Neonatal respiratory failure-An overview
Gortner L,Shen J
(1.DepartmentofPaediatricsandNeonatology,UniversityHospitaloftheSaarland,Homburg/Saar,Germany;2DepartmentofPaediatrics,DapingHospital&ResearchInstituteofSurgeryof ThirdMilitaryMedicalUniversity,Chongqing400042,China)
Respiratory disorders in the neonates still remain the reason for postnatal hospitalization.Main reasons for respiratory failure in the term neonate include transient tachypnea(TTN)of the neonate;in preterms,respiratory distress syndrome(RDS)is a dominating cause.The incidence of TTN has increased during the past two decades due to an increased rate of C-sections in western countries,whereas the rate of RDS could be lowered,mainly due to improved perinatal care.Overall prognosis in respiratory failure could be improved due to advanced ventilation techniques,systematic use of surfactant and the establishment of systematic ECMO-support.
1 Introduction
In Western countries,postnatal respiratory failure is the main cause of treatment in intensive care units in preterm and term neonates.Due to the continuously high prevalence of neonatal respiratory distress,research on neonatal respiratory failure is one main stay in neonatology.During the past decades,there was a reduction of respiratory distress syndrome(RDS)regarding age-defined groups of very preterm neonates.On the other hand,there had been an increase in respiratory distress in term neonates due to an increased frequency of C-sections in Western countries and resulting transient tachypnea of the neonate(TTN)[1].
As TTN is mainly caused by a delayed resorption of fetal lung fluid,which is produced actively by type-Ⅱ-cells from week 14 to 16 in utero;this active secretory process is slowed before delivery at term and postnatally immediately switches to a resorption of fetal lung fluid by the same cell system[2].This active process of resorption of fetal lung fluid is the main stay of postnatal pulmonary adaptation along together with an adequate surfactant concentration,mechanical forces at birth and at the vascular end,a decrease of pulmonary artery pressure[3].These components in concert work together that postnatal pulmonary adaptation is undisturbed in most neonates.However,they also explain the risk for respiratory distress syndrome in preterms,as the capacity to secret surfactant by type-Ⅱ-cells is largely decreased in an anatomically immature lung and active resorption of lung fluid may also be impaired[4].
As to the complex pathophysiology of respiratory failure in term neonates and preterms,the detailed description in both age groups will be given and currently used therapeutic interventions will be discussed.Before further giving an overview,some epidemiological figures will be mentioned in order to address the magnitude of the whole problem of neonatal respiratory failure facing needs for respiratory support.
2 Epidemiology
In Germany,systematic registers on respiratory failure depending on gestational age have been established more than two decades ago and allow to give information on trends of main respiratory disorders[1].Current data on TTN,i.e.delayed re-sorption of fetal lung fluid,is observed in 1 to 1.2%of all term neonates and increased by about 30%during the last two decades.This has been paralleled by an increase in the rate of C-section,which was about 16%by beginning of the 90 s and is currently observed in about 32%of all births[5].On the other hand two decades ago,about 80%of all preterms of a birth weight below 1 500 g(very low birth weight infants)needed mechanical ventilation,however currently,about only 50%of the same age group are on mechanical ventilation postnatally[6].The same direction has been observed for neonates with respiratory failure secondary to meconium aspiration syndrome(MAS),which currently is observed in about 1 to 5 000 neo-nates,this had been in the range of 1 to 1 000 about 20 years ago[7].Decreased rates in these disorders had been reached by sustained efforts in obstetrics by improved surveillance of pregnancies and possibly by an increased rate of C-section.In parallel,the rate of term neonates,which needed extracorporeal membrane oxygenation(ECMO)secondary to MAS dropped down during the last two decades from about 40%of all term neonates with ECMO to now about 5 to 8%[8-10].
An overview about the gestational age specific causes of respiratory failure during the last five years in the German federal states of Hesse and Saarland is given in table 1[1].
