由4-氰基苄溴引发的苯乙烯电子活化再生原子转移自由基聚合
张 良,于方丽,刘 方
(盐城工学院 材料工程学院,江苏 盐城 224051)
由4-氰基苄溴引发的苯乙烯电子活化再生原子转移自由基聚合
张良,于方丽,刘方
(盐城工学院 材料工程学院,江苏 盐城224051)
摘要:以4-氰基苄溴(CBB)为引发剂、FeCl3·6H2O/PPh3络合体系为催化剂、VC为还原剂实现了对苯乙烯(St)的电子活化再生原子转移自由基聚合(AGET ATRP)。研究表明,聚合过程中单体转化随反应时间增加而线性增长、数均分子量随单体转化率提高而线性增长、得到的聚合物分子量分布指数(PDI)在1.07~1.31之间。为进一步研究取代基团对聚合的影响,选用了4-甲基苄溴(MBB)为引发剂作了对比研究,所得聚合物的结构用核磁氢谱进行了验证。
关键词:电子活化再生原子转移自由基聚合;苯乙烯;4-氰基苄溴;引发剂
原子转移自由基聚合(ATRP)方法是1995年Matyjaszewski[1]和Sawamoto[2]课题组几乎同时发现的。这种聚合方法是以可逆的卤原子转移为基础,通过氧化还原反应,卤原子从有活性的烷基卤化物转移到低价的过渡金属化合物,形成烷基自由基和高价态的金属络合物。反应电烷基自由基先与单体发生加成反应,生成中间体,再从高价态的金属络合物中夺取卤素,通过自由基活性种与大分子有机卤化物休眠种之间的可逆动态平衡,实现对聚合反应的有效控制。ATRP技术为合成结构规整、分子量分布窄的指定分子量的聚合物提供了很好的方法。然而,普通ATRP因为使用相对不够稳定的低价态的金属离子络合物作催化剂使得这种方法存在一些缺陷,如需去除氧或氧化物等[3-8]。近些年有研究者通过电子活化再生改进原子转移自由基聚合(AGET ATRP)[9-14],有效克服了普通ATRP的一些缺陷。在典型的AGET ATRP体系中,烷基卤用作引发剂,高价态的金属离子络合物(Cu(II)络合物)作催化剂,低价态的催化剂活性中心(Cu(I)络合物)通过还原剂(如辛酸亚锡、维生素C等)原位生成[10-14]。