2-乙酰吡嗪缩肼基甲酸甲酯的镍和镉配合物的晶体结构及荧光性质
毛盼东 韩学锋 吴伟娜*, 王冠杰 王震 侯莹(河南理工大学物理化学学院,焦作454000)(河南理工大学人事处,焦作454000)
2-乙酰吡嗪缩肼基甲酸甲酯的镍和镉配合物的晶体结构及荧光性质
毛盼东1韩学锋*,2吴伟娜*,1王冠杰1王震1侯莹1
(1河南理工大学物理化学学院,焦作454000)
(2河南理工大学人事处,焦作454000)
合成并通过X射线单晶衍射、元素分析及红外光谱表征了配合物[Ni(L)2](1)和[Cd(HL)(CH3OH)(NO3)2](2)的结构(HL为2-乙酰吡嗪缩肼基甲酸甲酯)。单晶衍射结果表明,配合物1中,Ni(Ⅱ)离子与来自2个阴离子配体L-的N2O电子供体配位,形成扭曲的八面体配位构型。在配合物2中,Cd(Ⅱ)离子拥有双帽三棱柱配位构型,与1个中性配体HL,2个双齿配位硝酸根和1分子甲醇配位。此外还研究了配合物1和2的荧光及热性质。
肼基甲酸甲酯;吡嗪;荧光;晶体结构;热稳定性
Schiff bases as an important class of ligands play a crucial role in coordination chemistry and have been widely applied in different fields mainly due to their wide-spectrum biological applications[1-7].In recent years,there are many reports about the acylhydrazones, thiosemicarbazones derived from actyl-pyridine/ pyrazine,and their transition metal complexes because of high biological and pharmaceutical activities[3,8-10]. By contrast,as their structurally analogous,carbazates (R-O-CO-NH-NH2)and their metal complexes havebeen paid much less attention[6].
Generally,Cd(Ⅱ)ion is closely related to biochemistry,clinical diagnostics as well as environmental pollution[11].Furthermore,a large amount of Cd(Ⅱ)complexes have been reported for their fluorescence properties[12-13].Therefore,in this paper,Ni(Ⅱ)and Cd(Ⅱ)complexes with a Schiff base ligand derived from 2-acetyl pyrazine and methyl hydrazinocarboxylate have been synthesized and structural determined by single-crystal X-ray diffraction.In addition,the thermal stability and luminescent properties of the complexes are also investigated.
1 Experimental
1.1Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.1H NMR spectra of L was acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMS as internal standard.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer.
1.2Preparations of the ligand,complexes 1 and 2
As shown in Scheme 1,the ligand HL was produced by condension of 2-acetyl pyrazine(1.22 g, 0.01 mol)and methyl hydrazinocarboxylate(0.90 g, 0.01 mol)in anhydrous methanol solution(30 mL) with continuous stirring at room temperature for 3 h. The white solid was filtered and washed three times by cold methanol.Yield:1.13 g(58%).m.p.183~185℃.Elemental analysis Calcd.for C8H10N4O2(%):C: 49.48;H:5.19;N:28.85;Found(%):C:49.26;H: 5.34;N:29.00.FT-IR(cm-1):ν(C=O)1 748,ν(C=N) 1 613,ν(C=N)pyrazine1 558.1H NMR(400 MHz):δ 10.52(1H,s,NH),9.09(1H)/8.55~8.58(2H)for pyrazine-H,3.70(3H,s,CH3),2.24(3H,s,CH3).

Scheme 1 Synthesis route of HL
The complexes 1 and 2 were generated by reaction of the ligand HL(5 mmol)with equimolar of Ni(NO3)2·6H2O or Cd(NO3)2·6H2O in methanol solution(10 mL),respectively.Crystals suitable for X-ray diffraction analysis were obtained by evaporating the corresponding reaction solutions at room temperature.
1:Brown blocks.Anal.Calcd.for C16H18N8O4Ni (%):C:43.18;H:4.08;N:25.18.Found(%):C:43.05; H:4.00;N:25.33.FT-IR(cm-1):ν(N=C-O)1 606, ν(C=N)1 594,ν(C=N)pyrazine1 535.
2:Colorless blocks.Anal.Calcd.for C9H14N6O9Cd (%):C:23.36;H:3.05;N:18.16.Found(%):C:23.22; H:3.17;N:18.27.FT-IR(cm-1):ν(O-H)3 532,ν(C=O) 1 720,ν(C=N)1 579,ν(C=N)pyrazine1 523.ν1(NO3)1 494, ν4(NO3)1 311.
1.3X-ray crystallography
The X-ray diffraction measurement for complexes 1 and 2 were performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ=0.071 073 nm) by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[14].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[15].All nonhydrogen atoms were refined anisotropically.All the H atoms were positioned geometrically and refined using a riding model.Details of the crystal parameters, data collection and refinements for complexes 1 and 2 are summarized in Table 1.
CCDC:1424257,1;1424258,2.
2 Results and discussion
2.1Crystal structures description
Selected bond distances and angles,hydrogen bonds information for both complexes are listed in Table 2 and 3,respectively.As shown in Fig.1a,the central Ni(Ⅱ)ion in complex 1 is surrounded by two independent anionic ligands with N2O donor set,thus possesses a distorted octahedral coordination geometry.The enolization of C=O bond of the ligandcan be confirmed by the bond lengths of C-O being 0.125 3(4)and 0.124 1(4)nm,which are in excellent agreement with previously known semicarbazone complexes in the literature[6-7].The distances of Ni-N/ O bonds were in the range of 0.197 1(3)~0.209 5(2) nm,comparable with those in some reported complexes with similar donor set[3].As expected,there exist none classic hydrogen bonds in the crystal of 1. However,certain C-H…N and C-H…O interactions are helpful to construct three dimensional net work.