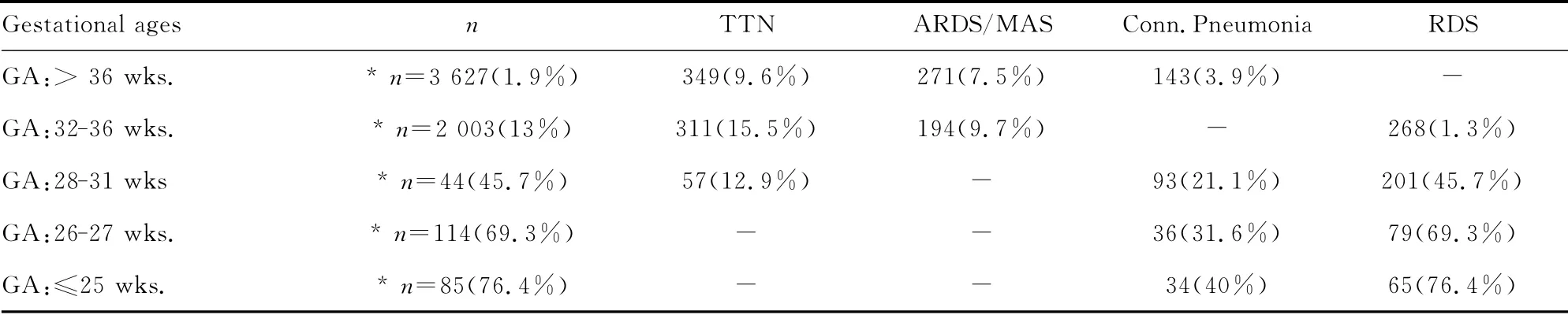
Table 1 Frequency of neonatal lung failure by gestational age and frequency of diagnosis
3 Respiratory failure in preterms
Respiratory distress syndrome[RDS;surfactant deficiency syndrome;hyaline membrane disease(HMD)]is the leading cause of respiratory failure in very preterm neonates(preterms with a gestational age below 32 completed weeks).Its pathophysiology is characterized by a primary surfactant deficiency in an anatomically immature lung[4].The anatomical structure of immature lungs is characterized by a lack of mature alveoli and instead of this by terminal saccular structures,which during transition to alveolarisation turn to be the terminal airways after development of mature alveoli.Due to a lack of pulmonary surfactant at the water/air interphase and thus resulting high surface tension,inadequately high pressures are necessary for adequate postnatal lung aeration.As with an adequate surfactant concentration,surface tension at the water/air interface is below 1 mN/m,surface tension without any surfactant components is in the range of 70 m N/m.As opening pressures are proportionally to the surface tension at the air/water interface,these inadequate high pressures lead to inadequate and unphysiologic high aeration pressures,which in cause physical trauma(volutrauma)of the lungs[4].Physical lung trauma in turn leads to lesions in distal airways and terminal sacs and thus causing entrance of plasma components into gas exchange spaces,which in turn inactivate surfactant and form hyaline membranes.Thus,histological findings in these lungs were the basis for the term hyaline membrane disease[11].The main pathophysiological features of RDS are given in figure 1.
The clinical course of RDS is characterized by tachy-and dyspnea with inadaequate ventilation pressures,which may lead to the viscous cycle mentioned above[4].Diagnosis by chest x-ray is necessary in order to describe lung pathology.RDS has been graded by Couchard and co-workers in stagesⅠto stageⅣ.A typical chest X-ray of gradeⅣRDS is given in figure 2.In most very preterm neonates,RDS is initially treated by application of nasal or nasopharyngeal CPAP(continuous positive airway pressure),in order to maintain adequate gas exchange[12].If CPAP therapy fails,intubation and administration of surfactant preparations obtained from animal lungs(cow or pig lungs)is indicated[6].Natural surfactant preparations have been obtained either lung lavage or by mincing of lung tissue,both procedures followed by a two-step lipid extraction using chloroform/methanol extraction.During the 80 s to the end of the 90 s of the last century,natural surfactant preparations from have been tested in a number of controlled clinical trials enrolling more 5 000 preterm neonates.Main effects of surfactant intratracheal administration of surfactant were described in metaanalyses by reduction in oxygen requirements and ventilation pressures as acute effects,a reduced rate of pneumothorax and by an increased survival without bronchopulmonary dysplasia[13].Maximum pulmonary effects of surfactant are obtained in case of combination with prenatal corticosteroids,e.g.betamethasone[12].
A new approach in administering surfactant,which had been introduced in Germany recently,is the surfactant administration via a gastric tube during continued CPAP therapy(avoid mechanical ventilation trial-AMV-trial)[14].If further controlled clinical trials will be in line with results from a German multicentre trial,this procedure may be an alternative to the conventional surfactant administration via an intratracheal tube.
Contrary to previously published controlled clinical trialsusing either CPAP with brief intubation and immediate return to CPAP(INSURE procedure)or intubation and surfactant administration via the intratracheal tube(support trial,COIN trial),where no advantage of primary CPAP could be demonstrated in extremely immature infants,the above-mentioned procedure may offer the advantage of completely avoiding mechanical ventilation with lowering the rate of bronchopulmonary dysplasia(BPD)[15-16].
