for each set of samples.
2.8 Tumor cell apoptosis detection
An Annexin V-FITC/PI Apoptosis Detection Kit was used to analyze the cells apoptotic mechanism of HepG2 induced by rose flavonoids. A total of 2 × 105cells was seeded into a 6-well culture plate and cultured for 24 h. After cell adherence, the original medium was discarded and a fresh medium containing different concentration gradients of RFW, RFE, and methotrexate (0, 80, 160 µg/mL)was added to the plate for continuous culture. The medium was discarded after 24 h, the plate was washed with PBS, and the cells were collected and centrifuged under the condition of 2 500 r/min for 3 min to obtain the supernatant. The supernatant was discarded, 1 mL of binding buffer was added to wash the cells, and 500 µL of binding buffer containing 5 µL of Annexin V-FITC and 5 µL of PI was then added. The plate was placed in dark place at room temperature for 10 min and was then tested by a flow cytometry [32-34]. The experimental data of each set were independently completed and three sets of parallel tests were set up.
2.9 Western blot
A total of 2 mL of cell suspension was evenly inoculated in a 6-well culture plate and cultured for 24 h. After cell adherence,the original medium was discarded and a fresh medium containing different concentration gradients of RFW, RFE or methotrexate(80, 160 µg/mL) was added to the plate for continuous culture. The medium was discarded after 24 h, the plate was washed with frozen PBS for 2-3 times, treated with lysis buffer (100 ×, PMSF:RIPA =1:100) for 15 min on ice of reaction and pyrolysis. The lysed cell masses were slowly removed with a scraper and centrifuged under the condition of 3 000 r/min for 10 min to obtain the supernatant. The supernatant was collected and measured with a BCA kit to detect the content of protein for determination of loading quantity of sample.The protein was separated by SDS-PAGE electrophoresis, and the NC membrane was used to transfer at voltage 90 V for 2 h, and was stained by Ponceau S Staining Solution to determine whether the membrane was successfully transferred. It was washed with PBS for 2-3 times, sealed with 5% skim milk powder for 1 h, washed with PBS, and then added with primary antibodies, sealed and stored in the refrigerator at 4 °C overnight. It was washed with PBS, added with secondary antibodies, then incubated for 2 h, washed with PBST for 3 times, and then rewashed with PBS for 3 times (5 min each time). The color developing agent was added, and optical density of the strip was analyzed by using western blot imaging analytic system [35-37].
2.10 Statistical analysis
All the data were expressed as mean ± SD. A one-way analysis of variance (ANOVA) was used to detect the significant difference between the experimental conditions and the control group. *P< 0.05 and **P< 0.01 were considered statistically significant.
3. Results and discussion
3.1 Flavonoid contents in Rose
As shown in Fig.1, (A) is the dried rose corolla, (B) is RFW, and(C) is RFE. The rutin standard curve was drawn, which resulted in a regression equationA= 0.011 5c+ 0.008 8 and a correlation coefficientR2= 0.999 7, indicating that the rutin concentration selected in the test had a good linear relationship with the light absorption value. The light absorption values of RFW and RFE were introduced into the rutin standard curve to calculate the content of flavonoids extracted from rose. As shown in Table.1 the extraction yield of crude rose flavonoids with extracted by distilled water and 70% ethanol solution were 24.70% and 28.34% , respectively. The content of RFW is(47.82 ± 0.152) mg RE/g, the content of RFE is (79.08 ± 0.195) mg RE/g.The content of RFE is 1.65 times of that in RFW. Marta et al. [38]used LC-MS/MS-MRM protocols to detect flavonoid aglycones was 14.46 µg/g inRosa rugosaThunb. In addition, studies have shown that roses also contain flavonols, quercetin, isoquercitrin, multinoside A, multiflorin B, anthocyanins, rutin, tiliroside, kaempferol-3-O-rutinoside and apigenin-7-O-glucoside, and so on [16,38-39].
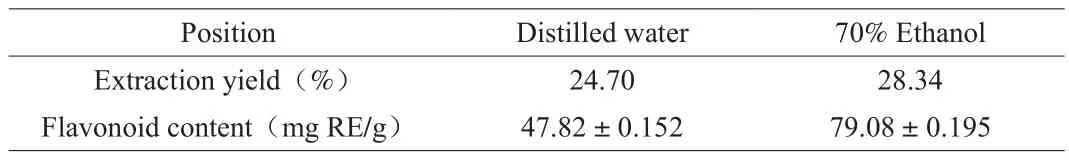
Table 1The rose flavonoid content in different extraction phases.
