Floating Escherichia coli by Expressing Cyanobacterial Gas Vesicle Genes
WANG Tianhe, KANG Li, LI Jiaheng, WU Wenjie, ZHANG Peiran, GONG Minghao,LAI Weihong, ZHANG Chunyan, CHANG Lei, PENG Yong, YANG Zhongzhou,LI Lian, BAO Yingying, XU Haowen, ZHANG Xiaohua, SUI Zhenghong,YANG Guanpin, and WANG Xianghong
Floatingby Expressing Cyanobacterial Gas Vesicle Genes
WANG Tianhe1), KANG Li1), LI Jiaheng1), WU Wenjie2), ZHANG Peiran1), GONG Minghao1),LAI Weihong1), ZHANG Chunyan1), CHANG Lei3), PENG Yong1), YANG Zhongzhou2),LI Lian4), BAO Yingying5), XU Haowen5), ZHANG Xiaohua1), SUI Zhenghong1),YANG Guanpin1), and WANG Xianghong6), *
1),,266003,2),,266100,3),,266100,4),,266003,5),,266003,6),,266003,
Gas vesicles are hollow, air-filled polyprotein structures that provide the buoyancy to cells. They are found in a variety of prokaryotes. In this study, we isolated a partial gas vesicle protein gene cluster containingandfrom, and inserted it into an expression vector and expressed it in. The gas vesicle was developed in bacterial cells, which made bacterial cells to float on medium surface. We also amplifiedandseparately and synthesized an artificial operon by fusing these two genes with the standardized gene expression controlling elements of. The artificial operon was expressed in, forming gas vesicles and floating bacteria cells. Our findings verified that the whole set of genes and the overall structure of gas vesicle gene cluster are not necessary for developing gas vesicles in bacteria cells. Two genes,and, of the gas vesicle gene cluster are sufficient for synthesizing an artificial operon that can develop gas vesicles in bacteria cells. Our findings provided a wide range of applications including easing the harvest of cultured microalgae and bacteria, as well as enriching and remediating aquatic pollutants by constructing gas vesicles in their cells.
gas vesicle; gene cluster; gas vesicle gene; buoyancy
1 Introduction
Gas vesicles are the components of gas vacuoles, which develop in cells of >150 unicellular aquatic prokaryotes including bloom forming cyanobacteria,, Archaeaand others (Pfeifer, 2012; Walsby, 1994). Gas vesicles have multiple functions, including preventing oxygen limitation and promoting light harvesting. Gas vesicles enable cyanobacteria and others to colonize the air-liquid interface and resist to the low- oxygen conditions. These functions of gas vesicles are regulated by the quorum sensing system (Oren, 2013; Ramsay and Salmond, 2012). In some species, the proteins forming gas vesicle embed in the cellular membrane, serving as a delivery vehicle there (Childs and Webley, 2012). Gas vesicles are hollow polyprotein structures, performing as the swimming bladders and providing the buoyancy to cells. Gas vesicles in some species take cylindrical structures with hollow conical end-caps. The most obvious difference between the vesicles of different species is their widths (Belenky., 2004). The genes encoding gas vesicle proteins are organized into a cluster in cyanobacterial genomes. The gas vesicle genes ofsp. from Lake Zürich encode two proteins, a small hydrophobic GvpA, about 7.5kDa in weight, which forms the ribs of the cylindrical gas vesicles (Offne., 1998), and a larger hydrophilic GvpC which attaches to the outer surface of the integrating gas vesicles (Strunk., 2011; Sivertsen., 2010; Englert and Pfeifer, 1993; Hayes., 1992, 1988) (Fig.1A). Thegenes in the cluster are two base pairs different each other; whilegenes vary in length and are characterized by either an internalor an internalregion (Beard., 2000, 1999). Differentgenes encode proteins which form gas vesicles with different diameters, making cells adapt to different pressures. For example, GvpC16 in combination with GvpC20 forms a narrow gas vesicle of 50nm in diameter, which float cells at a critical pressure of 1.1MPa; while GvpC20, either alone or in combination with GvpC28, forms a gas vesicles of 60nm in diameter, which float cells at 0.9MPa (Beard., 2002).9402 contains,and,and produces gas vesicles functioning at higher pressures than gas vesicles produced by others (Beard., 1999, Fig.1B).
