ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli
Research Paper
() Negatively Regulates Plant Immunity in Rice
Tan Jingyi#, Zhang Xiaobo#, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli
(State Key Laboratory of Rice Biology and Breeding, China National Rice Research Institute, Hangzhou 310006, China; These authors contributed equally to this work)
Identification of immunity-associated leucine-rich repeat receptor-like protein kinases (LRR-RLK) is critical to elucidate the LRR-RLK mediated mechanism of plant immunity. Here, we reported the map-based cloning of a novel rice() encoding a putative LRR-RLK protein (OsLRR-RLK41/OsSPL41) that regulated disease responses to the bacterial blight pathogenpv.(). An 8-bp insertion at position 865 bp in a mutant() allele led to the formation of purple-brown lesions on leaves. Functional complementation by the wild type allele () can rescue the mutant phenotype, and the complementary lines showed similar performance to wild type in a number of agronomic, physiological and molecular indices.was constitutively expressed in all tissues tested, and OsSPL41 contains a typical transmembrane domain critical for its localization to the cell membrane. The mutant exhibited an enhanced level of resistance toin companion of markedly up-regulated expression of pathogenesis-related genes such as,and, while the level of salicylic acid was significantly increased in. In contrast, the over-expression lines exhibited a reduced level of H2O2and were much susceptible towith down-regulated expression of pathogenesis-related genes. These results suggested that OsSPL41 might negatively regulate plant immunity through the salicylic acid signaling pathway in rice.
bacterial blight; leucine-richrepeat receptor-like protein kinase; plant immunity; reactive oxygen species; rice; spotted leaf
Plant spotted-leaf mutants spontaneously generate lesions without obvious stress, mechanical wounding, pest damage or pathogen infection (Huang et al, 2010; Zhu et al, 2020). Since the spotted-leaf lesions mostly resemble hypersensitive response (HR) lesions and are often associated with the alteration of disease reaction, spotted-leaf mutants are valuable for the study of plant immunity. Although the genetic control of the spotted- leaf phenotype generally follows the Mendelian law of inheritance with a few exceptions (Wu et al, 2008), the causal genes for the huge phenotypic variations involve a large variety of biological processes such as chlorophyll metabolism (Sun et al, 2011), cell cycle (Huang et al, 2016; Shi et al, 2019), ubiquitination (Zeng et al, 2004; Liu et al, 2015), hormone metabolism (Yuan et al, 2007), and reactive oxygen species (ROS) homeostasis (Shang et al, 2022), indicating the complexity of lesion formation in plants.
In rice (L.), nearly a hundred of spotted-leaf mutants have been identified and more than 30 relevant genes have been cloned (Huang et al, 2010; Zhu et al, 2020). The Sekiguchi lesion () mutant is the first reported rice spotted-leaf mutant with enhanced resistance to(Arase, 2005) and the causal gene (or) encodes a cytochrome P450 monooxygenase that catalyzes 5-hydroxylation of tryptamine to synthesize serotonin, suggesting thatplays a role in rice innate immunity mediated by the RacGTPase pathway (Fujiwara et al, 2010). Interestingly, the rice,,,,,(t) andare a few spotted-leaf mutants without detectable enhanced disease resistance but with varied expression levels of defense response genes (Yin et al, 2000; Yamanouchi et al, 2002; Campbell and Ronald, 2005; Chen et al, 2010). However, it should be noticed that the observation was achieved on the base of limited number of pathogens tested. Furthermore,is a unique rice mutant without detectable cell death at/around the spots which are considered due to the accumulation of unknown pigments (Huang et al, 2011).