3.2 Effect of rose flavonoids on the inhibition of tumor cell proliferation
MTT assay was used to determine the effects of rose flavonoids on growth of HepG2 cells. Fig. 2 shows the inhibition of cell growth after stimulating HepG2 cells with RFW and RFE for 24 h,respectively. The inhibition rate were enhanced with the increase of administered concentration, and the effect of RFE was obviously better than that of RFW at the same concentration. When the concentration reached 160 µg/mL, the inhibition effect of RFE was significantly higher than that of methotrexate, with the inhibition rate being up to 83.76% .The inhibition rate of 10, 20, 40, 80 and 160 µg/mL of the RFW on HepG2 cells were 15.17% , 18.11% , 25.88% , 39.31% and 53.47% ,respectively, while the same concentration of the RFE on HepG2 cells were 22.38% , 30.08% , 38.14% , 49.15% and 83.76% , indicating a dose-dependent pattern. The IC50value was (138.22 ± 1.38) µg/mL for RFW, and (75.55 ± 0.87) µg/mL for RFE, respectively.

Fig. 1 Dried rose corolla (A) and the crude extract of rose flavonoids:RFW (B), RFE (C).
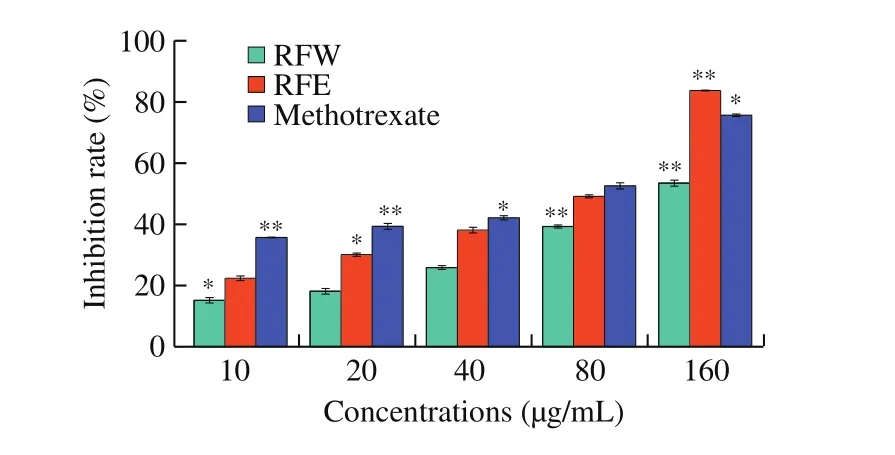
Fig. 2 The growth-inhibitory effects of rose flavonoids on HepG2 cells were assayed by MTT assay. The results are expressed as means ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the control.
The MTT test is the most conventional and effective method for detecting cell proliferation and cell viability [27]. The test results show that the inhibitory effect of rose flavonoids extracted with different solvents on HepG2 cells is dose-dependent. The higher the dose is, the stronger the cytotoxicity exhibited. This shows that rose flavonoids have good anti-tumor properties, and the effect of RFE is markedly better than that of RFW.
3.3 Effects of rose flavonoids on morphological changes of HepG2 cells
Adherent cells growth in normal process will be stimulated by their own secretion or nutrients in the medium, spread on the surface of the medium through precellular attachment factor, and extend to the normal morphology, and their adhesion will be decreased when cells are stimulated by external conditions [40]. Fig. 3 showed the changes of cell morphology after treating HepG2 cells with rose flavonoids for 24 h. The cells growth of the control group was in good condition, the cell adherence was firm, and the cell masses were tightly connected and evenly grew with good diopter. With the increase of drug concentration, the cell growth was obviously inhibited. When the concentration of rose flavonoids was 40 µg/mL,the cell density was obviously decreased and the adhesion became worse. When the concentration reached 80 µg/mL, cells became round gradually, the suspended cells were gradually increased, and a large number of edge shrinkage, cell breakage, floating appeared. When the concentration reached 160 µg/mL, more than 90% of the cells became round and lost their original morphology.