正因为AGET ATRP使用高氧化态的金属氧化物作催化剂,大大降低卤化物失活引起反应失败的几率,使得合成均聚物或嵌段共聚物过程控制性更好,而且聚合过程中对氧气不是特别敏感,催化剂更易于制备、存储和使用[9]。在ATRP过程中,引发剂在决定增长聚合物链的数目上起着重要作用,苄卤结构由于与苯乙烯结构的相似性,经常用作苯乙烯的引发剂[15]。含不同取代结构的苄卤已成功用于普通ATRP聚合[16-32],然而,根据现有文献,尚没有苄卤衍生物用于苯乙烯的AGET ATRP报道,尽管引发剂的结构对聚合速率、活性会产生重要影响[33]。在本研究中,使用4-氰基苄溴(CBB)作为引发剂,FeCl3·6H2O/PPh3/VC作为催化体系(该体系具有低毒、来源丰富、生物相容性好的优点)[34-37],进行苯乙烯的本体AGET ATRP,得到了结构规整且分子量分布窄的聚合物。
1实验部分
1.1 原料与试剂
苯乙烯(国药集团化学试剂有限公司,纯度≥99%):经5%NaOH溶液洗涤3次,去离子水洗涤3次,加无水硫酸镁干燥过夜,减压蒸馏保存在-18 ℃冰箱环境中;FeCl3·6H2O(>99%)、 PPh3(>99%)、 VC(>99.7%)皆购于国药集团化学试剂有限公司,未经提纯直接使用;4-氰基苄溴(CBB,纯度>98%)和4-甲基苄溴(MBB,纯度>98%)皆从百灵威集团有限公司购买,未经提纯直接使用;四氢呋喃(THF)及其它试剂皆从国药集团化学试剂有限公司购买并直接使用。
1.2 聚合与纯化
按配比将FeCl3·6H2O、PPh3、St、CBB、VC加入干燥圆底烧瓶并搅拌,用氩气鼓泡20 min以除去溶液中的氧气。烧瓶封口后置于油浴中在设定的温度下搅拌反应,到预定的反应时间后,通过冰水冷却,反应物用THF稀释后倒入大量甲醇中析出聚合物(Vmethanol/VTHF=100/1)。经过滤并真空干燥后作为分析测试之用。
1.3 分析与测试
