金钗石斛水溶性多糖分子量分布及单糖组份分析
金钗石斛水溶性多糖分子量分布及单糖组份分析
姚晓东1,2,刘章泉1,汪巍1,周旭美1,2
(1.遵义医学院 药学院,贵州 遵义563099;2. 遵义医学院 院士工作站糖化学与糖生物学实验室,贵州 遵义563099)
[摘要]目的 考察金钗石斛水溶性多糖分子量分布并分析其单糖组成。方法 金钗石斛水溶性粗多糖采用水提醇沉法获得,适量双蒸水复溶,再次醇沉,然后采用Savag法除去蛋白得到精制多糖。金钗石斛精制多糖的分子量分布采用高效凝胶渗透色谱(HPGPC)测得;多糖经水解、还原、乙酰化后采用气相色谱(GC)确定单糖组成。7种标准单糖(包括1种酮糖)同法处理。结果 精制金钗石斛多糖主要由5部分组成,其保留时间分别为9.269、9.564、14.628、15.410和15.840 min,由面积归一化法计算各组分的百分含量依次是26.65%、32.36%、8.98%、20.70%和11.31%,其分子量则为9.2× 104、8.1 × 104、4.5 × 102、1.2 × 102、6.1 × 10 Da。乙酰化标准单糖实现基线分离,精制金钗石斛多糖由鼠李糖、阿拉伯糖、木糖、甘露糖、葡萄糖和半乳糖6种酮糖组成,其摩尔比是:1.00:1.65:2.58:1.08:1.83:1.15。结论 金钗石斛多糖主要由多糖及寡聚糖组成,其单糖组成为6种酮糖。
[关键词]分子量分布;HPGPC;单糖组成;GC;多糖;金钗石斛
There are about 700 genus, 20000 species plants in Orchidaceae, and mainly located in tropical and subtropical regions in the world[1]. About 1000Dendrobiumspecies in the world, among which there are 76 species (including 74 species and 2 varieties) in China[2].DendrobiumnobileLindl., famous in Chishui, Guizhou Province, was awarded “National Product of Geographical Indication” in 2006. Dendrobine is an active alkaloid content extracted fromDendrobiumnobileLindl., and the index content distinguishing different Dendrobii Caulis[3]. Polysaccharide ofDendrobiumnobileLindl. is another research hot-spot in recent years, meanwhile, is a potential antioxidant[4], antitumor agent[5], anti-aging[6], hyperlipidemia modulator[7], and so on . The polysaccharide activity is determined by its molecular weight and structure, while the kinds and amounts of monosaccharide are the first step of structure elucidation. Hence, we report a method to clarify the molecular weight distribution and analyze the monosaccharide composition of wsDNP, so as to establish a theoretical foundation for wsDNP structure analysis and the development ofDendrobiumnobileLindl.
1Sample, equipment and reagents
1.1SampleDendrobiumnobileLindl. (Batch No: CSWL20140325G-3) was collected in Chishui, Guizhou Province, and identified by Professor Yang Jianwen. The voucher specimen was deposited in the Laboratory of Glycochemistry and Glycobiology, Academic Workstation of Zunyi Medical University (Batch No: LGGDNL20150330D-1).
1.2EquipmentAn Agilent 1260 HPLC system consisted of automatic injector,column oven, quaternary pump, vacuum degasser, and Refractive Index Detector (RID). An Agilent 7 890N GC system consisted of automatic injector, column oven, and flame ionization detector (FID). The data were collected and analyzed by Agilent ChemStation. Rotary evaporator (RE2000A, Shanghai Yarong, Shanghai, China). Freeze dryer (Modulyod-230, Thermoelctron corporation, USA).
1.3ReagentsEthanol,Chloroform,n-Butanol, Trifluoroacetic Acid (TFA), Sodium Borohydride, Sodium Nitrate,Acetic Acid, Methanol, Acetic Anhydride, Sinopharm Chemical Reagents Co., Ltd products, Analytical Reagents (AR). Dextran serial standards (molecular weight 1000, 5000, 12000, 250000, 50000Da) were Fluka products.