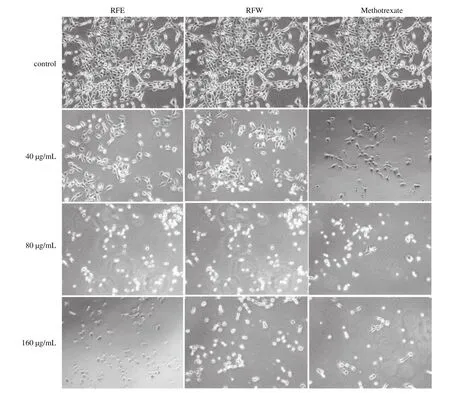
Fig. 3 The morphological changes of HpeG2 cells treated with RFW and RFE respectively. Observation were made under an inverted optic microscope (100 ×).
The observation of cell morphology further proved the results of the MTT test that the effects of different concentrations of rose flavonoids on HepG2 cells were in a concentration-dependent pattern.The low concentration of rose flavonoids (10-40 µg/mL) can inhibit the growth of HepG2 cells, while the high concentration of rose flavonoids (80-160 µg/mL) can destroy the cell structure and promote cell apoptosis. The effect of RFE is slightly better than that of RFW and is equivalent to the effect of methotrexate. Therefore, we use low-dose and high-dose samples to study tumor cell metastasis and apoptosis, respectively.
3.4 Effect of rose flavonoids on HepG2 cell migration
To investigate the effect of rose flavonoids on migration of HepG2 cells, a wound-healing assay was used in this study. Scratches were made on the cell surface with a pipette tip, and the width of scratches was measured under microscope. Cells were cultured after scratching for 24 h. As shown in Fig. 4, when treating cells with increased concentration of RFW and RFE respectively, and the scratch-healing width at 24 h decrease in a dose-dependent pattern.Compared with the initial scratches, the scratches in the control group(0 µg/mL) basically healed with the healing rate of up to 70.83% .With the increase in the concentration of rose flavonoids, the healing rate was decreased gradually in the group. When the concentration was 10 µg/mL, the healing rate was 38.11% in the RFW group and 22.58% in the RFE group; when the concentration was 20 µg/mL, the healing rate was 36.67% in the RFW group and 20.83% in the RFE group; and when the concentration was 40 µg/mL, the healing rate was 23.33% in the RFW group and almost zero in the RFE group.The above results showed that rose flavonoid could effectively inhibit migration of HepG2 cells at low concentration, and the effect of the RFE was better than that of the RFW.