转化率用称重法测定;聚合物分子量分布用Waters公司1515型GPC测定,流动相为四氢呋喃,柱温30 ℃,PMMA标样校正;1H NMR用INOVA 300 MHz NMR核磁共振仪,CDCl3为溶剂测定。
2结果与讨论
2.1 苯乙烯的AGET ATRP本体聚合
图1表示的是在110 ℃苯乙烯的AGET ATRP动力学点ln([M]0/[M])和反应时间的关系(聚合条件为[St]0∶[I]0∶[FeCl3·6H2O]0∶[PPh3]0∶/[VC]0= 200∶1∶1∶3∶1;St=3 mL)。由图1看出反应呈一阶线性关系,表明聚合过程中增长自由基浓度恒定;由图1还可看出,在聚合初始有大约2 h的诱导期,这与以前报道中以三价铁离子为催化中心的催化体系中观察到的现象一致[36-37]。根据3价铁离子催化的AGET ATRP反应机理,还原剂使催化中心原位生成,接下来的反应机理同ATRP,即引发剂与催化剂反应生成自由基,可以引发聚合[38],在聚合初期,如果原位生成的催化中心数量不足,而存在大量氧化态的铁离子络合物,反应平衡会向左移,导致自由基浓度不足,从而产生诱导期。
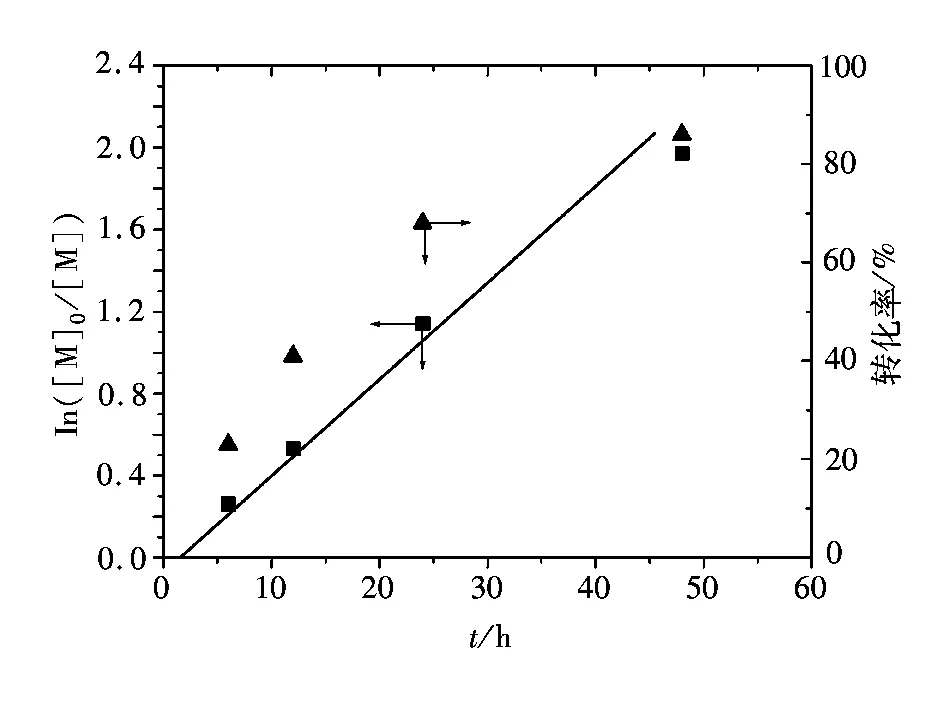
图1 110 ℃苯乙烯本体AGRT ATRP反应速率及单体转化率与聚合时间的关系Fig.1 ln([M]0/[M]) and conversion as a function of time for the AGET ATRP of St at 110 ℃ in bulk
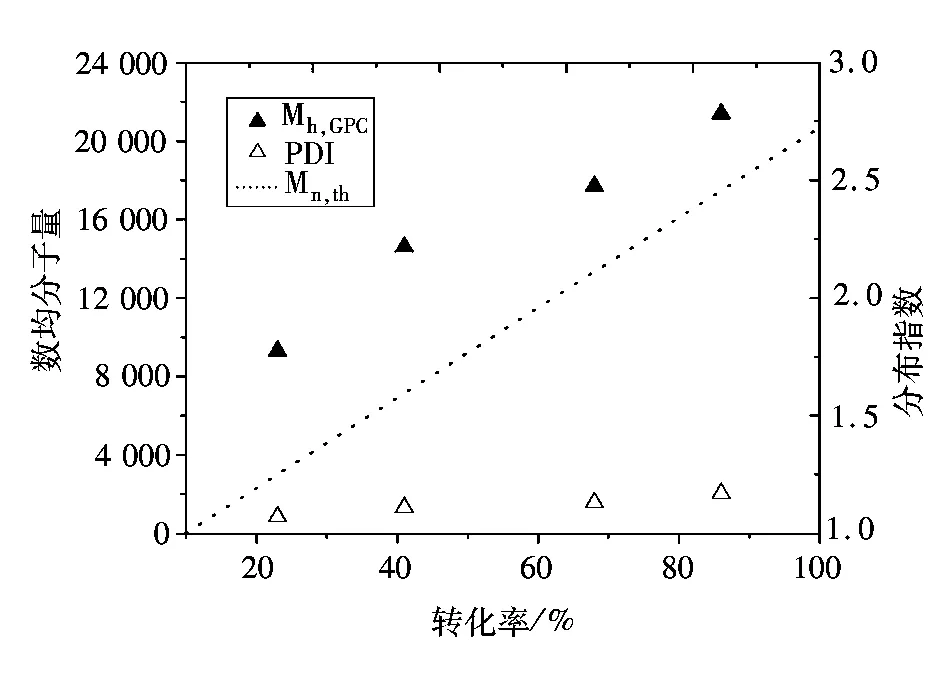
图2 110 ℃本体聚合数均分子量和分子量分布指数与转化率的关系Fig.2 Number-average molecular weight (Mn,GPC) and molecular weight distribution versus the conversion for the AGET ATRP of St at 110 ℃ in bulk