A fragment containingandhas been tentatively expressed inpreviously (Hayes and Powell, 1995); however, expressing the partial gas vesicle gene cluster and synthesizing artificial functional operons with the genes of gas vesicles have not been reported to our knowledge. The gas vesicle proteins may assemble automatically into gas vesicles (Ezzeldin., 2012); however, the assembling progress is not clearly described. In this study, we isolated a partial gas vesicle gene cluster from9402, which containedandand a putative promoter ahead of, and expressed the partial cluster in. We also amplifiedandseparately and synthesized an artificial operon by fusing these genes with standardized gene expression controlling elements of. It was found that both the partial gas vesicle gene cluster and the synthesized operon determined gas vesicle development in.
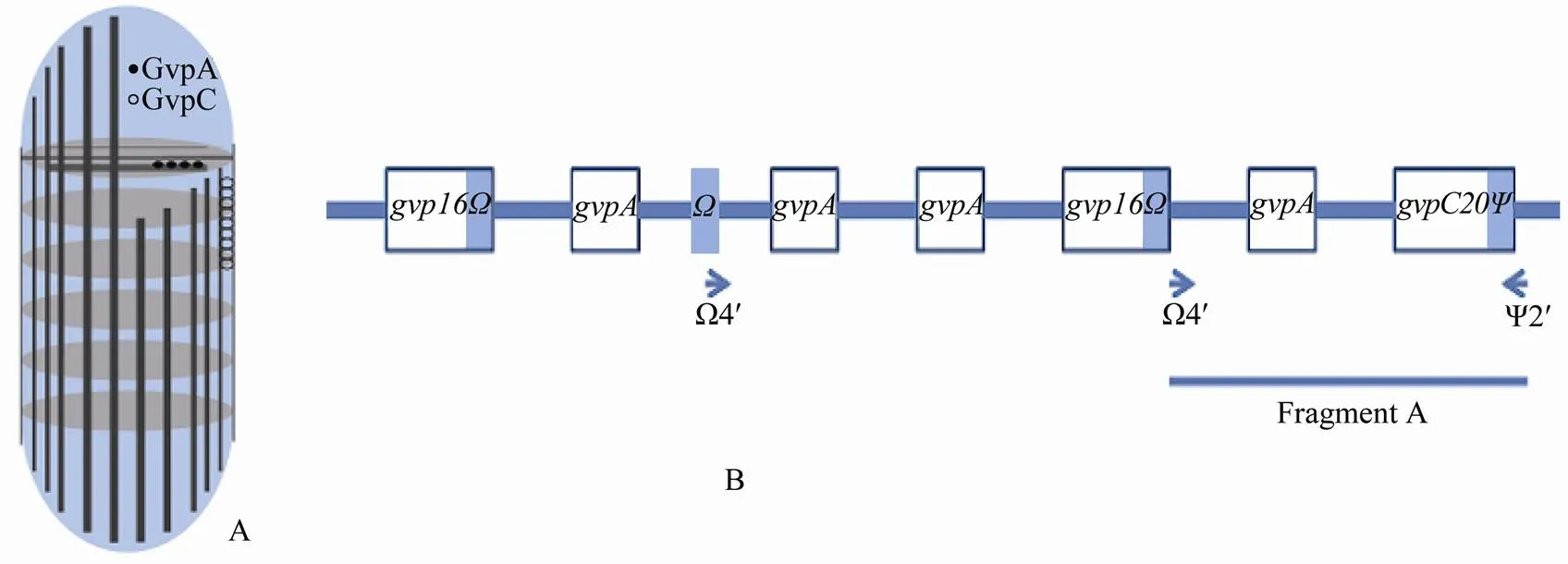
Fig.1 The sketch map of a gas vesicle (A) and the gas vehicle gene cluster (B) of P. rubescens 9402. The gas vesicle is a hollow structure. Horizontal ribs consisting of GvpA stratify inside; while vertical piles consisting of GvpC coat outside. Air is entrapped into the gas vesicle providing buoyancy to the vesicles themselves and bacteria containing the vesicles indirectly. Boxes in B indicate the open reading frames; while lines indicate the intergenic spacers (not proportional). Bars represent either Ω region alone or at the 3’ end of gvpC16Ω or Ψ region at the 3’ end of gvpC20Ψ. Primer Ω4’ and Ψ2’ amplify fragment A which is expected to be 1207bp in length. To gvpA from left and to gvpC20Ψ from right, two regions, 131bp and 179bp in length, respectively, flank fragment A. Primer Ω4’ matches two positions; however only the second near gvpA works in PCR amplification.