Table 1 Crystal data and structure refinem ent for com p lexes 1 and 2

Fig.1 Diamond drawing of 1(a)and 2(b)with 30%thermal ellipsoids;(c)Coordination enviorment of Cd(Ⅱ)ion in complex 2 (coordination atoms shown with 30%thermal ellipsoids);(d)Extend 2D supromolecular structure along c axis in comp lex 2

Table 2 Selected bond lengths(nm)and angles(°)in com p lexes 1 and 2

Table 3 Hydrogen bonds information in comp lexes 1 and 2
By contrast,the molar ratio of the ligand HL and metal is 1∶1,and the ligand is neutral tridentate in complex 2(Fig.1b)with C-O bond length being 0.121 1(5)nm.The Cd(Ⅱ)ion is also coordinated with two bidentate nitrate anions and one methanol molecule,giving bicapped-triangular prism geometry(Fig.1c).In the crystal,intermolecular O9-H9…N2ivand N4-H4…O4v(Symmetry codes:ivx-1/2,-y+1,z+ 1/2;vx,y-1,z)hydrogen bonds link the complexes into extended 2D supromolecular structure(Fig.1d).
2.2IR spectra
The ν(C=O)of the free ligand is 1 748 cm-1,it shifts to lower frequency value in complex 2,confirming the coordination of the carbonyl group[3].However,such absorption is disappeared in complex 1, meanwhile,new(N=C-O)stretching vibration absorption is observed at 1 606 cm-1,revealing that the C=O in O=C-N moiety has enolizated and the oxygen atom coordinates to the Ni(Ⅱ)ion[6-7].The ν(C=N)bands of the imine group and pyrazine ring in the ligand HL shift to lower frequency values in the complexes, indicating that the N atoms of both units take part in the coordination[8].Meanwhile,the bands at 1 494 and 1 311 cm-1in complex 2 could be assigned to the two split bands ν1and ν4of the coordinated nitrate group, respectively,showing that the nitrate group is bidentate[16].It is in accordance with the crystal structure study.
2.3Thermal decomposition process of complexes 1 and 2
For detecting the thermal stabilities of complexes 1 and 2,thermal gravimetric(TG)analyses were carried out from the room temperature to 800℃with the linear heating rate of 10℃·min-1under argon atmosphere.Complex 1(Fig.2a)is thermally stable up to about 300℃,indicating there exist no solvent molecules in the complex.A sharp weight loss for complex 1 could be observed from 300 to 330℃, then gradually smooth until to about 700℃,corresponding to the decomposition of two organic ligands. However,the first stage occurs with weight loss of 3.62%below 150℃for complex 2,contributing to the loss of one coordinated methanol molecule(Calcd. 6.89%).The second process of weight loss appears between 150 to 780℃,considered as the decomposition of the ligand L and two nitrate anions.The remainders of the complexes 1 and 2 might be the metal oxides because the residue weights(11.53% and 15.08%)are agreement with the calculated values of 10.12%,and 15.72%,respectively.