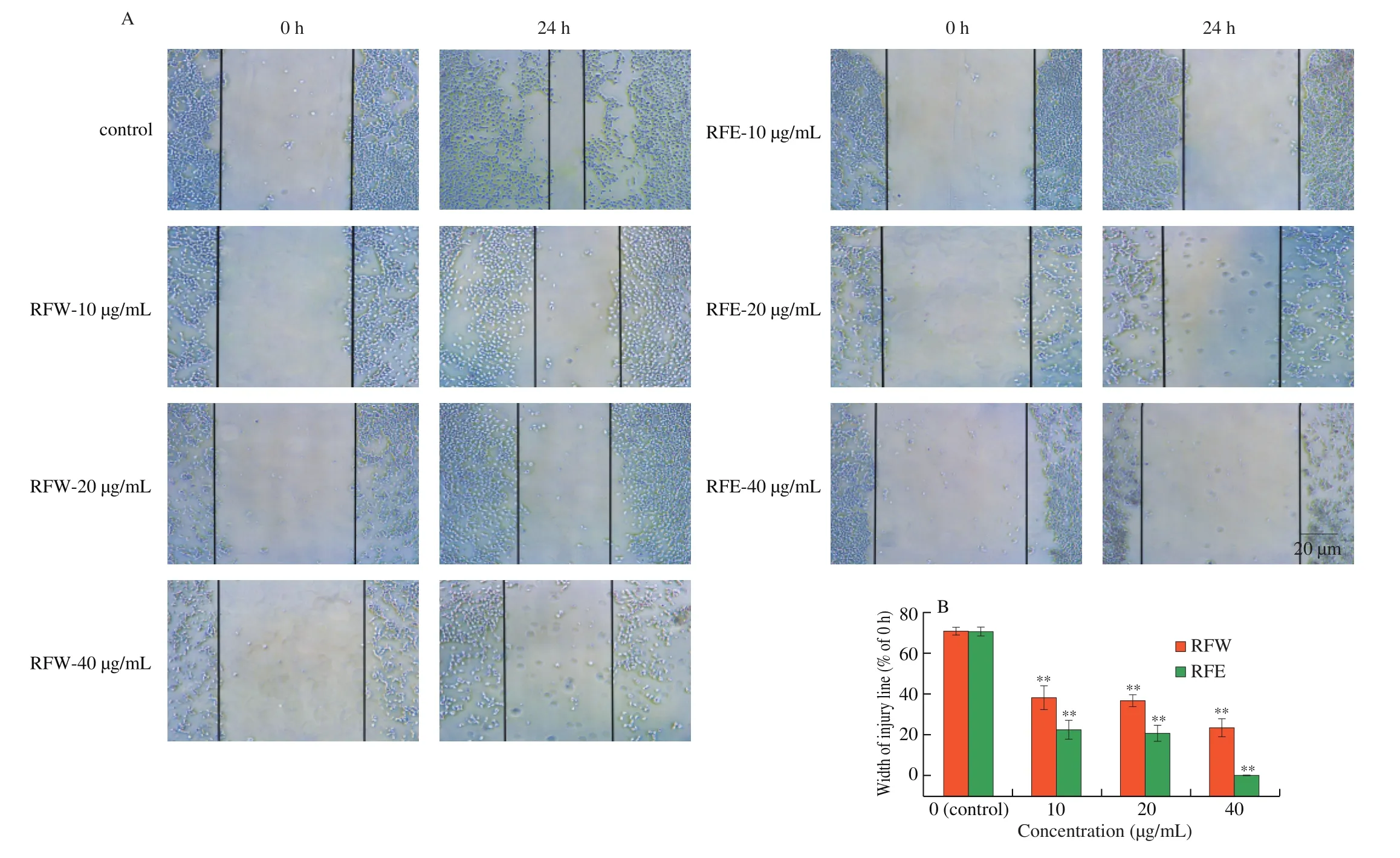
Fig. 4 Effect of rose flavonoids on migration assay of HepG2 cells. (A) Representative images of control and RFW or RFE-treated cells (200 ×). (B) The width of the injury line was measured and plotted as a percentage of the width at 0 h. Data means ± SD from three independent experiments *P < 0.05, **P < 0.01 compared with the control.
3.5 Effect of rose flavonoids on HepG2 cell invasion
Cell invasion is an important factor to inhibit tumor metastasis. In order to further study the effect of rose flavonoids on invasion ability of HepG2 cells, anin vitroexperiment of Transwell cell invasion was conducted in this study. Per-stained HepG2 cells were treated for 24 h with rose flavonoids of different concentrations, and cells that transmigrated through were examined under fluorescence microscope.As shown in Fig. 5, with the increase of concentration, the number of HepG2 cells penetrate through Matrigel was gradually decreased.The migration rate of the control group (0 µg/mL) was considered as 100% . When the concentration of rose flavonoids was 10, 20 and 40 µg/mL, respectively, the invasion rates were 13.33% , 10.27% and 7.22% in the RFW group and 11.38% , 9.72% , 3.33% in the RFE group, respectively. The results indicated that when the concentration of rose flavonoids was between 10 µg/mL and 40 µg/mL, it had a good effect on inhibiting tumor metastasis, and the effect of the RFE group was significantly better than that of the RFW group.
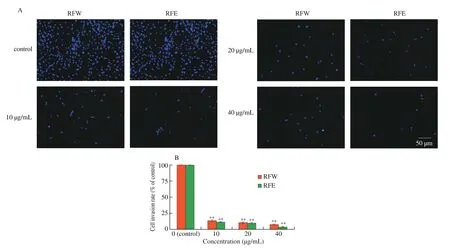
Fig. 5 Effect of rose flavonoids on invasion ability of HepG2 cells. (A) Representative images of control and RFW or RFE-treated cells under fluorescence microscope (40 ×). (B) Invasion was quantified by counting the number of cells that migrated to the opposite side of the transwell membrane, within randomly selected microscope fields. Data means ± SD from three independent experiments.