2Methods
2.1Crude wsDNP preparation and purificationDendrobiumnobileLindl. was dried in an oven set at 60 ℃ for 24 h under-0.08 MPa,then grinded appropriately. A 5 fold (w/v) ethanol was added and extracted 3 times, 2 h each time. The residue was dried by airing, then extracted by hot water for 5 times, 3 h each time. Water solution was filtered with a 4-layer gauze and mixed, then condensed using a rotary evaporator. And 4-fold (v/v) pre-cold 95% ethanol was added and well-mixed, then stored at 4 ℃ for 12 h. The ethanol solution was centrifuged at 4000 rpm for 10 min, and the residue was dissolved into hot water again, equivalent volume Savag solution (Chloroform:n-Butanol = 4:1, v/v) was added, at the same time, mixed evenly. The upper solution was treated twice the way described as above mentioned, and condensed using the rotary evaporator into an appropriate volume, then, dialyzed using a semipermeable membrane whose cut-off molecular weight (COMW) is 3500 Da for 24 h. The solution in the semipermeable membrane was condensed, then dried with a freeze dryer. At last, the purified wsDNP was obtained and readily for the following procedure.
2.2HPLC sample preparation of purified wsDNPAbout 2~3 mg purified wsDNP was put into an EP tube, and 1 ml deionized water was added, sonicated to dissolve, then centrifuged at 10000 rpm for 10 min, supernatant was HPLC loading sample.
2.3HPLC analysis procedureThe molecular weight distribution of purified wsDNP was carried out on the Agilent 1260 HPLC coupled with RID. An Ultrahydrogel 250 column (30 cm, i.d. 0.78 cm) was used to separate the wsDNP. The mobile phase was 0.1 mg/ml NaNO3, flow rate was 0.6 mg/ml, and the sample injection volume was 40 μl using the automatic injector. Column temperature was set at 30 ℃.
2.4Purified wsDNP and Standard monosaccharide hydrolysis, reduction and acetylation[8]About 1.0 mg purified wsDNP was put into an ampoule, and 1.5 ml 2.0 mol/L TFA was added, incubated for 4 h in the oven set at 110 ℃. Once finished, the mixture solution was evaporated to dry, 1 ml distill water was added and once again so as to remove residual TFA. 2 ml distill water was added into the fully hydrolysis sample, and 10 mg sodium borohydride was added to reduce for 3 h at room temperature. Until the reaction finished, the excess sodium borohydride was destroyed by adding adequate acetic acid. Then the mixture solution was evaporated to dry, 4 ml methanol was added for 4 times in order to remove the boric acid completely. To acetylate, 1 ml acetic anhydride was added, sonicated then incubated for 1 h at 110 ℃, when finished, 4 ml toluene was added for 4 times to remove the excess acetic anhydride. The acetylated products were extracted with 1 ml chloroform and washed using equivalent volume distill water, the lower layer was dried by anhydrous sodium sulfate under dark circumstance for 12 h, and appropriate volume was injected into a GC to analyze the sample.
The standard monosaccharides (rhamnose, glucose, galactose, mannitol, xylose, arabinose and fructose) were treated the same way as described above.
2.5GC Analysis procedureThe standard monosaccharides and the hydrolyzed wsDNP were carried out on the Agilent 7 890N GC. A DB-5 capillary column (30 m, i.d. 0.32 mm) was used to separate the acetylated product. Nitrogen, hydrogen and air flow rate was 45, 30, 400 mg/ml, respectively. The gas flow rate was 1.0 mg/ml, the sample injection volume was 0.3 μl with split (2:1) using the automatic injector, and the standard monosaccharide injection volume was 0.1 μl with split (10:1). Injection temperature was set at 240 ℃. The oven conditions were as follows: initial temperature was 120 ℃, and ramped 5 ℃·min-1to 210 ℃, then hold on 5 min.
3Results
3.1Molecular weight distribution of purified wsDNP Polysaccharide composition of wsDNP was mainly consisted of two groups, i.e., whose retain time were about 9 min and 15 min.And the detailed content ratio was 26.65%, 32.36%, 8.98%, 20.70% and 11.31%, whose retain time was 9.269, 9.564, 14.628, 15.410 and 15.840 min, respectively. The molecular weight was 9.2 × 104, 8.1 × 104, 4.5 × 102, 1.2 × 102, and 6.1 × 10 Da calculated according to standard curve of dextran molecular weight. The standard dextran curve was showed in Fig 1. The HPLC chromatogram of wsDNP was in Fig 2.