图2表示的是110 ℃本体聚合数均分子量和分子量分布指数与转化率的关系。由图2看出,聚合物数均分子量随单体转化而线性增长,分子量分布指数比较窄(1.07~1.17)。图2还标出了数均分子量的理论值,这些理论值都要低于实验值,表明引发效率相对较低。通常高活性的引发剂在聚合初期会产生大量自由基,自由基相互耦合会终止而不是用于引发聚合,导致引发效率降低[15]。
2.2 反应温度对聚合的影响
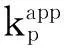
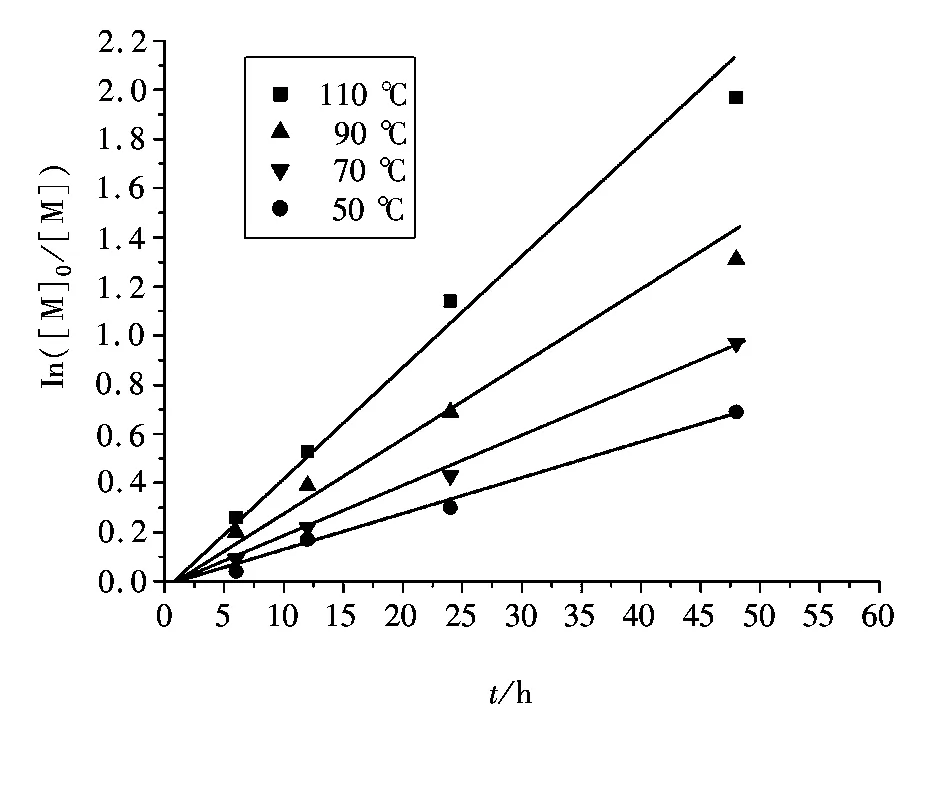
图3 本体聚合不同温度下AGRT ATRP反应速率与聚合时间的关系Fig.3 Kinetic plot for St polymerization in bulk at different temperatures
2.3 引发剂用量对聚合的影响
在苯乙烯的AGRT ATRP中,热聚影响不可忽视[37],表1为10 ℃本体聚合反应12 h引发剂用量对聚合的影响。由第1组反应1看出,不加引发剂单体转化率为6.5%,数均分子量为3 200。引发的不充分会导致数均分子量的提高,而增加引发剂的用量会导致单体转化率的提高。根据表1,[St]0∶[I]0∶[FeCl3·6H2O]0∶[PPh3]0∶[VC]0的最佳比率为200∶2∶1∶3∶1。
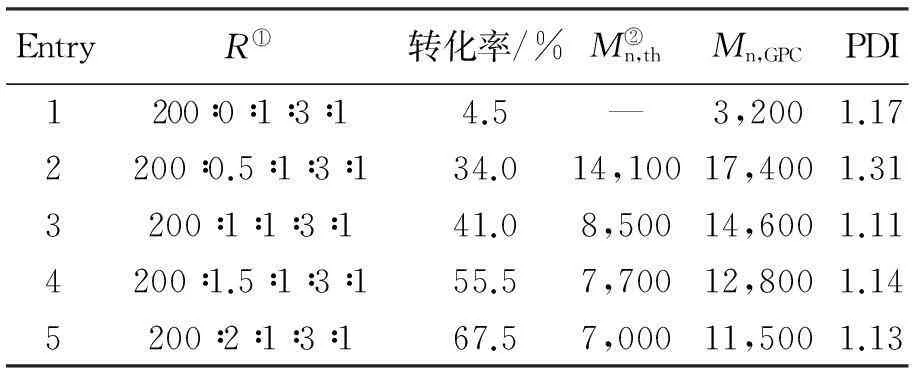
表1 110 ℃本体聚合反应12 h引发
注:①R=[St]0∶[I]0∶[FeCl3·6H2O]0∶[PPh3]0∶[VC]0
②Mn,th=([St]0/[I]0)×Mw,St×转化率%
2.4 取代基团对聚合的影响
为了进一步研究取代基团对聚合的影响,还选用MBB作为引发剂进行了苯乙烯的AGET ATRP聚合(反应条件为[St]0∶[I]0∶[FeCl3·6H2O]0∶[PPh3]0∶[VC]0= 200∶1∶1∶3∶1),结果见表2。根据表2,使用MBB作为引发剂,聚合物数均分子量及分布指数都显著增加,产生这样现象可以解释为供电子基团的电子效应使得碳-溴键难于断裂,导致引发剂的活性降低[33]。