Wound-healing assay and Transwell invasion test proved that low-dose rose flavonoids can inhibit the migration and metastasis of HepG2 cells effectively. Previous researches had found that quercetin and resveratrol can inhibit cell metastasis well [28], and these substances are also the main aglycons in rose flavone [15,16].
3.6 Effect of rose flavonoids on tumor cell apoptosis
In order to assess the effect of rose flavonoids on cell apoptosis,HepG2 cells were treated with rose flavonoids of different concentrations for 24 h, and the ratio of early and late apoptotic cells was tested by Annexin V-FITC and PI staining kit in this study. As shown in Fig. 6, most of the cells in the control group were normal,only 7.4% were damaged or apoptotic. In comparison with the control, when the drug concentration was 80 µg/mL, cells gradually began to apoptosis, with 65% of cells in condition, 24.5% in the early apoptotic status, and 8.95% in the late apoptotic status for the RFW group. Meanwhile in the RFE group, 53.5% of cells were normal,34.4% were in early apoptotic status, and 11.2% were in late apoptotic status. When the concentration reached 160 µg/mL, most cells became apoptotic. The inhibition rate of the RFE group reached 92.89% ,much higher than that of the RFW group (around 50% ) as well as the methotrexate group (around 75% ).
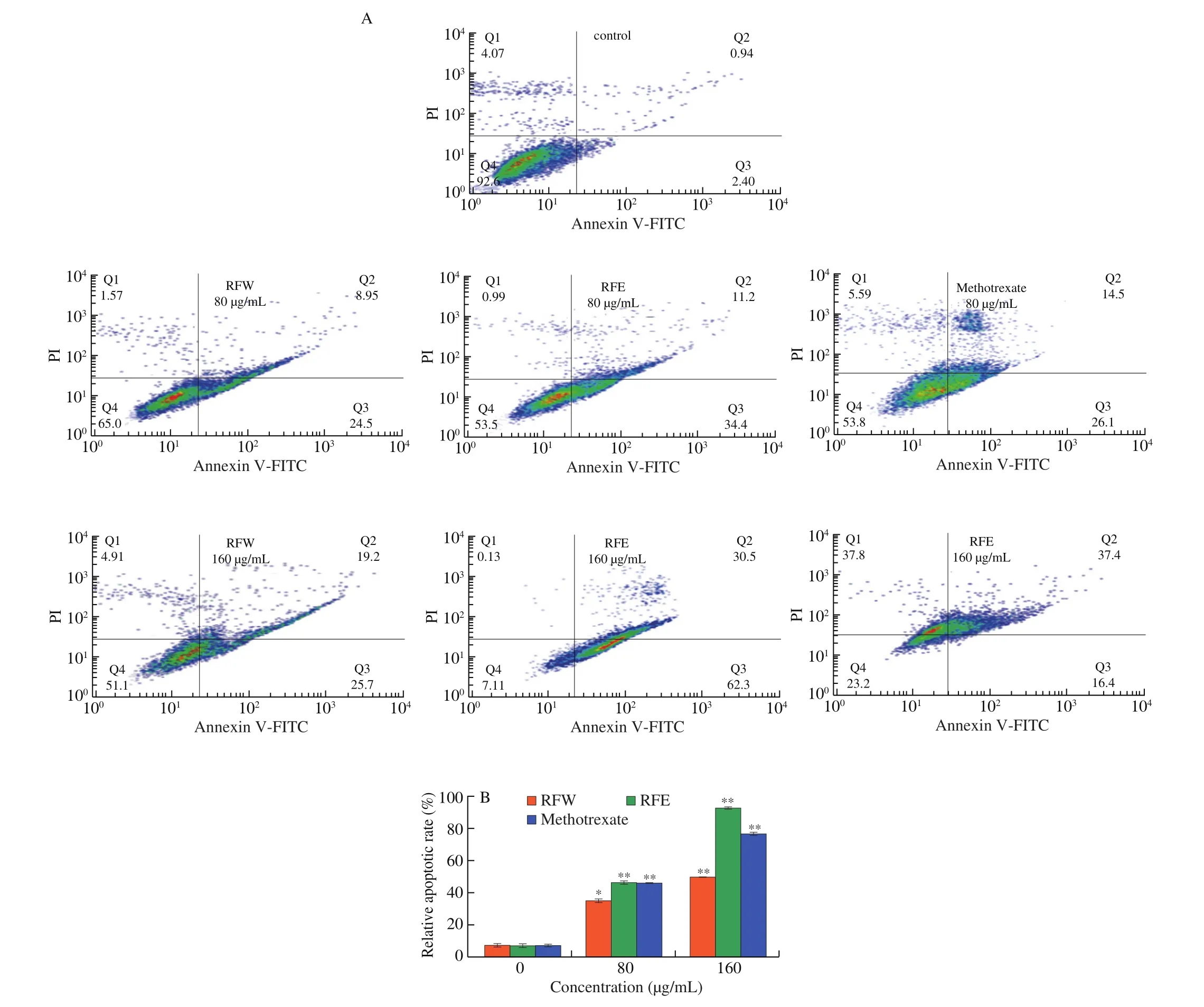
Fig. 6 The effects of rose flavonoids on apoptosis of HepG2 cells. (A) Flow cytometry of HepG2 cells exposure to RFW or RFE for 24 h. (B) The proportion of damaged and apoptotic HepG2 cells. Data shown represent means ± SD for three independent experiments. *P < 0.05, **P < 0.01 compared with the control.
Cell apoptosis is a continuous and irreversible process. This process is divided into 3 stages: a derivative stage, an effector stage and a degradative stage [42]. The results of apoptosis showed that rose flavonoids could induce apoptosis of HepG2 cells in a dose-dependent pattern. The effect of RFE was better than that of RFW, and even methotrexate when administrating at a same concentration of 160 µg/mL.Yun et al. [42]used seed extract ofCoix lacryma-jobito induce HepG2 cell apoptosis and obtained similar results, and proved that the extract up-regulated and prolonged the expression of caspase protein,but had no clear effect on the expression of Bcl-2. Studies have found the