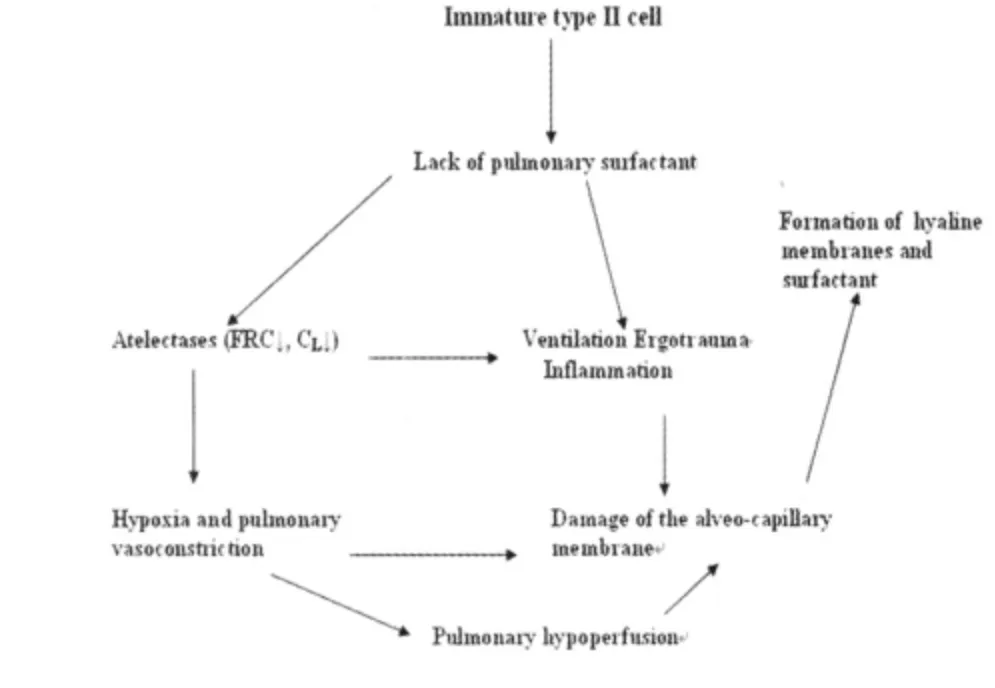
Figure 1 Scheme on the pathogenesis of RDS
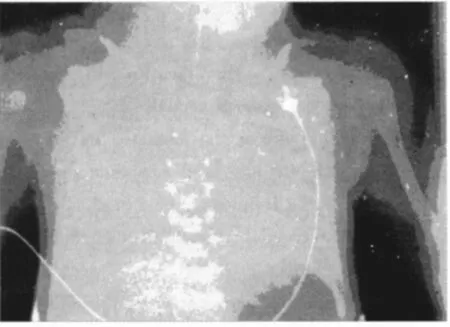
Figure 2 Chest X-ray of RDS
On the other hand,if there is repetitive surfactant administration is necessary,the preterm neonates require twice intubation,as the gastric tube of cause is not continuously positioned in the trachea.Thus,further studies will be necessary to judge on whether this method becomes alternative for the classical surfactant administration.
Thus,the typical therapy up to now is an early surfactant administration following intratracheal intubation either following birth in infants needing immediate respiratory support or later in the course of respiratory failure in case of unsuccessful CPAP therapy[6].Currently,most natural surfactant preparations in are given at a dose of 100 mg/kg.A second surfactant dose is scheduled if fraction of inspired oxygen(FiO2)is further increased to 30%to 40%mostly 12 to 24 hours after the first dose[6,13].As best effects of surfactant have been demonstrated in lungs,which have been conditioned by prenatal administration of corticosteroids,all pregnant women with threatening preterm delivery before 34 weeks of gestation should receive one or two courses with betamethasone at 12 mg[12].There now is a discussion ongoing,if late preterms,e.g.infants of 34 to 36+6/7 weeks of gestation,should also receive prenatal steroids,as a substantial number also in this age group develops respiratory failure.However,trials are on the way to answer this question.Apart from the effects of prenatal corticosteroids on lung failure,the administration of betamethasone or alternatively dexamethasone also leads to a reduced rate of intraventricular hemorrhage and necrotizing enterocolitis(NEC)by reduction the relative risk of about 50%.Thus,apart from its beneficial effects on lung failure,prenatal corticosteroids also reduce further severe postnatal complications,which might be the cause of long-term developmental outcome impairments[17].
New development in surfactant preparations for therapy of neonatal RDS have been introduced among others in the USA,where a mixture of phospholipids was added a surfactant protein B(SP-B)-like acting polypeptide(KL4-surfactant).However,up to now,no superiority of the specific surfactant on short-and long-term outcome has been demonstrated[18].The problem in this substance might be that,this specific protein has not the full biological activity of SP-B,thus,the KL4-surfactant(Surfaxin?)needs further critical evaluation[19].
In several countries,surfactant preparations obtained from animal lungs have been developed,among others Brazil,China,Cuba,South Korea.
During the last 20 years,more than 1 million preterm and term neonates have been treated with surfactant preparations from different origin.Out of these 5 000 had been enrolled in controlled clinical trial.
Thus,surfactant therapy for neonatal RDS is the best controlled intervention in neonatal intensive care.Despite this,no universal final consensus on initial dosing,dosing intervals and indications for surfactant re-administration have been adapted.However,the above-mentioned dose and time schedules are used in the most countries[6].
3.1 Further respiratory disorders in very preterm neonates In about 1/4 of all ventilated preterm neonates below 32 weeks of gestation,clinical or lab evidence for the presence of a neonatal pulmonary or systemic infection can be found.Thus,neonatal pneumonia(classified according to the suggestions by Sherman[20])is observed in a considerable part of preterm neonates.These neonates show a different pattern of response to natural surfactant treatment.The effect of surfactant on lowering oxygen requirements is reduced and the effect is lasting less long.Main factors for this difference with the response in RDS are an increased leakage of the alveolar-capillary membrane,which is in part secondary to prematurity and in the other part secondary to the systemic inflammatory response in the lung and hemodynamic factors.In affected patients,early diagnostics on specific causative germs,especially in case of preterm premature rupture of membranes(PPROM)and an adequate antibiotic treatment is mandatory.During the last decades in Western countries,the most prevalent germs were group B streptococci andE.coli[21].Apart from classical microbiological approach and measuring C-reactive protein and white blood cell counts,nowadays,molecular recovery of bacteria,e.g.using16S-ribosomal subunit and molecular markers of infections in the host,is able to extend our diagnostic repertoire[22].