2 Materials and Methods
2.1 Culture of
9402 (CCAP1460/9) was purchased from CCAP (NERC Culture Collection of Algae and Protozoa) and cultured in BG11 (Bright and Walsby, 2000) at 16℃ and under 3000 lux following a rhythm of 12h light and 12h dark.
2.2 Amplification of Gas Vesicle Gene(s)
As shown in Fig.1B, primers Ω4’ (5’-CCT TAA GTG ATA TAA AAG ATC TCC AAG CCA TTC CCC ATT CTA T-3’) and Ψ2’ (5’-AAC TGC AGA TAA TAA TAC TAC TAG TCT GCC GAG TTA GGG ATT AGC-3’) (Beard., 2000, 1999) were used to amplify the genomic DNA of9402. The amplification was carried out by denaturing at 94℃ for 4min followed by 30 cycles of denaturing at 94℃ for 30s, annealing at 65℃ for 30s and extending at 72℃ for 1min and an extra extension at 72℃ for 5min. The PCR product was phenol-chloroform extracted and precipitated with ethanol and stored at −20℃.
We also amplifiedandseparately with primer pair A1 (5’-GAA TTC GCG GCC GCT TCT AGA GTC ACA CAG GAA AGT ACT ATA TGG CCG TTG A-3’) and A2 (5’-CTG CAG CGG CCG CTA CTA GTA TTA GAC CGA AGG AAC AGC CGC CTG-3’) and primer pair C-20-1 (5’-GAA TTC GCG GCC GCT TCT AGA GTC ACA CAG GAA AGT ACT AGA TGG CTT TAA AAG ACG A-3’) and C-20-2 (5’-CTG CAG CGG CCG CTA CTA GTA TTA ACA GGA ATA TAA ACG CGA TGG-3’). The amplification ofwas carried out by denaturing at 94℃ for 4min followed by 30 cycles of denaturing at 94℃ for 30s, annealing at 70℃ for 30s and extending at 72℃ for 20s and an extra extension at 72℃ for 5min. The amplification ofwas carried out by denaturing at 94℃ for 4min followed by 30 cycles of denaturing at 94℃ for 30s, annealing at 68℃ for 30s and extending at 72℃ for 30s and an extra extension at 72℃ for 5min.
2.3 Construction of Recombinant Plasmids
A recent study showed that some strong promoters likePfunction in the expression ofcluster which encodes the major gas vesicle structural proteins GvpA and GvpC in(Marschaus and Pfeifer, 2012). We believed that our partial gene cluster contained also its own promoter(s). Therefore, we did not place our partial cluster under the control of anpromoter but rather fused this partial cluster with a terminator only. The fragment A was double digested withRI andI, ligated with a double terminator, BBa_B0015, an iGEM part (http://partsregistry.org/) consisting of two concatenate transcriptional terminators, BBa_B0010 and BBa_ B0012, double digested withI andI in advance. In order to avoid intensive expression which may cause the death of host cells (Hayes, 1995), the ligation product was inserted into a low-copy number expression vector pSB4K5 (http://partsregistry.org/) double digested withRI andI early, yielding a recombined plasmid pSB4K5-(Fig.2A).