anti-tumor effect of apple flavonoids by using apple flavonoids to induce cancer cells. Compared with apple flavonoids, rose flavonoids have more obvious anti-tumor ability. The anti-tumor ability of rose flavone may be due to the induction of cells to produce ROS, which is a key mediator of inducing apoptosis [23], but the specific mechanism needs to be further studied. In summary, rose flavonoids are with great potential to be further developed and utilized as anticancer drugs.
3.7 Effect of rose flavonoids on modulating apoptotic associated proteins level of HepG2 cell
Thep53,Bcl-2andcaspasefamilies are the main apoptosisrelated genes. After treating HepG2 cells with the rose flavonoids for 24 h. Western blotting was used to test the expression of apoptotic related proteins. p53 is an important regulator of apoptosis, which can promote cell apoptosis by activating the expression of positive regulatory factors of apoptosis. Bcl-2 and Bax proteins belong to the Bcl-2 family, among which Bcl-2 is anti-apoptotic protein and Bax is pro-apoptotic protein [41,42]. Both pro-caspase 3 and pro-caspase 9 belong to the caspase family, among which pro-caspase 9 is involved in the initiation of cell apoptosis and pro-caspase 3 in the execution of cell apoptosis [43,44].
As shown in Fig. 7, the expression levels of p53and Bax were gradually up-regulated with the increase of rose flavonoids concentration, whereas the expressions of Bcl-2, pro-caspase 3, and pro-caspase 9 were down-regulated. When the HepG2 cells were treated with 80 and 160 µg/mL RFW respectively,p53 was upregulated by 1.33 and 1.77 times and Bax was up-regulated by 1.51 and 1.65 times, Bcl-2 was down-regulated to 61% and 18% , procaspase 3 was down-regulated to 93% and 60% , pro-caspase 9 was down-regulated to 82% and 68% respectively. However, when the HepG2 cells were treated with 80 and 160 µg/mL RFE respectively,p53 was up-regulated by 1.44 and 1.98 times and Bax was upregulated by 1.42 and 1.68 times, respectively. Bcl-2 was downregulated to 35% and 5% , pro-caspase 3 was down-regulated to 51% and 44% , and pro-caspase 9 was down-regulated to 69% and 51% ,respectively. In comparison, when the concentration of methotrexate was 160 µg/mL, p53 and Bax were up-regulated by 2.51 and 2.36 times, and Bcl-2, pro-caspase 3, and pro-caspase 9 were downregulated by 7% , 33% , and 31% , respectively.