A number of studies are on the way to describe neonatal response to early onset infections using molecular signature taken fromLeukocytes of newborns with chorioamnionitis and other causes of prenatal infections[23].However,up to now,no valid biomarker has been identified in order to stratify therapy with respect to modulation of inflammation.
3.2 Bronchopulmonary dysplasia(BPD) BPD is a complication of RDS or neonatal pneumonia observed in mostly in very preterm neonates.The current definition of moderate to severe BPD includes the necessity for oxygen supplements at week 36 postmenstrual age to obtain an adequate oxygenation[24].Typical changes in X-ray include a milk-glass like appearance of the lungs——a typical example is given in figure 3.
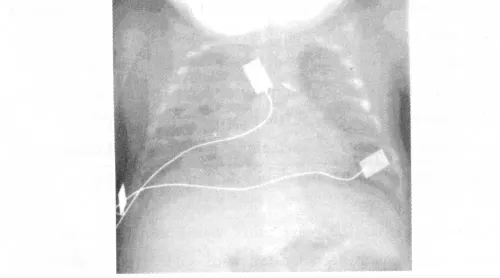
Figure 3 Chest X-ray of BPD
The main risk factor for BPD is the degree of immaturity i.e.low gestational age.Further modifying factors are intrauterine growth restriction,male gender,postnatal asphyxia and genetic factors[25].The latter factors may account for about 1/3 of pathogenesis of BPD and up to now,mainly include polymorphisms in genes coding for surfactant proteins,modulators of inflammatory response and growth factors[24].A scheme on the pathogenesis of BPD is given in figure 4 including above mentioned pre-and post-natal factors.
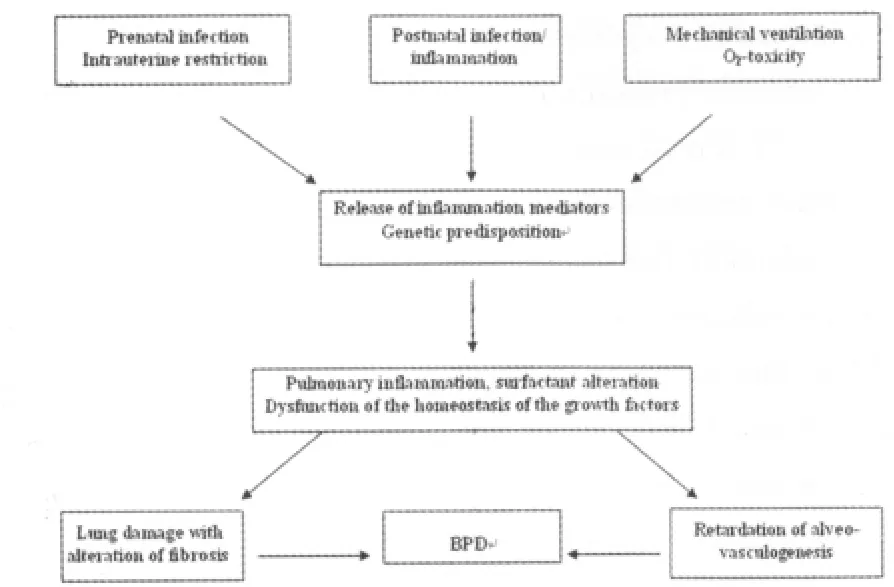
Figure 4 Scheme on the pathogenesis of BPD
About 15%to 20%of preterms below 32 weeks of gestation develop a BPD,which is characterized on long-terms by an increased risk for recurrent airway infections and bronchial asthma[25].Furthermore,it has been shown that BPD is associated with an increased risk for spastic paresis and cognitive impairment[26].Thus,BPD has to be seen as a systemic disorder,which does not only affect the lungs,but also possibly due to an inflammatory response,central nervous system.Thus,a number of studies have been performed during the last decades in order to prevent or cure BPD.During the 80s,dexamethasone was introduced into BPD-therapy.The pathophysiological rationale was the observation,that BPD is characterized by an inflammatory reaction.However,it turned out that long-term results included an impaired cognitive outcome after systemic high-dose dexamethasone[27].Therefore,low-dose hydrocortisone became a therapeutic option.Dexamethasone should only be used in very specific situation in week 2 or 3,when none of the mentioned treatment options are able to wean the babies from the ventilators.The basis to prevent BPD is a critical indication for mechanical ventilation and adequate surfactant usage.
Furthermore,early caffeine has been shown to reduce the rate of BPD[27].However,as caffeine is given as a routine,these data did not indicate a breakthrough in BPD-therapy.Studies currently are on the way to evaluate the effect of vitamin A given early,e.g.during the first days of life in order to prevent BPD.
Very current experimental therapeutic options for BPD include regenerative therapeutic measures including growth factors and stem cell administration in experimentally induced disease[28].Up to now,it is too early to clearly state on the clinical relevance of regenerative therapies for BPD.