Fig 1 Molecular weight curve of standard dextran
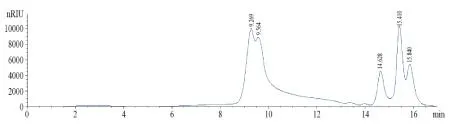
Fig 2 The HPLC chromatogram of wsDNP
3.2Monosaccharide composition of purified wsDNP The single standard monosaccharide and mixed standard monosaccharides were injected into the GC system respectively, whose retain time were 16.537 min (rhamnose), 16.792 min (arabinose), 17.141 min (xylose), 21.824 min (mannose), 22.072 min (glucose), and 22.297 min (galactose), the GC chromatograms were showed in Fig 3.
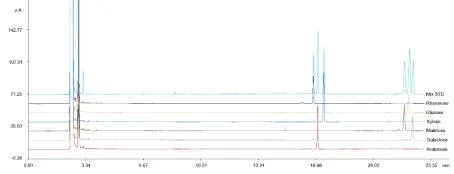
Fig 3 The GC chromatogram of Standard monosaccharides and the mixed monosaccharides.
The hydrolyzed purified wsDNP contains 6 aldose, namely, rhamnose, arabinose, xylose, mannose, glucose and galactose, and its molar ratio was 1.00:1.65:
2.58:1.08:1.83:1.15, whose GC chromatogram were showed in Fig 4. Besides, there was an unknown peak (tR= 18.119) in the sample GC chromatogram.
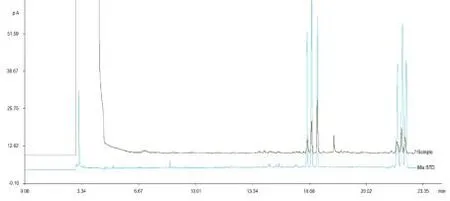
Fig 4 The GC chromatogram of mixed monosaccharide and hydrolyzed wsDNP
4Discussion
The molecular weight range of ultrahydrogel 250 column we selected was from 1× 103Da to 8× 104Da[9], while our results showed a glycan portion whose molecular weight was 6.1 × 10 Da, which was obviously a mistake. It was reasonable that molecular weight calculated according to standard dextran curve may have deviation. Meanwhile, the molecular weight of wsDNP was different because of extraction condition, plant resource, and so on[5]. However, it was successfully to determine the polysaccharide content ratio of wsDNP.
In our study, 6 aldose were successfully detected, while the ketose,fructose were not detected, unfortunately. The reason maybe that fructose was reduced to produce two alditol, i.e., glucitol and mannitol[10]. An alternate monosaccharide compositions determination method was PMP (1-phenyl-3-methyl-5-pyrazolone)-HPLC[11]. Whether the fructose during the reduction disappeared or not, our result couldn’t answer that question. Besides, the existing mannitol peak in the sample GC chromatogram is a latent index of ketose existence in wsDNP.
Our result showed that there was a peak (tR=18.119 min) in the sample GC chromatogram, which maybe an unknown monosaccharide, a solution to that question is an available Gas Chromatography coupled with Mass Spectrometry (GC-MS), and a verification work will be further done.
[Reference]
[1] Lang K. Orchlldaceae (1). Flora ReipublicaePopularisSinicae [M].Beijing: Science Press, 1999:1.
[2] Ji Z. Orchlldaceae (3). Flora ReipublicaePopularisSinicae [M].Beijing: Science Press, 1999:67.
[3] Li S, Wang C, Guo S. Determination of Dendrobin in Dendrobiumnobile by HPLC Analysis [J]. Chin Pharm J, 2009, 45(4):359-363.
[4] Luo A, He X, Zhou S, et al. In vitro antioxidant activities of a water-soluble polysaccharide derived from DendrobiumnobileLindl. extracts[J]. Int J Biol Macromol, 2009, 45 (4):359-363.