Fig.2 TG curves for complexes 1(a)and 2(b)
2.4UV spectra

Fig.3 UV spectra of the ligand HL(a),1(b)and 2(c)in CH3OH solution at room temperature
The UV spectra of HL,complexes 1 and 2 in CH3OH solution(concentration:1×10-5mol·L-1)were measured at room temperature(Fig.3).The spectra of HL features only one main band located around 290 nm(ε=10 674 L·mol-1·cm-1),which could be assigned to characteristic π-π*transition of pyrazine unit[3]. Similar bands are observed at 289 nm(ε=12 422 L· mol-1·cm-1)in the complex 2.However,there arethree bonds in spectra of 1 at 252(ε=7914 L·mol-1· cm-1),296(ε=16 464 L·mol-1·cm-1)and 385 nm(ε= 12 538 L·mol-1·cm-1).The former two could be contributed to the characteristic π-π*transition of pyrazine and imine unit,respectively[16],while the final one is probably due to the ligand-to-metal charge transfer(LMCT)[3].This indicates that an extended conjugation is formed in anionic ligand after complexation in complex 1.
2.4Fluorescence spectra
The fluorescence spectra of the ligand HL, complexes 1 and 2 have been studied in CH3OH solution (concentration:1×10-5mol·L-1)at room temperature. The results show that the emission spectra of all three compounds exhibit one emission peak at 380 nm when excited at 340 nm,while complex 2 shows another peak at 550 nm.The behavior of Cd(Ⅱ)ion coordinated to the ligand is regarded as that of emissive species resulted in a CHEF(chelation enhancement of the fluorescence emission)effect[17].
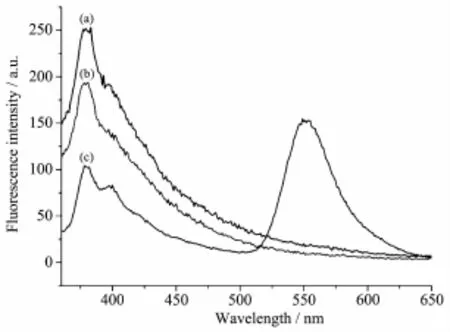
Fig.4 Fluorescence emission spectra of the ligand L(a), 1(b)and 2(c)in CH3OH solution at room temperature
[1]Alagesan L,Bhuvanesh N S P,Dharmaraj N.Dalton Trans., 2013,42:7210-7223
[2]Ye X P,Zhu T F,Wu W N,et al.Inorg.Chem.Commun., 2014,47:60-62
[3]CHEN Yan-Min(陈延民),XIE Qing-Fan(解庆范),LIU Jin-Hua(刘金花),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(1):74-80
[4]Singh P,Singh D P,Singh V P.Polyhedron,2014,81:56-65
[5]NathM,VatsM,Roy P.Eur.J.Med.Chem.,2013,59:310-321
[6]Milenkovic'M,Cantoni G,Bacchi A,et al.Polyhedron, 2014,80:47-52
[7]Milenkovic'M,Bacchi A,Cantoni G,et al.Inorg.Chim.Acta, 2013,395:33-43
[8]Chang H Q,Jia L,Xu Z Q,et al.Inorg.Chem.Commun., 2015,57:8-10
[9]Li M X,Zhang L Z,Yang M,et al.Bioorg.Med.Chem.Lett., 2012,22:2418-2433
[10]Li M X,Zhang L Z,Zhang D,et al.Eur.J.Med.Chem., 2011,46:4383-4390
[11]WANG Zhao-Lu(王召璐),FENG Hui-Yun(冯慧云),LI Yan (李艳),et al.Chinese J.Inorg.Chem.(无机化学学报),2015, 31(10):2-7
[12]WANG Gui-Xian(王桂仙),ZHANG Qi-Wei(张启伟),WEN Yi-Hang(温一航).Chinese J.Inorg.Chem.(无机化学学报), 2014,30(11):2571-2576
[13]LI Ke(李可),LI Shu-Jing(李书静),ZHANG Xiao-Peng(张小鹏),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30 (7):1647-1652
[14]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[15]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen, Germany,1997.
[16]Song X Q,Zang Z P,Liu W S,et al.J.Solid State Chem., 2009,182:841-848
[17]Vicente M,Bastida R,Lodeiro C,et al.Inorg.Chem.,2003, 42:6768-6779
Ni(Ⅱ)and Cd(Ⅱ)Com p lexes w ith a Schiff Base Ligand Derived from 2-Acetyl Pyrazine and Methyl Hydrazinocarboxylate:Crystal Structures and Fluorescence Properties
MAO Pan-Dong1HAN Xue-Feng*,2WU Wei-Na*,1WANG Guan-Jie1WANG Zhen1HOU Ying1
(1Department of Physics and Chemistry,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2Personnel Services,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Two complexes,namely[Ni(L)2](1)and[Cd(HL)(CH3OH)(NO3)2](2)(HL is derived from 2-acetyl pyrazine and methyl hydrazinocarboxylate)have been synthesized and characterized by single-crystal X-ray diffraction,elemental analysis and IR spectroscopy.X-ray diffraction analysis results show that the central Ni(Ⅱ)ion in complex 1 is surrounded by two independent anionic ligands with N2O donor set,thus possesses a distorted octahedral coordination geometry.However,in complex 2,the Cd(Ⅱ)ion with bicapped-triangular prism geometry is coordinated with one neutral ligand HL,two bidentate nitrate anions and one methanol molecule.In addition, the thermal stability and luminescent properties of the complexes are also studied in detail.CCDC:1424257,1; 1424258,2.
methyl hydrazinocarboxylate;pyrazine;fluorescence;crystal structure;thermostability
O614.24+2;O614.81+3
A
1001-4861(2016)01-0161-06
10.11862/CJIC.2016.008
2015-09-15。收修改稿日期:2015-10-25。
国家自然科学基金(No.21404033,21401046,21001040)和河南省教育厅自然科学基金(No.12B150011,14B150029)资助项目。
*通信联系人。E-mail:108242720@qq.com,wuwn08@hpu.edu.cn;会员登记号:S06N6704M112。