Many enzymes including leucine-rich repeat receptor- like protein kinase (LRR-RLK) modulate the lesion formation and immunity in rice. Besides SL, SPL11 encodes an E3 ubiquitination ligase (Zeng et al, 2004), which interacts with SPIN6 for its degradation via the 26S proteasome-mediated pathway (Liu et al, 2015). SPIN6, a Rho GTPase-activating protein, is able to catalyze the GTP-bound OsRac1 into GDP-bound state,thus regulating immunity and cell death in rice (Liu et al, 2015). In addition, SDS2 (SPL11 CELL DEATH SUPPRESSOR 2) phosphorylates SPL11, which in turn ubiquitinates SDS2 for degradation (Fan et al, 2018). Furthermore, SDS2 can phosphorylate the receptor-like cytoplasmic kinase OsRLCK118 and the phosphorylated-OsRLCK118 trans-phosphorylates OsRbohB, leading to ROS generation and defense gene expression (Fan et al, 2018). SPIN6 and SDS2, two positive regulators of immunity, are highly accumulated and result in spontaneous HR-like cell death and enhanced resistance in rice(Liu et al, 2015; Fan et al, 2018). It has been reported that the mitogen-activated protein kinase kinase kinase(MAPKKK), SPL3/OsEDR1 (enhanced disease resistance 1)/OsACDR1 (accelerated cell death and resistance 1), modulates HR-like lesion formation (Kim et al, 2009; Shen et al, 2011; Wang et al, 2015). Firstly, loss-of- function ofactivates immunity and cell death in rice likely associated with the alteration of hormone levels in the mutants (Shen et al, 2011; Wang et al, 2015). Secondly, over-expression ofin rice confers spontaneous HR-like cell death and enhanced disease resistance (Kim et al, 2009). However, the mechanism of this MAPKKK- regulated cell death and immunity has yet to be further characterized.
In the present study, we reported the identification of a novel() in rice. We showed that an 8-bp insertion in the coding region ofwas responsible for the mutant phenotype. The enhanced resistance topv.() was associated with markedly up-regulated expression of pathogenesis-related genes such as,and, as well as increased level of salicylic acid (SA) in. In contrast, over-expression ofincreased the susceptibility towith reduced level of H2O2, suggesting thatmight negatively regulate plant immunity in rice.
RESULTS
OsSPL41 encodes a novel LRR-RLK protein
The mutation ofwas previously delimited in a 147-kb region on the long arm of rice chromosome 4 (Li et al, 2015). To pinpoint the target locus, we carried out the whole genome sequence of IR64 and. Sequence comparison analysis in the candidate region showed that only an 8-bp (5ʹ-CTGGCGAA-3ʹ) insertion at position 865 bp was detected in the open reading fragment ofin the mutant (Fig. 1-A). Therefore,is likely the candidate gene responsible for the spotted-leaf phenotype. To validate the result, we carried out a complementation test by introducing the wild type (WT) allele ofinto embryogenic calli derived frommature seeds (Fig. 1-B), and a total of 12 positive transformants were obtained. Among them, 11 transformants displayed a spotted-leaf phenotype similar to WT (Fig. 1-C and -D). In addition, the contents of photosynthetic pigments such as chlorophyll and carotenoid in the complementary lines recovered to the WT levels as well (Fig. 1-E). Furthermore, the performance of major agronomic traits including plant height, the number of effective panicles per plant, panicle length, the number of grains per panicle, seed-setting rate and 1000-grain weight in the complementary lines was similar to WT (Fig. S1), and the photosynthetic parameters of the complementary lines recovered to the WT levels (Fig. S2). Taken together, the results suggested thatwas the causal gene responsible for the mutant phenotype.