We synthesized an artificial operon by using a three antibiotic assembly (Shetty., 2011), which containedand, a promoter J23106 (an iGEM part, http:// partsregistry.org/), an introduced RBS and a transcriptional terminator B0015 in order to verify the feasibility of forming gas vehicle by expressing gas vehicle genes under the control of standardized elements of. The construct was named as p5B1K3-gvpA-(Fig.2B).

Fig.2 Two recombination plasmids, pSB4K5-gvpA-gvpC20Ψ(A) and pSB1K3-gvpA-gvpC20Ψ(B), constructed in this study. Fragment A, a partial gas vesicle gene cluster amplified from the genome of P. rubescens with primer Ω4’ and Ψ2’ contains gvpA, gvpC20Ψ and a putative promoter in front of gvpA, and Eco RI and Xba I sites in left and Spe I and Pst I sites in right. The transcriptional terminator BBa_B0015, a double terminator in a plasmid with AmpR and KanR marker, contains Eco RI and Xba I sites in left and Spe I sites in right. The low copy plasmid pSK4K5-I52002 contains Eco RI and Xba I sites in the left and Spe I and Pst I sites in the right of an insert I52002. After double digestion (Fragment A with Eco RI and Spe I, BBa_B0015 with Xba I and Pst I and pSK4K5-I52002 with Eco RI and Pst I) and purification, fragment A, BBa_B0015 and pSB4K5 are merged together, yielding recombinant pSB4K5-gvpA-gvpC20Ψ. With the same strategy, gvpA was ligated with promoter BBa_J23106 in plasmid J61002, and gvpC20Ψ was ligated with terminator BBa_B0015 first, and then two genes and two control elements were inserted into high copy vector pSB1K3, yielding recombinant pSB1K3-gvpA-gvpC20Ψ.
2.4 Bacterial Transformation
Bacterial transformation was carried out following the standard protocols (Berger and Kimmel, 1987).
2.5 Observation ofFloating
The constructs were transferred into eitherJM109 (pSB4K5-) orTop10 (pSB1K3--). In order to quantify the cell numbers at different layers of medium, the transformant was inoculated into a sampling apparatus we devised ourselves and cultured without rotation to the logarithmic growth phase (about 24h). The cells were sampled by punching needles of a few small syringes into the large syringe at different heights and drawing a desirable amount of culture into small syringes each in an up-to-bottom order. The sampled cells were diluted to 1mL with the bacteria cell number counted with blood counting plate. The engineered bacterial cells were treated with lysozyme and SDS, stained with uranyl acetate and observed under an electron microscope (JEM-1200EX-TEM, JEOL) for checking the existence of gas vesicles.
3 Results
3.1 Isolation of Partial Gas Vesicle Gene Cluster,and
Fragment A (1207bp in length) was amplified with primers Ω4’and Ψ2’. To the left and right ends of fragment A, the reorganization sites ofRI,I,I andI, were introduced, respectively, as were underlined in primer Ω4’ and Ψ2’, which facilitated the construction of recombination plasmid. Theandwere amplified with primer pairs A1 and A2, C-20-1 and C-20-2, which were 0.3kb and 0.6kb in length, respectively.