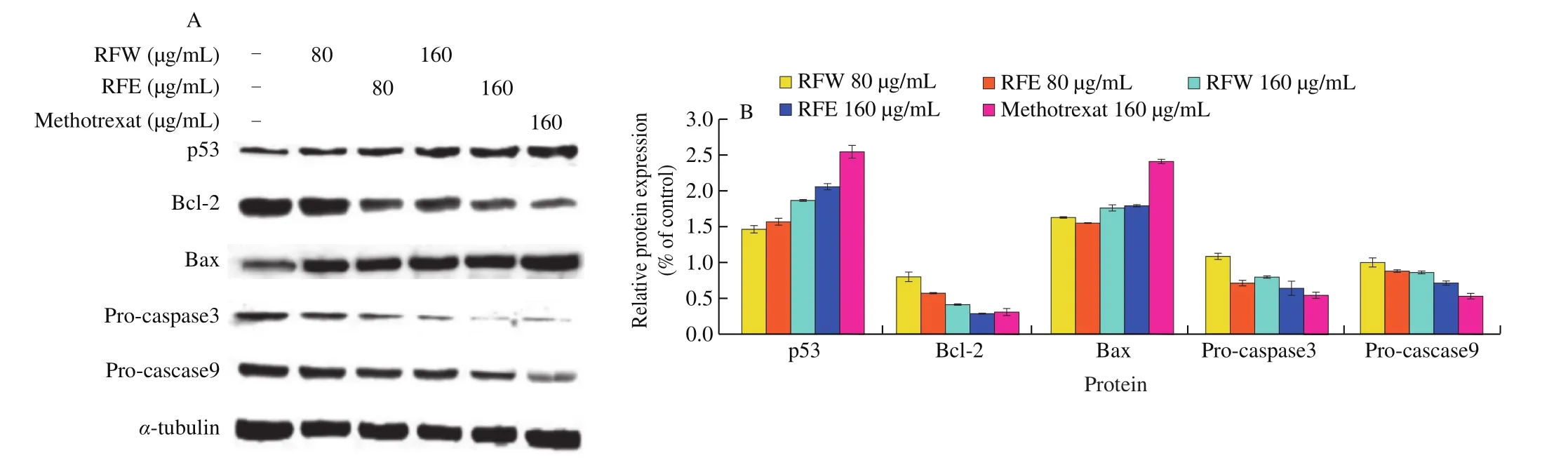
Fig. 7 The effect of rose flavonoids on western blot of HepG2 cells. (A) Changes in the protein content of HepG2 cells exposure to RFW or RFE for 24 h.(B) Optical intensity of bands were quantified compared with control. Data shown represent means ± SD for three independent experiments.
To sum up, rose flavonoids can destroy HepG2 cells by upregulating the expressions of pro-apoptotic proteins p53 and Bax, and down-regulating levels of pro-caspase 3 and pro-caspase 9, as well as inhibit the expression of anti-apoptotic protein Bcl-2, therefore promoting release of caspase 3 and caspase 9. These data are in consistent with the analytic results of apoptosis experiments, further indicating that rose flavonoids can induce apoptosis of HepG2 cells.
4. Conclusion
This study indicated that rose flavonoids have a remarkable antitumor activity through inhibiting tumor cell growth and reducing cell attachment. The study on HepG2 cells found that low concentration of rose flavonoids(10-40 µg/mL)could inhibit migration and metastasis, while high concentration of rose flavonoids(80-160 µg/mL) could destroy cell morphology, promote apoptosis by regulating expression of apoptotic related proteins. Hopefully,this study will provide a theoretical basis for further research and utilization of rose flavonoids.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This study was supported by the natural science foundation of Shandong province ZR2017BH053 and the youth doctor cooperation foundation of Qilu University of Technology (Shandong Academy of Sciences) 2017BSH2017.
- 食品科学与人类健康(英文)的其它文章
- Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
- Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
- Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria