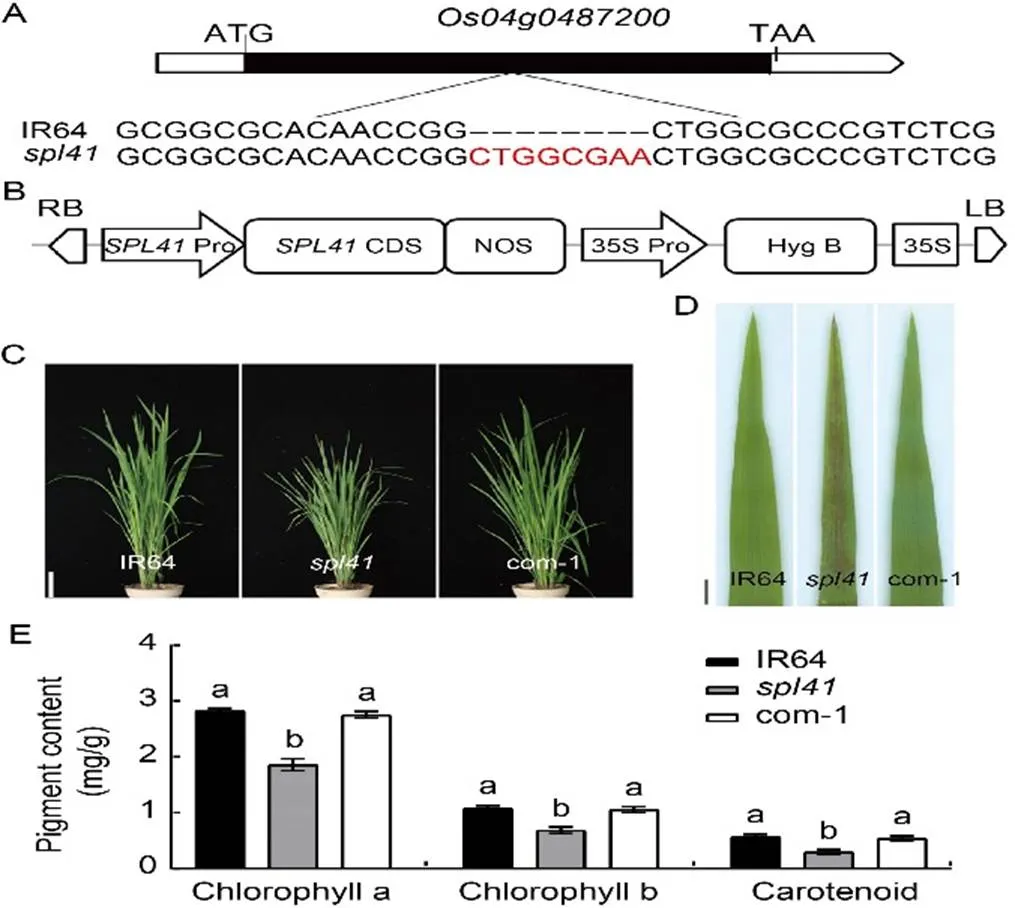
Fig. 1. Identification and functional validation of.
A, An 8-bp insertion (red letters) was detected in the mutant allele of. ATG and TAA refer to initiation codon and termination codon, respectively.
B, Structure of complementary construct p1300-OsSPL41-com. RB, Right border; LB, Left border; Pro, Promoter; CDS, Coding sequence; Hyg, Hygromycin; NOS, Terminator.
C, Phenotypes of IR64,and complementary line (com-1) at the tillering stage. Scale bar, 10 cm.
D, Leaf phenotypes of IR64,and complementary line (com-1) at the tillering stage. Scale bar, 1 cm.
E, Contents of chlorophyll a, chlorophyll b and carotenoid in IR64,and complementary line (com-1) at the tillering stage.
Based on the annotation in the RAP-DB database (https:/rapdb.dna.affrc.go.jp),encodes a putative serine leucine-like and LRR-RLK (tentatively termed as OsRLK41 or OsSPL41), containing 622 amino acid residuals with a molecular weight of 66 kDa (Fig. S3). The 8-bp insertioncaused a 19 amino acid substitution immediately after the insertion position and terminated at position 310 bp, leading to a putative truncated protein defective in the intracellular domain (Fig. S3). The WT allele ofin IR64 (ssp.) consists of 2 893 bp including an intronless 1 869 bp coding sequence, which is identical to the Nipponbare (ssp.) allele. OsSPL41 consists of an extracellular leucine rich repeat N-terminal (LRRNT) domain, an extracellular leucine rich repeat (LRR) domain, a transmembrane (TM) domain, and an intracellular protein kinase (PK) domain.
We analyzed and compared the homologous protein sequences of SPL41 from,,,,,,,and. Among these species, OsSPL41 has the similarity with the homologues ranged from ObSPL41 (91%), ZmSPL41 (89%), SiSPL41 (84%), SbSPL41 (82%), EgSPL41 (66%), CgSPL41 (65%), VvSPL41 (56%) and AtSPL41 (51%), indicating that SPL41 is highly conservative in plants especially in monocots (Fig. S4-A). Phylogenetic analysis showed that SPL41 homologues can be classified into monocot and dicot independent groups, indicating that SPL41 may have conserved unique functions in monocots, and gene divergence may have occurred after the evolution of monocots and dicots (Fig. S4-B).
Altered physiological/biochemical reactions related to ROS scavenging system in spl41
Lesion formation is mostly associated with the dysfunction of ROS scavenging system (Nanda et al, 2010). To validate it, we measured the contents of malondialdehyde (MDA), H2O2and soluble proteins, as well as the activities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD) and ascorbate peroxidase (APX) in the WT IR64, mutantand complementary lines. The results showed that the H2O2contents of WT and the complementary lines were similar, but was increased markedly in(Fig. 2-A). The activities of CAT, SOD, APX and POD were similar in WT and the complementary lines, but were significantly different from those of(Fig. 2-B to -E), indicating the disruption of homeostasis of ROS scavenging system in. The level of soluble proteins recovered to the WT level in the complementary lines whereas markedly decreased in the mutant (Fig. 2-F). Furthermore, The MDA content ofwas notably higher than those of WT and the complementary plants (Fig. 2-G), indicating a higher degree of membrane lipid peroxidation in the mutant. Taken together, the mutation resulted in the abruption of ROS scavenging system, accumulations of H2O2and MDA, HR-like cell death and lesion formation in.
OsSPL41 negatively regulates disease resistance to Xoo
In the transformation of the over-expression construct p1300-UBI-OsSPL41, a total of 10 transformants were obtained. The over-expression lines displayed normal green leaves and growth status similar to the WT Nipponbare (Fig. 3-A and -B). However, the expression ofwas notably increased in the over-expression lines compared with WT (Fig. 3-C). Two representative over-expression lines (OE3 and OE5) with similar growth status to WT were selected for further study on relevant physiological parameters. The results showed that the contents of H2O2and MDA were significantly decreased in the over-expression lines compared with WT (Fig. 3-D and -E). The activities of CAT in the over-expression lines were markedly higher than that of WT, while the activities of SOD in the over-expression lines were significantly lower than that of WT (Fig. 3-F and -G).