3.2 Gas Vesicle Development in Bacterial Cells
The recombinant plasmid pSB4K5-was constructed with fragment A, which was introduced intoJM109. When the transformant was cultured for 24h, the cells were found to float on the top of medium (Fig.3A). The transformant was also cultured in a sampling device with cells at different heights. It was found that most bacteria cells containing the recombinant plasmid aggregated at the top of medium, which was different from the control containing pSB4K5 (Fig.3B). The recombinant plasmid pSB1K3--was transferred intoTop10. It was found that bacteria cells floated on the top of medium (Fig.3C). Cell counting showed that most cells aggregated at the top of medium; while the control cells containing pSB1K3 appeared at different heights with the most at the bottom (Fig.3D). The development of gas vesicles incontaining pSB4K5--was also verified under an electron microscope (Fig.4).
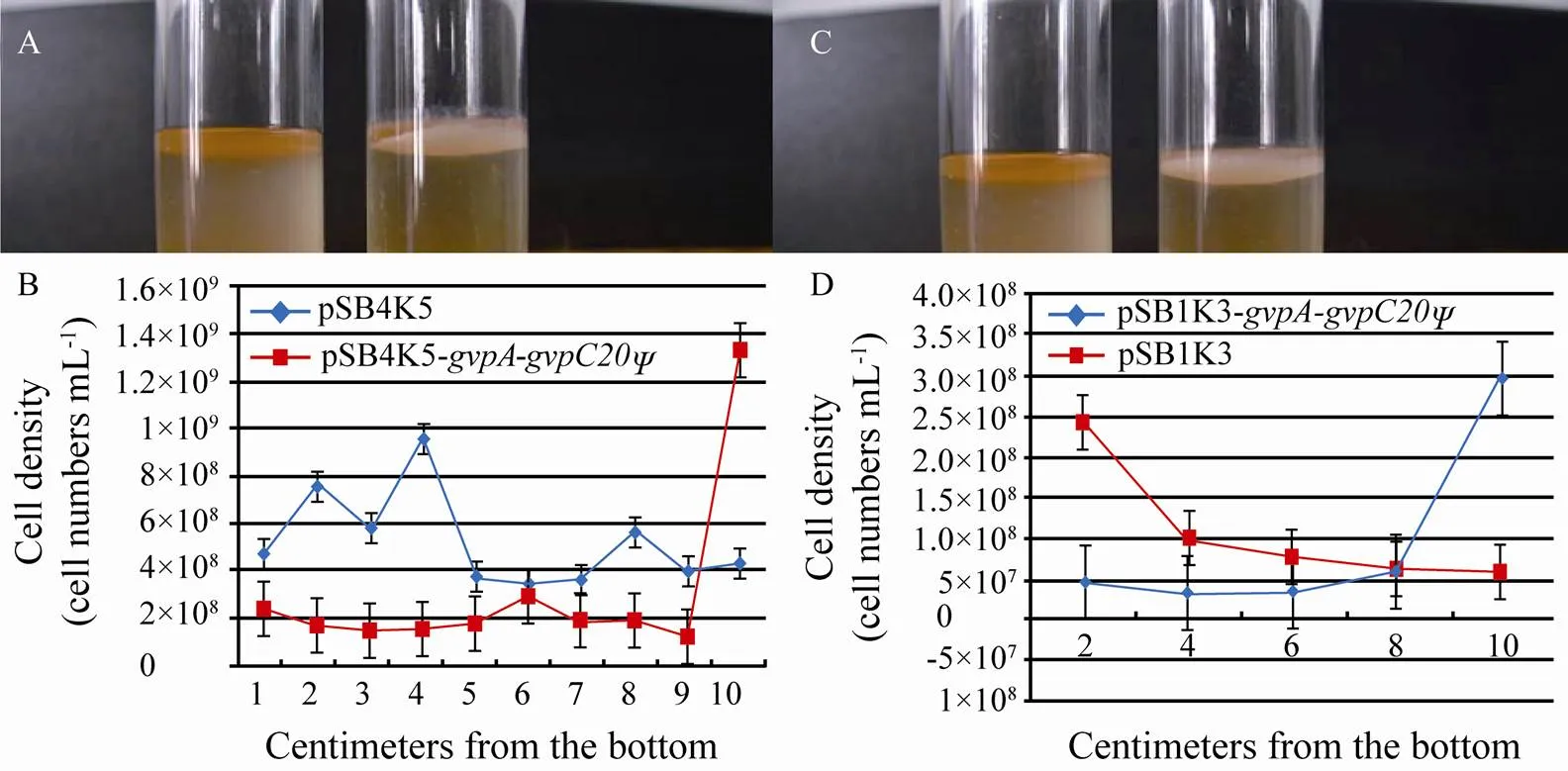
Fig.3 Observation and counting of floating E. coli JM109 cells containing pSB4K5-gvpA-gvpC20Ψand E. coli Top10 cells containing pSB1K3-gvpA-gvpC20Ψ. E. coli JM109 cells containing pSB4K5-gvpA-gvpC20Ψ float on the top of medium; while the control cells do not (A, observation; B, cell number counting). E. coli Top10 containing pSB1K3- gvpA-gvpC20Ψ float on the top of medium; while the control cells do not (C, observation; D, cell number counting).