4 Respiratory disorders in term neonates
4.1 Transient tachypnea of the neonate(TTN) Transient tachypnea of the neonate has been observed during the past decade with an increased frequency.This might be explained by the fact that in Western countries,there was an increase of Csection on demand.Thus,actual rates of C-section are in the rage of 30%to 32%in Germany currently[29].TTN is observed in about 1 out of 100 term neonates,e.g.neonates of>37 weeks of gestation.
The pathogenesis of TTN is mainly explained by a delayed resorption of fetal lung fluid[2,30].Relevant mechanisms of resorption of fetal lung fluids mainly include mechanical chest compression during spontaneous delivery(about 30%of fetal lung fluid).However,the largest part of fetal lung fluid is resorbed by an active process,which has been described to be mainly dependant on the activation of sodium channels in the epithelium(ENaC).ENaC-activity is regulated by catecholamines(beta-2-receptors and vasopressin)[31-32].As both substances increase during the spontaneous parturition,one central factor for resorption of fetal lung fluid is lacking in Csection without previous labor.Thus the lack of labor and the absence of chest compressions are the pathophysiological cause for an increased risk for TTN after primary C-section at 37 to 38 weeks of gestation.Further mechanisms for the resorption of fetal lung fluid following birth include an adequate surfactant concentration at the air-liquid-interface and high transpulmonary pressures during the first tidal breathings[19].A typical chest X-ray is given in figure 5.
In a currently published study,pathophysiology of TTNfurther could be described in part by a reduced number of laminar bodies,e.g.extracellular particles of surfactant released from type-Ⅱ-cells.This might indicate that apart from abovementioned pathogenetic factors a borderline surfactant deficiency also might be operative[33].
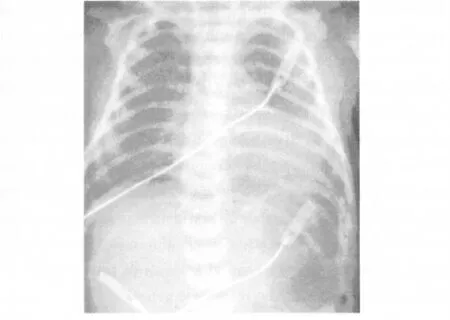
Figure 5 Chest x-ray of TTN
As described above,TTN in neonates with C-section without previous labor below 39 weeks of gestation is a considerable risk.Thus,recommendations now tend towards a later C-section,e.g.at 39 weeks,in order to lower the risk of TTN[29].However,pharmacological studies also focused on the affect of prenatal steroids given in case of delivery via C-section before 39 weeks of gestation.It could be shown that after prenatal betamethsone,the rate of TTN could be lowered.However,it has to be taken into account that corticosteroids may show longterm effects,which are not clearly excluded until now.Thus prenatal corticosteroid therapy has not been adapted as standard in perinatal care for prevention of TTN in term neonates.The postnatal therapy of TTN includes the maintenance of an adequate oxygenation and haemodynamics.In case of more severe courses of TTN,administration of CPAP and mechanical ventilation might be necessary.It has to be mentioned that in case of severe courses of TTN leading to hypoxic respiratory failure,a high rate of pulmonary hypertension might occur.Thus,echocardiographic controls to rule out persistent pulmonary hypertension are mandatory in severely affected neonates[34].
In case of a regular course of TTN,clinical signs of respiratory distress will disappear during the first 24 to 48 hours.Apart from above-mentioned respiratory therapy,some groups administered furosemide to lower oxygen requirements and minimized respiratory distress,however,without clear-cut success until now[34].As an alternative,beta-2-mimetics have been administered systemically during the past decades,however,also without success.A recently published studying showed that inhalative administration of salbutamol resulted in an improved gas exchange in term neonates with TTN[35].Towards the same direction a study using restricted fluid administration for TTN can be interpreted[36].Thus,due to an increased incidence of TTN,further studies currently are on the way to confirm a bove-mentioned data on beta-2-agonists and fluid restriction.
4.2 Meconium aspiration syndrome(MAS) Meconium stained amniotic fluid is observed in about 10%to 20%of births at term.In post-term neonates,the frequency even may be higher[37-38].If fetal distress occurs,meconium stained amniotic fluid may enter the airways and lungs,leading to the belowmentioned pathophysiology[39-40].
As meconium contains bile acids,albumin and other proinflammatory and surfactant inactivating components,the typical sequence after inhalation of meconium stained amniotic fluid is a respiratory failure.This may be further be aggravated by fetal distress,which in turn may lead to meconium passage from the infant′s colon to the amniotic fluid.As meconium additionally has a viscous property,inflammation and surfactant inactivation are further complicated by atelectasis due to occlusion of small airways.Overall in turn,this leads to a severe respiratory failure potentially accompanied by hypoxic-pulmonary vasoconstriction(Euler-Liljestrand-reflex)[39,41].An overview on pathophysiology of MAS is given in figure 6.