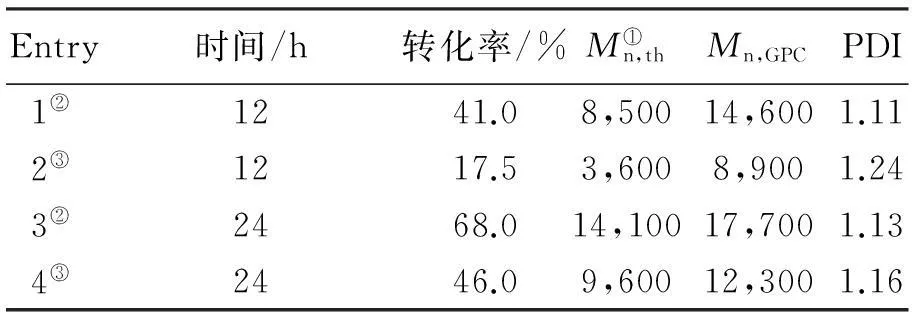
表2 110 ℃本体AGET ATRP引发剂
注:①Mn,th=200×Mw,St×转化率%
②CBB作为引发剂
③MBB作为引发剂
2.5 聚合物结构表征
根据AGET ATRP机理,在得到的聚合物中引发剂基团留在聚合物链的α端,而ω端为卤素封端,这可以通过本实验所得聚苯乙烯的核磁氢谱(图4~图5)验证。图4中(7.60~6.20)×10-6(a)的出峰信号对应CBB上的苯环质子氢,而(2.60~1.20)×10-6(b)处的出峰为聚苯乙烯主链上亚甲基、次甲基的质子氢信号,(4.75~4.25)×10-6(c)处的出峰为聚苯乙烯ω端-C(Ph)H-Br结构的质子氢信号,这表明在实验聚合体系中引发剂的溴原子确已引入聚合物的ω端。
3结论
以苄卤衍生物CBB为引发剂、FeCl3·6H2O/PPh3为催化剂、VC为还原剂,对苯乙烯进行了本体聚合,聚合符合AGET ATRP机理,并具有“活性”特征。结果表明,反应温度、引发剂加入量、引发剂取代基的结构均显著影响聚合的控制性。
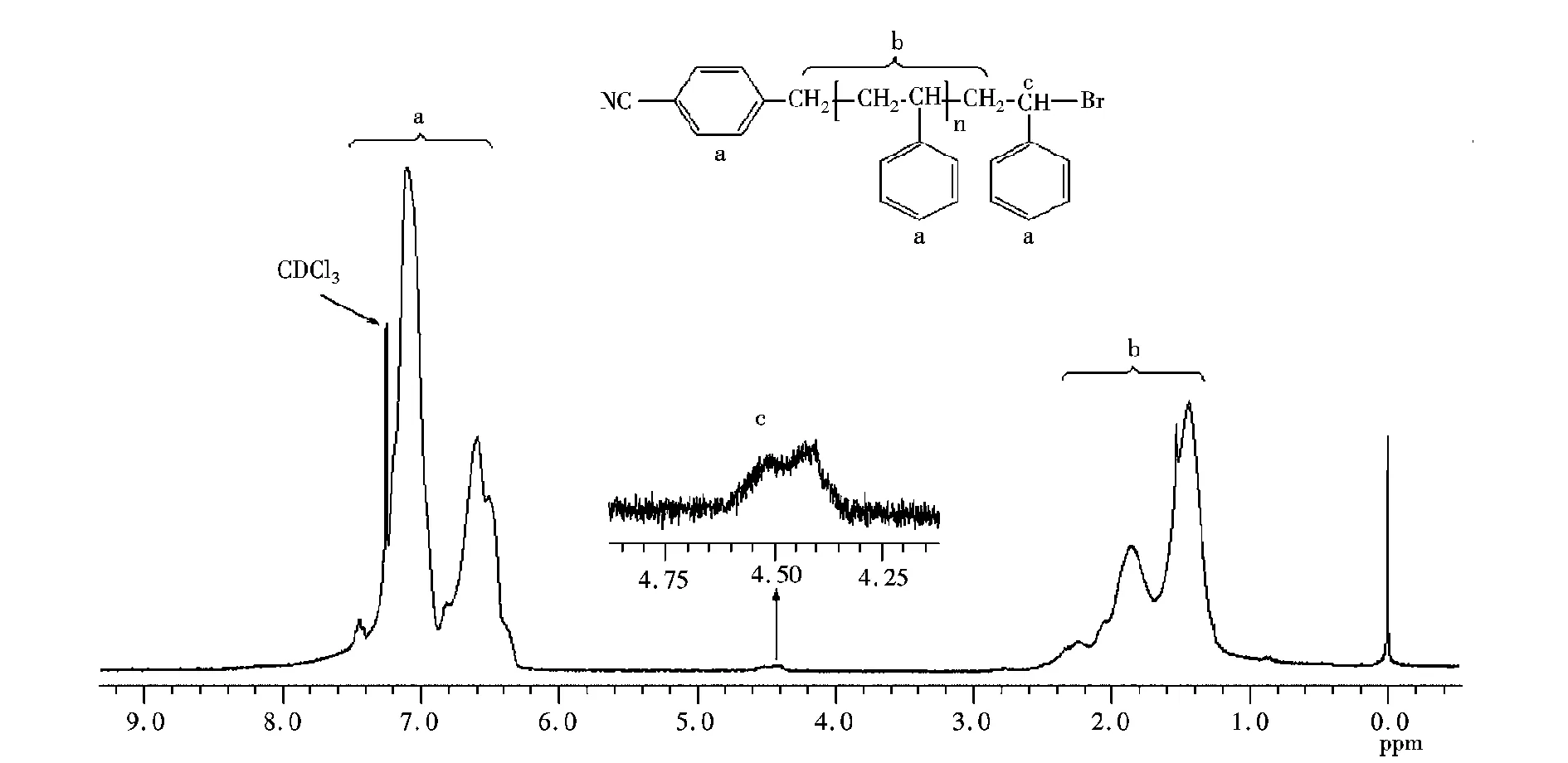
图4 CBB引发的聚苯乙烯的核磁氢谱(样品数均分子量4070,分布指数1.19)Fig.4 1H NMR spectrum of PSt initiated with CBB in bulk. Sample:Mn,GPC=4070 g/mol, PDI=1.19
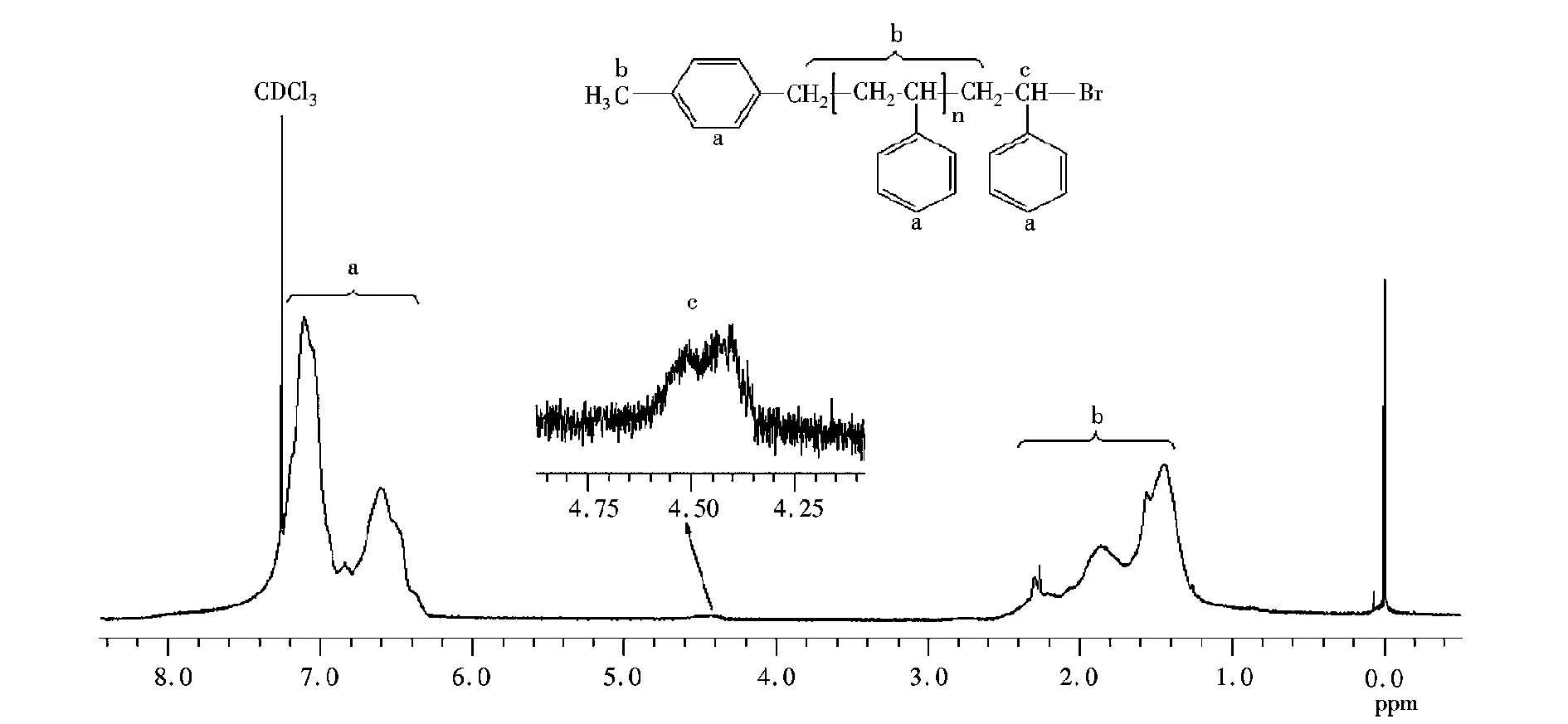
图5 MBB引发的聚苯乙烯的核磁氢谱(样品数均分子量2860,分布指数1.21)Fig.5 1H NMR spectrum of PSt initiated with MBB in bulk. Sample:Mn,GPC=2860 g/mol, PDI=1.21
参考文献:
[1]Matyjaszewski K, Wang J S. Controlled Living Radical Polymerization-Halogen Atom-Transfer Radical Polymerization Promoted by A Cu(I)/Cu(II) Redox Process[J].Macromolecules, 1995,28(23):7 901-7 910.
[2]Sawamoto M, Kato M, Higashimura T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine)ruthenium(II) /Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System:Possibility of Living Radical Polymerization[J].Macromolecules, 1995,28(5):1 721-1 723.
[3]Zhu S M, Yan D Y. Atom Transfer Radical Polymerization of Methyl Methacrylate Catalyzed by IronII Chloride/Isophthalic Acid System[J].Macromolecules, 2000,34(22):8 233-8 238.
[4]Hong S C, Matyjaszewski K. Fundamentals of Supported Catalysts for Atom Transfer Radical Polymerization (ATRP) and Application of an Immobilized/Soluble Hybrid Catalyst System to ATRP[J].Macromolecules, 2002,35(20):7 592-
7 605.