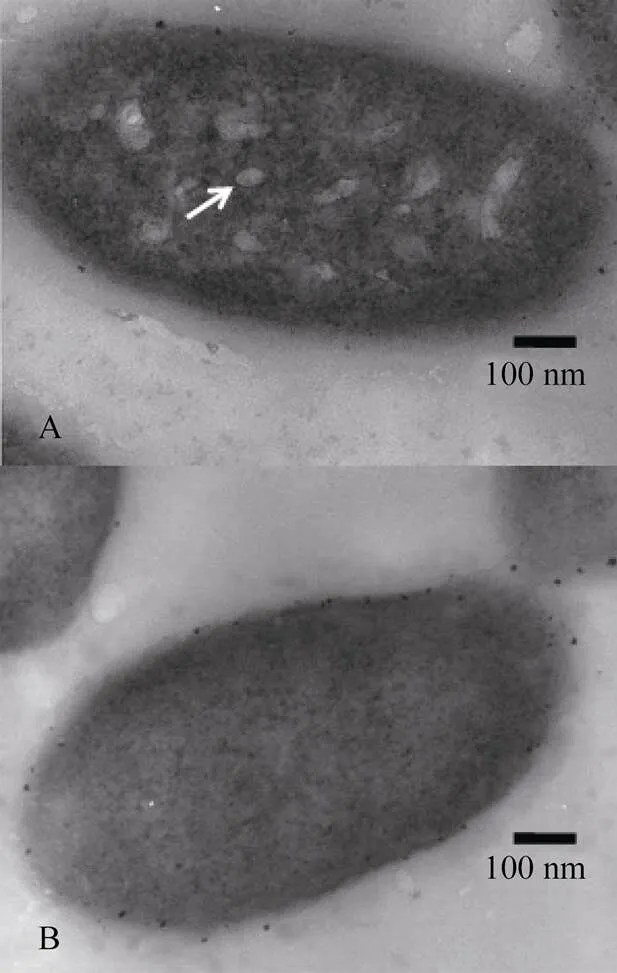
Fig.4 Gas vesicles developed inJM109 containing pSB4K5--(A). The white arrow points to a gas vesicle; Magnification, 60000; No gas vesicles were found in control cells containing pSB4K5 (B).
4 Discussion
We isolated a partial gas vesicle gene cluster containingandfromand synthesized an artificial operon usingandand standardized gene expression controlling elements of. The expression of both partial gas vesicle gene cluster and artificial operon developed the gas vesicles in. In a recent research in haloarchaea, a few over expressed gas vesicle genes (,,and) were found to associate with the formation of gas vesicles (Tavlaridou., 2013). Our findings verified that the whole gene set and the overall structure of the gas vesicle gene cluster were not necessary for developing gas vesicles in bacteria cells. Moreover,and, two genes of the gas vesicle gene cluster were sufficient for synthesizing an artificial operon controlling the development of gas vesicles in bacteria.