[5] Wang J, Luo J, Zha X, et al. Comparison of antitumor activities of different polysaccharide fractions from the stems of DendrobiumnobileLindl[J]. Carbohydrate Polymers, 2010, 79 (1):114-118.
[6] Wang L. Nobile dendrobium polysaccharides attenuate learning and memory deficits Induced by lipopolysaccharide and its mechanisms in rats[D]. Zunyi:Zunyi Medical College, 2011.
[7] Li X, Gong Q, Wu Q, et al. Effects of Dendrobiumnobile Polysaccharide on Hyperlipemia and Liver Fatty Degeneration in Rats [J]. Chin Pharm J, 2010, 45(15):1142-1144.
[8] Pettolino F, Walsh C, Fincher G, et al. Determining the polysaccharide composition of plant cell walls[J]. Nat Protoc, 2012, 7 (9):1590-1607.
[9] Nascimento G, Corso C, Werner M, et al. Structure of an arabinogalactan from the edible tropical fruit tamarillo (Solanumbetaceum) and its antinociceptive activity[J]. CarbohydrPolym, 2015, 116:300-306.
[10] Zhang W. Biochemistry technologies of Glycoconjugates(2nd edition)[M]. Zhejiang: Zhejiang University Press, 1999: 42-44.
[11] Wang P, Zhang L, Yao J, et al. An arabinogalactan from flowers of Panaxnotoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling[J]. CarbohydrPolym, 2015, 121:328-335.
[收稿2015-04-23;修回2015-05-22]
(编辑:谭秀荣)
基础医学研究
Molecular weight distribution and monosaccharide composition analysis for water-soluble polysaccharide fromDendrobiumnobileLindl
YaoXiaodong1,2,LiuZhangquan1,WangWei1,ZhouXumei1,2
(1.Pharmacy School of Zunyi Medical University, Zunyi Guizhou 563099,China;2.Laboratory of Glycochemistry and Glycobiology, Academic Workstation of Zunyi Medical University, Zunyi Guizhou 563099,China)
[Abstract]Objective To explore the molecular weight distribution and analyze monosaccharide composition of water-soluble polysaccharide of Dendrobium nobile Lindl. (wsDNP).Methods Crude wsDNP was obtained by hot-water extraction and high concentration ethanol precipitation. Purified wsDNP was acquired using water dissolving and ethanol precipitating, then the Savag method was used for deproteinization. The molecular weight distribution of purified wsDNP was carried out on a High Performance Gel Permeation Chromatography (HPGPC), then subjected to hydrolysis, reduction, and acetylate, and injected into a Gas Chromatography (GC). 7 standard monosaccharides (include one ketose) were treated the same procedure as above mentioned.Results The purified wsDNP included five portion, whose retain time were 9.269, 9.564, 14.628, 15.410 and 15.840 min, and their content ratio were 26.65%, 32.36%, 8.98%, 20.70% and 11.31% according to Area Normalization Method. The estimated molecular weights were 9.2 × 104, 8.1 × 104, 4.5 × 102, 1.2 × 102, and 6.1 × 10 Da, respectively. Acetylated standard monosaccharides were separated on baseline. The purified wsDNP included 6 aldose, namely, rhamnose, arabinose, xylose, mannose, glucose and galactose, and its molar ratio was 1.00:1.65:2.58:1.08:1.83:1.15. Conclusion The wsDNP consists polysaccharide and oligosaccharide, and its monosaccharide composition was 6 aldose.
[Key words]molecular weight distribution;HPGPC;monosaccharide composition;GC;polysaccharide; Dendrobium nobile Lindl
[文献标志码][中图法分类号]Q539A
[文章编号]1000-2715(2015)03-0239-05
[基金项目]贵州省遵义医学院院士工作站建设项目(NO:C-768);教育部春晖计划项目(NO:B-035);遵义医学院招标课题(NO:F-681);遵义医学院硕士启动基金资助项目(NO:F-714)。[通信作者]周旭美,女,教授,硕士生导师,研究方向:药物质量标准建立及新药研发,E-mail:zmczxm@163.com。