[5]Shen Y, Tang H, Ding S. Catalyst Separation in Atom Transfer Radical Polymerization[J].Prog Polym Sci, 2004,29(10):
1 053-1 078.
[6]Wang Y, Kwak Y W, Buback J, et al. Determination of ATRP Equilibrium Constants under Polymerization Conditions[J].ACS Macro Lett, 2012,1(12):1 367-1 370.
[7]汪永彬.原子转移自由基聚合(ATRP)的研究进展[J].化学推进剂与高分子材料,2014,12(1):32-36.
[8]李艳明,孔娟,杨柏林,等.PS-PMMA-PBMA三嵌段聚合物的ATRP合成及表征[J].涂料工业,2013,43(7):10-14.
[9]Jakubowski W, Matyjaszewski K. Activator Generated by Electron Transfer for Atom Transfer Radical Polymerization[J].Macromolecules, 2005,38(10):4 139-4 146.
[10]Pietrasik H, Dong H, Matyjaszewski K. Synthesis of High Molecular Weight Poly(styrene-co-acrylonitrile) Copolymers with Controlled Architecture[J].Macromolecules, 2006,39(19):6 384-6 390.
[11]Magenau A J, Strandwitz N C, Gennaro A, et al. Electrochemically Mediated Atom Transfer Radical Polymerization[J].Science, 2011,332(6025):81-84.
[12]Zhang Y, Wang Y, Peng C H, et al. Copper-Mediated CRP of Methyl Acrylate in the Presence of Metallic Copper: Effect of Ligand Structure on Reaction Kinetics[J].Macromolecules, 2012,45(1):78-86.
[13]张良,刘方.由NBS引发的MMA的室温电子活化再生原子转移自由基聚合(AGET ATRP)[J].盐城工学院学报:自然科学版,2014,27(3):13-17.
[14]钱涛,汪娟娟,张庆华,等.ARGET ATRP制备甲基丙烯酸短全氟酯共聚物及其性能[J].浙江大学学报,2013,47(8):1 470-1 474.
[15]Matyjaszewski K. Atom Transfer Radical Polymerization (ATRP)-Current Status and Future Perspectives[J].Macromolecules, 2012,45(10):4 015-4 039.
[16]Lu J M, Xia X W, Guo X, et al. Synthesis, characterization, and property of end-functionalized telechelic PSt via ATRP[J].J Appl Polym Sci, 2008,108(5):3 430-3 434.
[17]Lu J M, Xu Q F, Yuan X, et al. Synthesis, characterization, and fluorescence of end-functionalized polystyrene initiated by 4-chloromethyl benzoic acid and ethyl 4-chloromethyl benzenecarboxylate via ATRP[J].J Appl Polym Sci, 2007,104(1):75-80.
[18]Xu Q F, Lu J M, Yang Z, et al. Synthesis and fluorescent properties of end-functional PSt initiated by 5-chloromethyl-2-methoxy-benzaldehyde via ATRP[J].Polym J, 2007,39(3):213-219.
[19]Zhang L, Xu Q F, Li N J, et al. First example of polystyrene end-functionalized with excited state intramolecular proton transfer (ESIPT) fluorophore by ATRP method[J].E-Polymers, 2008,8(1):1 845-1 854.
[20]Narrainen A P, Hutchings L R, Ansari I, et al. Multi-end-functionalized polymers: additives to modify polymer properties at surfaces and interfaces[J].Macromolecules, 2007,40(6):1 969-1 980.
[21]Kopping J T, Tolstyka Z P, Maynard H D. Telechelic aminooxy polystyrene synthesized by ATRP and ATR coupling[J].Macromolecules, 2007,40(24):8 593-8 599.
[22]Broyer R M, Quaker G M, Maynard H D. Designed amino acid ATRP initiators for the synthesis of biohybrid materials[J].J AmChem Soc, 2008,130(3):1 041-1 047.
[23]Colak D G, Cianga I, Muftuoglu A E, et al. Synthesis and characterization of mid- and end-chain functional telechelics by controlled polymerization methods and coupling processes[J].J Polym Sci A Polym Chem, 2006,44(2):727-743.
[24]Colak D G, Cianga I, Yagci Y, et al. Novel poly(phenylene vinylenes) with well-defined poly(epsiloncaprolactone) or polystyrene as lateral substituents: synthesis and characterization[J].Macromolecules, 2007,40(15):5 301-5 310.
[25]Kiskan B, Gacal B, Tasdelen M A, et al. Design and synthesis of thermally curable polymers with benzoxazine functionalities[J].Macromol Symp, 2006,245(1):27-33.