Cyanobacterial genes may be controlled by expression regulating elements different from those functioning in bacteria (Chungjatupornchai., 1999). Both the partial gas vesicle gene cluster andandgenes we isolated may contain regulating elements in their flanking regions; however, these regions did not interfere with the expression of both the partial gene cluster and the artificial operon in. It is necessary to verify the possible influence of these regions and delete them completely in our future studies. It is well known that over expression of a protein may cause the death of host cells (Hayes, 1995). In this study, we expressed both the partial gas vesicle gene cluster inby inserting it into a low copy plasmid, pSB4K5, and an artificial operon in a high copy plasmid, pSB1K3. Expression of gas vesicle proteins was not found to cause the death of host cells.
The gas vesicle can be applied in measuring cellular turgor pressure and determining phylogeny (Miklas- zewska., 2012; Holland and Walsby, 2009). Our research provides a wide range of applications. For example, the partial gas vesicle gene cluster or the artificial operon could be transferred into cultured algae, easing their harvesting. It is also expected that algal or bacterial species modified genetically with the gas vesicle genes may grow in water and float on surface, which can help to enrich nutrients or remediate pollutants in aquatic environments.
5 Conclusions
A partial gas vesicle protein gene cluster containingandwas isolated from, and an artificial operon was synthesized containingandand standardized gene expression controlling elements of. The expression of both partial gas vesicle gene cluster and artificial operon was sufficient to develop gas vesicles in. The whole gene set and the overall structure of gas vesicle gene cluster were not necessary for the development of gas vesicles in bacterial cells.
Acknowledgements
This work was partially supported by Undergraduate Institution of Marine Biological Science, Ocean University of China (OUC). Prof. Xiaohua Zhang, College of Marine Life Sciences, OUC, provided9402. Prof. Xiaohua Zhang and Prof. Guanpin Yang, College of Marine Life Sciences, OUC, provided the cost of sequencing. Prof. Guanpin Yang helped prepare the manuscript. This work has won International Genetically Engineered Machine Competition (iGEM) Group of OUC a Gold Award in 2012. Authors appreciate all those who have helped iGEM Group of OUC directly or indirectly.
Beard, S. J., Handley, B. A., and Walsby, A. E., 2002. Spontaneous mutations in gas vesicle genes ofsp. affect gas vesicle production and critical pressure., 215:189-195.
Beard, S. J., Davis, P. A., Iglesias-Rodrıguez, D., Skulberg, O. M., and Walsby, A. E., 2000. Gas vesicle genes insp. from Nordic lakes: Strains with weak gas vesicles possess a longer variant of., 146 (8): 2009- 2018.
Beard, S. J., Handley, B. A., Hayes, P. K., and Walsby A. E., 1999. The diversity of gas vesicle genes infrom Lake Zürich., 145: 2757-2768.
Belenky, M., Meyers, R., and Herzfeld, J., 2004. Subunit structure of gas vesicles: A MALDI-TOF mass.,86:499-505.
Berger, S. L., and Kimmel, A. R., 1987.. Academic Press Inc., Orlando, FL, 812pp.
Bright, D. I., and Walsby, A. E., 2000. The daily integral of growth bycalculated from growth rate in culture and irradiance in Lake Zürich., 146 (2):301-316.
Childs, T. S., and Webley, W. C., 2012.assessment of halobacterial gas vesicles as avaccine display and delivery system.,30 (40): 5942-5948.
Chungjatupornchai, W., Senawong, T., and Panyim, S., 1999. Isolation and characterization ofPCC7942 promoters: tRNAprogene functions as a promoter.,38 (4): 210-216.
Englert, C., and Pfeifer, F., 1993. Analysis of gas vesicle gene expression inreveals thatandare both gas vesicle structural proteins., 268 (13): 9329-9336.
Ezzeldin, H. M., Klauda, J. B., and Solares, S. D., 2012. Modeling of the major gas vesicle protein, GvpA: From protein sequence to vesicle wall structure., 179:18-28.