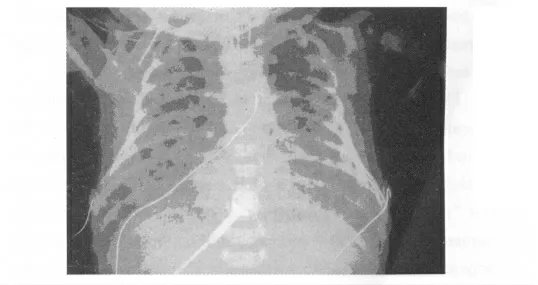
Figure 6 Chest X-ray of MAS
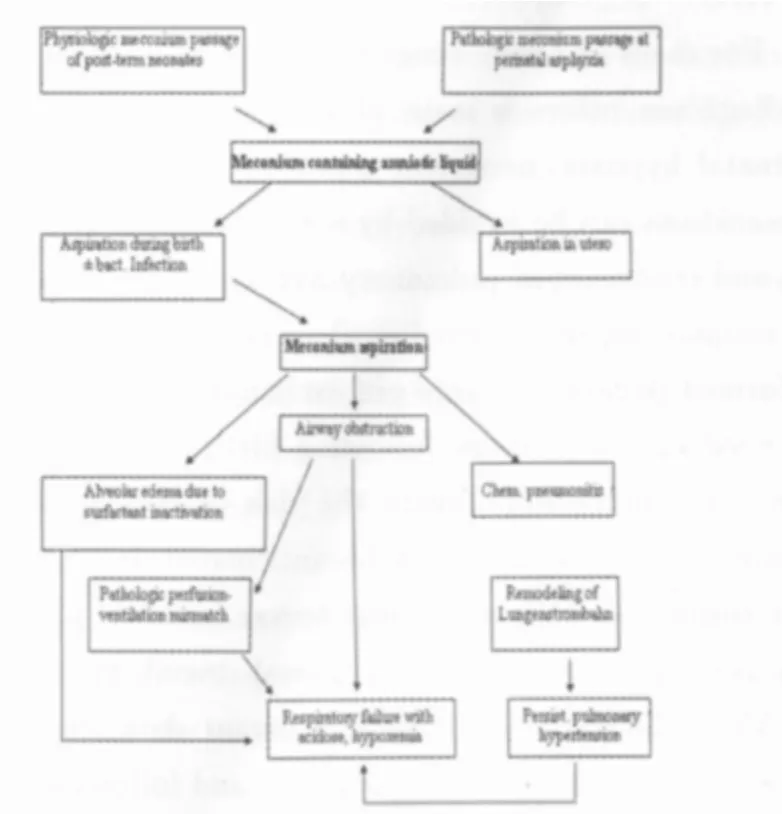
Figure 7 Scheme on the pathogenesis of MAS
Thus,the typical history of a neonate with severe meconium aspiration syndrome includes the presence of meconium in amniotic fluid,a typical X-ray with atelectasis and hyperinflation and inflammatory infiltrates.In most severe cases,the chest X-ray shows atelectasis or RDS-like changes.An example for this is given in figure 7.
As MAS frequently is complicated by a bacterial superinfection,this fact should be considered on diagnosis and treat-ment.
Severe causes lead to a severe respiratory failure necessitating mechanical ventilation.In case of pulmonary hypertension,the administration of nitric oxide is indicated[42].Furthermore,surfactant saline lavage may be used to remove meconium from the airways and therapeutic surfactant administration can be considered in most severe cases.If the above-mentioned therapy is not successful,i.e.gas exchange cannot be maintained,extracorporeal membrane oxygenation(ECMO)is indicated[10].
Overall,prognosis due to improved perinatal care and postnatal neonatal intensive care has been improved,thus,mortality in Western countries currently is below 1%[38].
4.3 Congenital anomalies of the lung and disorders in surfactant proteins Neonatal respiratory failure due to congenital anomalies of the lung is most often observed to occur secondary to congenital diaphragmatic hernia(CDH).CDH is observed at a rate of about 1∶4 000 to 1∶5 000 and is seen at rate of 4∶1 on the left-hand side with most frequently in the postero-lateral position[43].About 20%of the neonates with congenital diaphragmatic hernia have other malformations,among others of the central-nervous system and the heart[44].
The diagnosis can be verified by chest X-ray,demonstrating an eventeration of abdominal cavity contents into the chest.Clinically,neonates present with an“empty abdomen”,which is accompanied in case of left side diaphragmatic hernia by a“pseudo-dextrocardia”.In case of lung hypoplasia,which frequently occurs in case of congenital diaphragmatic hernia,an early and severe respiratory failure is observed,which in most cases,is accompanied by persistent pulmonary hypertension(PPHN)[37].
For cases of severe congenital diaphragmatic hernia,prenatal diagnosis offers a main stay for improving prognosis:If postnatal hypoxia,persistent pulmonary hypertension and severe acidosis can be avoided by early intubation,gentle ventilation and treatment of pulmonary hypertension,a stabilization of the neonate can be achieved until surgery,which only should be performed in case of stable gas exchange.In most cases,this is observed 12 to 48 hours following birth[10].If surgery can be performed successfully,again the risk of relapse of persistent pulmonary hypertension may be anticipated.In cases of intractable respiratory failure or most severe forms of persistent pulmonary hypertension,extracorporeal membrane oxygenation(ECMO)should be considered.Current data show that even severe cases of pulmonary hypoplasia and following respiratory failure,ECMO-therapy increases survival up to 60%[10].Longterm consequences of congenital diaphragmatic hernia include gastro-esophageal reflux disease with secondary pulmonary involvement.In case of severe postnatal hypoxia due to severely impaired gas exchange,hypoxic-ischemic injury must be considered[45].