[26]Sahin E, Camurlu P, Toppare L, et al. Conducting copolymers of thiophene functionalized polystyrenes with thiophene[J].J Electroanal Chem, 2005,579(2):189-197.
[27]Yurteri S, Cianga A, Demirel A L, et al. New polyphenyleneg-polystyrene and polyphenylene-g-polystyrene/poly(epsiloncaprolactone) copolymers by combined controlled polymerization and cross-coupling processes[J].J Polym Sci A Polym Chem, 2005,43(4):879-896.
[28]Yurteri S, Cianga I, Yagci Y. Synthesis and characterization of poly(epsilon-caprolactone)-b-polystyrene macromonomer by combined ring-opening and atom transfer radical polymerizations and its use for the preparation of grafted polyphenylenes by Suzuki polycondensation[J].Des Monomers Polym, 2005,8(1):61-74.
[29]Cianga I, Mercore V M, Grigoras M, et al. Synthesis and characteristics of polymacromonomers composed of alternating binaphthalene-phenylene main chain and polystyrene side chains[J].Polymer, 2007,48(22):6 501-6 509.
[30]Tarkuc S, Sahin E, Toppare L, et al. Synthesis characterization and electrochromic properties of a conducting copolymer of pyrrole functionalized polystyrene with pyrrole[J].Polymer, 2006,47(6):2 001-2 009.
[31]Sahin E, Camurlu P, Toppare L, et al. Synthesis and characterization of thiophene functionalized polystyrene copolymers and their electrochemical properties[J].Polym Int, 2005,54(12):1 599-1 605.
[32]Cianga I, Mercore V M, Grigoras M, et al. Poly(thienylphenylene)s with macromolecular side chains by oxidative polymerization of well-defined macromonomers[J].J Polym Sci A Polym Chem, 2007,45(5):848-865.
[33]Tang W, Kwak Y, Braunecker W, et al. Understanding atom transfer radical polymerization: effect of ligand and initiator structures on the equilibrium constants[J].J Am Chem Soc, 2008,130(32):10 702-10 713.
[34]Luo R, Sen A. Electron-transfer-induced iron-based atom transfer radical polymerization of styrene derivatives and copolymerization of styrene and methyl methacrylate[J].Macromolecules, 2008,41(12):4 514-4 518.
[35]Zhang L F, Cheng Z P, Shi S P, et al. AGET ATRP of methyl methacrylate catalyzed by FeCl3/iminodiacetic acid in the presence of air[J].Polymer, 2008,49(13-14):3 054-3 059.
[36]Zhang L F, Cheng Z P, Tang F, et al. Iron(III)-mediated atom transfer radical polymerization of methyl methacrylate using activators generated by electron transfer[J].Macromol Chem Phys, 2008,209(16):1 705-1 713.
[37]Zhang L F, Cheng Z P, Zhang Z B, et al. Fe(III)-catalyzed AGET ATRP of styrene using triphenyl phosphine as ligand[J].Polym Bull, 2010,64(3):233-244.
[38]Min K, Gao H F, Matyjaszewski K. Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET)[J].J Am Chem Soc, 2005,127(11):3 825-3 830.
(责任编辑:李华云)
AGET ATRP of Styrene Using 4-Cyanobenzyl
Bromide as the Initiator
ZHANG Liang, YU Fangli, LIU Fang
(School of Materials Engineering, Yancheng Institute of Technology, Yancheng Jiangsu224051, China)
Abstract:4-Cyanobenzyl bromide was used as the initiator for the atom transfer radical polymerization using activators generated by electron transfer (AGET ATRP) of styrene in a FeCl3·6H2O/PPh3/VC catalytic system. Factors that can affect the AGET ATRP system, such as the reaction temperature and amount of initiator, were discussed. The rates of polymerization exhibited first-order kinetics with respect to the monomer. A linear increase in the number-average molecular weight with increased monomer conversion was observed in the initiation systems. The polydispersity indices of the polymer were relatively low (1.07~1.31). To study further the effect of the substituent group of the initiator on polymerization, 4-methylbenzyl bromide was also used as an initiator for the polymerization system. The structures of the polymers were characterized by proton nuclear magnetic resonance spectroscopy.
Keywords:AGET ATRP; Styrene (St); 4-Cyanobenzyl bromide (CBB); Initiator
作者简介:张良(1977-),男,江苏建湖人,讲师,博士,主要研究方向为可控/活性自由基聚合。
收稿日期:2014-12-08
中图分类号:TQ316.32
文献标识码:A
文章编号:1671-5322(2015)02-0009-06
doi:10.16018/j.cnki.cn32-1650/n.201502002