Hayes, P. K., and Powell, R. S., 1995. Thecluster ofhas multiple copies of a gene encoding GvpA.Archives of, 164: 50-57.
Hayes, P. K., Buchholz, B., and Walsby, A. E., 1992. Gas vesicles are strengthened by the outer-surface protein GvpC.Archives of,157:229-234.
Hayes, P. K., Lazarus, C. M., Bees, A., Walker, J. E., and Walsby, A. E., 1988. The protein encoded byis a minor component of gas vesicles isolated from the cyanobacteriaandsp., 2: 545-552.
Holland, D. P., and Walsby, A. E., 2009. Digital recordings of gas-vesicle collapse used to measure turgor pressure and cell- water relations of cyanobacterial cells., 77 (2): 214-222.
Marschaus, L., and Pfeifer, F., 2012. A dual promoter region with overlapping activator sequences drives the expression of gas vesicle protein genes in haloarchaea., 158 (11): 2815-2825.
Miklaszewska, M., Waleron, M., Morin, N.,Calusinska, M., Wilmotte, A., de Marsac, N. T., Rippka, R., and Waleron, K.,2012. Elucidation of the gas vesicle gene clusters in cyanobacteria of the genus(Oscillatoriales, Cyanophyta) and correlation with ITS phylogeny., 47 (3): 233-244.
Offner, S., Ziese, U., Wanner, G., Typke, D., and Pfeifer, F., 1998. Structural characteristics of halobacterial gas vesicles.,144: 1331-1342.
Oren, A., 2013. The function of gas vesicles in halophilic archaea and bacteria: Theories and experimental evidence., 3 (1): 1-20.
Pfeifer, F., 2012. Distribution, formation and regulation of gas vesicles., 10: 705-715.
Ramsay, J. P., and Salmond, G. P. C., 2012. Quorum sensing- controlled buoyancy through gas vesicles: Intracellular bacterial microcompartments for environmental adaptation.,5 (1): 96-98.
Sivertsen, A. C., Bayro, M. J., Belenky, M., Griffin, R. G., and Herzfeld, J., 2010. Solid-state NMR characterization of gas vesicle structure., 99 (6): 1932-1939.
Shetty, R.,Lizarazo, M.,Rettberg, R., and Knight, T. F., 2011. Assembly of biobrick standard biological parts using three antibiotic assembly., 498: 311-326.
Strunk, T., Hamacher, K., Hoffgaard, F., Engelhardt, H., Zillig, M. D., Faist, K., Wenzel, W., and Pfeifer, F., 2011. Structural model of the gas vesicle protein GvpA and analysis of GvpA mutants, 81 (1): 56-68.
Tavlaridou, S., Faist, K., Weitzel, K., and Pfeifer, F., 2013. Effect of an overproduction of accessory Gvp proteins on gas vesicle formation in., 17 (2): 277-287.
Walsby, A. E., 1994. Gas vesicles.,58 (1): 94-144.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2344-3
ISSN 1672-5182, 2015 14 (1): 84-88
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
(April 3, 2013; revised May 22, 2013; accepted September 25, 2014)
* Corresponding author. Tel: 0086-532-82031711 E-mail: wangxianghong67@yahoo.com.cn
 Journal of Ocean University of China2015年1期
Journal of Ocean University of China2015年1期
- Journal of Ocean University of China的其它文章
- The Influence of El Niño on MJO over the Equatorial Pacific
- Research on the Interannual Variability of the Great Whirl and the Related Mechanisms
- Brightness Temperature Model of Sea Foam Layer at L-band
- Parametric Instability Analysis of Deepwater Top-Tensioned Risers Considering Variable Tension Along the Length
- DPOI: Distributed Software System Development Platform for Ocean Information Service
- Nonlinear Contact Between Inner Walls of Deep Sea Pipelines in Buckling Process