Disorders of the lung surfactant system as a further cause of congenital anomalies leading to respiratory failure mainly include genetic variations of genes encoding for surfactant proteins mainly for SP-B[46].These neonates present with the clini-cal picture of pulmonary alveolar proteinosis,which represents life-threatening neonatal respiratory disorder.Affected neonates present with severe postnatal respiratory failure requiring mechanical ventilation early following birth and exhibit high volumes of tracheal secretions,which contain all surfactant components apart from SP-B[47].As this distinct protein is essential for surfactant function and thus for maintenance of lung mechanics,an adequate therapy could not have been established yet.Few neonates with SP-B deficiency have been given lung transplants,however,without favorable long-term results[48].
Apart from SP-B deficiency,neonatal alveolar proteinosis further may be caused by granulocyte macrophage colony stimulating factor(GM-CSF)-receptor defects and ATP-binding cassette(ABCA)3 deficiency[49].Prognosis is as poor as stated for SP-B deficiency.Diagnosis in all aforementioned disorders can be established by biochemical analysis of tracheal aspirates and genetic analyses of the respective genes.
Reference:
[1]Gortner L,Tutdibi E.Respiratory disorders in preterm and term neonates:an update on diagnostics and therapy[J].Z Geburtshilfe Neonatol,2011,215(4):145-151.
[2]Bland RD.Lung liquid clearance before and after birth[J].Semin Perinatol,1988,12(2):124-133.
[3]Barker PM,Olver RE.Invited review:Clearance of lung liquid during the perinatal period[J].J Appl Physiol,2002,93(4):1542-1528.
[4]Jobe A.Respiratory distress syndrome——new therapeutic approaches to a complex pathophysiology[J].Adv Pediatr,1983,30:93-130.
[5]Morrison JJ,Rennie JM,Milton PJ.Neonatal respiratory morbidity and mode of delivery at term:influence of timing of elective caesarean section[J].Br J Obstet Gynaecol,1995,102(2):101-106.
[6]Sweet D,Bevilacqua G,Carnielli V,et al.European consensus guidelines on the management of neonatal respiratory distress syndrome[J].J Perinat Med,2007,35(3):175-186.
[7]Greenough A,Pulikot A,Dimitriou G.Prevention and management of meconium aspiration syndrome——assessment of evidence based practice[J].Eur J Pediatr,2005,164(5):329-330.
[8]Brown KL,Sriram S,Ridout D,et al.Extracorporeal membrane oxygenation and term neonatal respiratory failure deaths in the United Kingdom compared with the United States:1999 to 2005[J].Pediatr Crit Care Med,2010,11(1):60-65.
[9]Kugelman A,Gangitano E,Taschuk R,et al.Extracorporeal membrane oxygenation in infants with meconium aspiration syndrome:a decade of experience with venovenous ECMO[J].J Pediatr Surg,2005,40(7):1082-1089.
[10]Schaible T,Hermle D,Loersch F,et al.A 20-year experience on neonatal extracorporeal membrane oxygenation ina referral center[J].Intensive Care Med,2010,36(7):1229-1234.
[11]Roberton NR.Management of hyaline membrane disease[J].Arch Dis Child,1979 November,54(11):838-844.
[12]Jobe AH,Mitchell BR,Gunkel JH.Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant on preterm infants[J].Am J Obstet Gynecol,1993,168(2):508-513.
[13]Gortner L,Herting E.Leitlinie:Surfactanttherapie des Atemnotsyndroms Frühgeborener(RDS).AWMF online(www awmf org/leitlinien/detail/II/024-021 html)2009 February;
[14]Gopel W,Kribs A,Ziegler A,et al.Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants(AMV):an open-label,randomised,controlled trial[J].Lancet,2011,378(9803):1627-1634.
[15]Morley CJ,Davis PG,Doyle LW,et al.Nasal CPAP or intubation at birth for very preterm infants[J].N Engl J Med,2008,358(7):700-708.
[16]Carlo WA,Finer NN,Walsh MC,et al.Target ranges of oxygen saturation in extremely preterm infants[J].N Engl J Med,2010,362(21):1959-1969.
[17]Roberts D,Dalziel S.Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth[J].Cochrane Database Syst Rev,2006,3:CD004454.
[18]Moya F,Sinha S,Gadzinowski J,et al.One-year follow-up of very preterm infants who received lucinactant for prevention of respiratory distress syndrome:results from 2 multicenter randomized,controlled trials[J].Pediatrics,2007,119(6):e1361-1370.
[19]Ramanathan R,Rasmussen MR,Gerstmann DR,et al.A randomized,multicenter masked comparison trial of poractant alfa(Curosurf)versus beractant(Survanta)in the treatment of respiratory distress syndrome in preterm infants[J].Am J Perinatol,2004,21(3):109-119.
[20]Sherman MP,Goetzman BW,Ahlfors CE,et al.Tracheal asiration and its clinical correlates in the diagnosis of congenital pneumonia[J].Pediatrics,1980,65(2):258-263.
[21]Stoll BJ,Hansen NI,Sanchez PJ,et al.Early onset neonatal sepsis:the burden of group B Streptococcal and E.coli disease continues[J].Pediatrics,2011,127(5):817-826.
[22]Ohlin A,Backman A,Ewald U,et al.Diagnosis of neonatal sepsis by broad-range 16S real-time polymerase chain reaction[J].Neonatology,2011,101(4):241-246.
[23]Buhimschi CS,Bhandari V,Dulay AT,et al.Proteomics mapping of cord blood identifies haptoglobin“switch-on”pattern as biomarker of early-onset neonatal sepsis in preterm newborns[J].PLoS One,2011,6(10):e26111.
[24]Jobe AH.The new bronchopulmonary dysplasia[J].Curr Opin Pediatr,2011,23(2):167-172.
[25]Gortner L,Misselwitz B,Milligan D,et al.Rates of bron-chopulmonary dysplasia in very preterm neonates in Europe:results from the MOSAIC cohort[J].Neonatology 2011,99(2):112-117.
[26]Van Marter LJ,Kuban KC,Allred E,et al.Does bronchopulmonary dysplasia contribute to the occurrence of cerebral palsy among infants born before 28 weeks of gestation[J].Arch Dis Child Fetal Neonatal Ed,2011,96(1):F20-29.
[27]Thomas W,Speer CP.Nonventilatory strategies for prevention and treatment of bronchopulmonary dysplasia——what is the evidence[J].Neonatology,2008,94(3):150-159.
[28]Zhang X,Wang H,Shi Y,et al.The role of bone marrowderived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice[J].Cell Biol Int,2012,36(6):589-594.
[29]Tutdibi E,Gries K,Bucheler M,et al.Impact of labor on outcomes in transient tachypnea of the newborn:population-based study[J].Pediatrics,2010,125(3):577-583.
[30]Avery ME,Gatewood OB,Brumley G.Transient tachypnea of newborn.Possible delayed resorption of fluid at birth[J].Am J Dis Child,1966,111(4):380-385.
[31]Aslan E,Tutdibi E,Martens S,et al.Transient tachypnea of the newborn(TTN):a role for polymorphisms in the beta-adrenergic receptor(ADRB)encoding genes[J].Acta Paediatr,2008,97(10):1346-1350.
[32]O′Brodovich HM.Immature epithelial Na+channel expression is one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome[J].Proc Assoc Am Physicians,1996,108(5):345-355.
[33]Machado LU,Fiori HH,Baldisserotto M,et al.Surfactant deficiency in transient tachypnea of the newborn[J].J Pediatr,2011,159(5):750-754.
[34]Yurdakok M.Transient tachypnea of the newborn:what is new[J].J Matern Fetal Neonatal Med,2010,23 Suppl 3:S24-26.
[35]Armangil D,Yurdakok M,Korkmaz A,et al.Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn[J].J Pediatr,2011,159(3):398-403.
[36]Stroustrup A,Trasande L,Holzman IR.Randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn[J].J Pediatr,2012,160(1):38-43.
[37]Bloss RS,Aranda JV,Beardmore HE.Congenital diaphragmatic hernia:pathophysiology and pharmacologic support[J].Surgery,1981,89(4):518-524.
[38]Vivian-Taylor J,Sheng J,Hadfield RM,et al.Trends in obstetric practices and meconium aspiration syndrome:a population-based study[J].BJOG,2011,118(13):1601-1607.
[39]Karatekin G,Kesim MD,Nuhoglu A.Risk factors for meconium aspiration syndrome[J].Int J Gynaecol Obstet,1999,65(3):295-297.
[40]Khazardoost S,Hantoushzadeh S,Khooshideh M,et al.Risk factors for meconium aspiration in meconium stained amniotic fluid[J].J Obstet Gynaecol,2007,27(6):577-579.
[41]Wiswell TE,Fuloria M.Management of meconiumstained amniotic fluid[J].Clin Perinatol,1999,26(3):659-668.
[42]Gadzinowski J,Kowalska K,Vidyasagar D.Treatment of MAS with PPHN using combined therapy:SLL,bolus surfactant and iNO[J].J Perinatol,2008,28 Suppl 3:S56-66.
[43]Downard CD,Wilson JM.Current therapy of infants with congenital diaphragmatic hernia[J].Semin Neonatol,2003,8(3):215-221.
[44]Doyle NM,Lally KP.The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia[J].Semin Perinatol,2004,28(3):174-184.
[45]Valfre L,Braguglia A,Conforti A,et al.Long term fol-low-up in high-risk congenital diaphragmatic hernia survivors:patching the diaphragm affects the outcome[J].J Pediatr Surg,2011,46(1):52-56.
[46]Nogee LM,de Mello DE,Dehner LP,et al.Brief report:deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis[J].N Engl J Med,1993,328(6):406-410.
[47]Nogee LM.Alterations in SP-B and SP-C expression in neonatal lung disease[J].Annu Rev Physiol,2004,66:601-623.
[48]Palomar LM,Nogee LM,Sweet SC,et al.Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure[J].J Pediatr,2006,149(4):548-553.
[49]Bonella F,Bauer PC,Griese M,et al.Pulmonary alveolar proteinosis:new insights from a single-center cohort of 70 patients[J].Respir Med,2011,105(12):1908-1916.