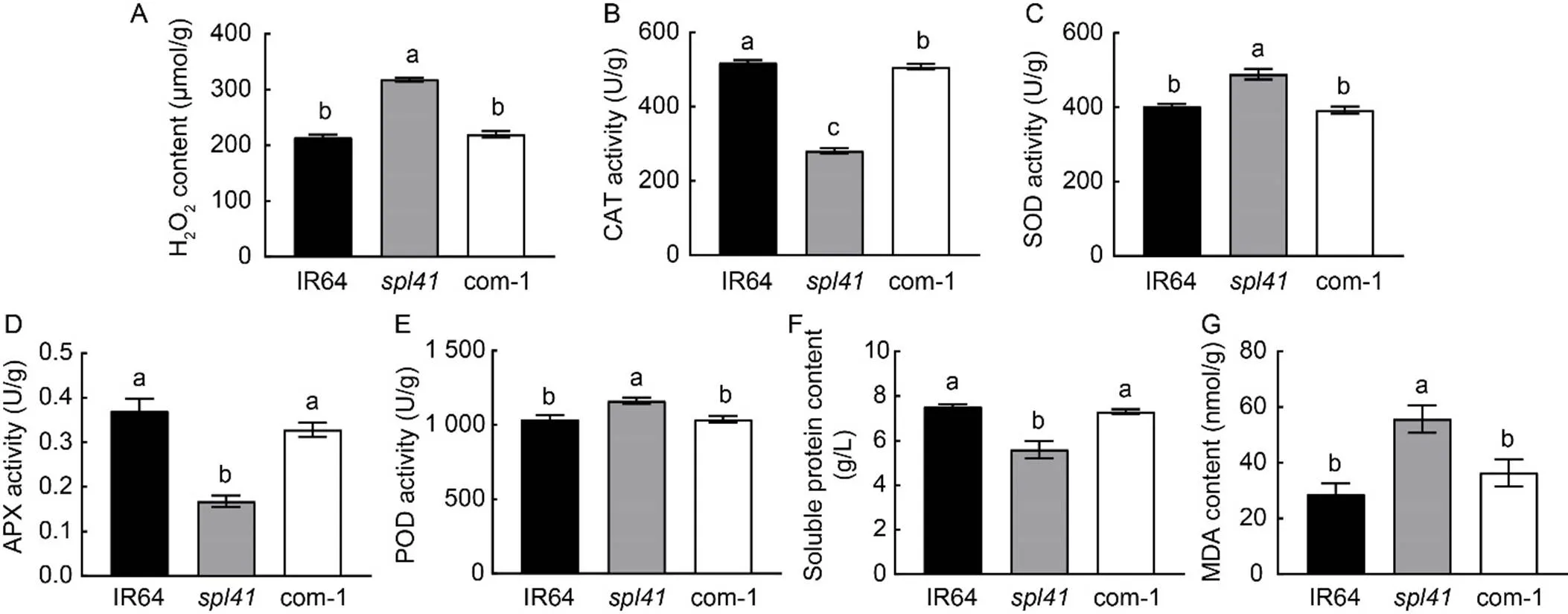
Fig. 2. Physiological parameters in IR64,and complementary line (com-1).
A‒G,Hydrogen peroxide (H2O2) content (A), catalase (CAT) activity (B), superoxide dismutase (SOD) activity (C), ascorbate peroxidase (APX) activity (D), peroxidase (POD) activity (E), total soluble protein content (F) and malondialdehyde (MDA) content (G) in IR64 (wild type),and complementary line (com-1). Data are Mean ± SD (= 3). Different lowercase letters above the bars indicate significant differences by the Duncan’s multiple test (< 0.05).
To determine the effects of over-expression ofon the level of disease resistance, the WT Nipponbare and the over-expression lines (OE3 and OE5) were inoculated with a bacterial blight pathogen race PXO280 at the tillering stage. The results showed that both OE3 and OE5 displayed longer lesion length than WT (Fig. 4-A), suggesting the weakened plant disease resistance. Furthermore, the over-expression lines exhibited significantly down-regulated expression of several important pathogenesis-related genes such as,andcompared with WT(Fig. 4-B). The results suggested that over-expression ofincreased disease susceptibility and OsSPL41 negatively regulated defense responses toin rice.
OsSPL41 regulates programed cell death in rice
To determine whether cell death presented at/around the lesions, we first carried out trypan blue staining on WT IR64,and complementary lines at the late- tillering stage. The results showed that a large number of blue staining was detected at/around the lesions on the leaves of, but only a small number of blue staining was observed on the leaves of WT and the complementary plants (Fig. 5-A). To determine whether any DNA fragmentation occurred in the mutant, we performed a TDT-mediated dUTP Nick End Labeling (TUNEL) assay on IR64 and. Compared with IR64,showed stronger green signals after staining (Fig. 5-B), indicating that severe DNA fragmentation did occur in the mutant. To verify the expression of metacaspase (MC) family-related genes inleaves, we performed qRT-PCR analysis on a set of eight MC genes associated with programmed cell death. The results showed that the relative expression levels of,,andwere significantly up-regulated while the relative expression levels of,andwere markedly down-regulated incompared with WT, indicating that the expression of programed cell death related MC family genes was interrupted in(Fig. 6-C). Taken together, the results demonstrated that OsSPL41 was likely involved in the regulation of programed cell death in rice.
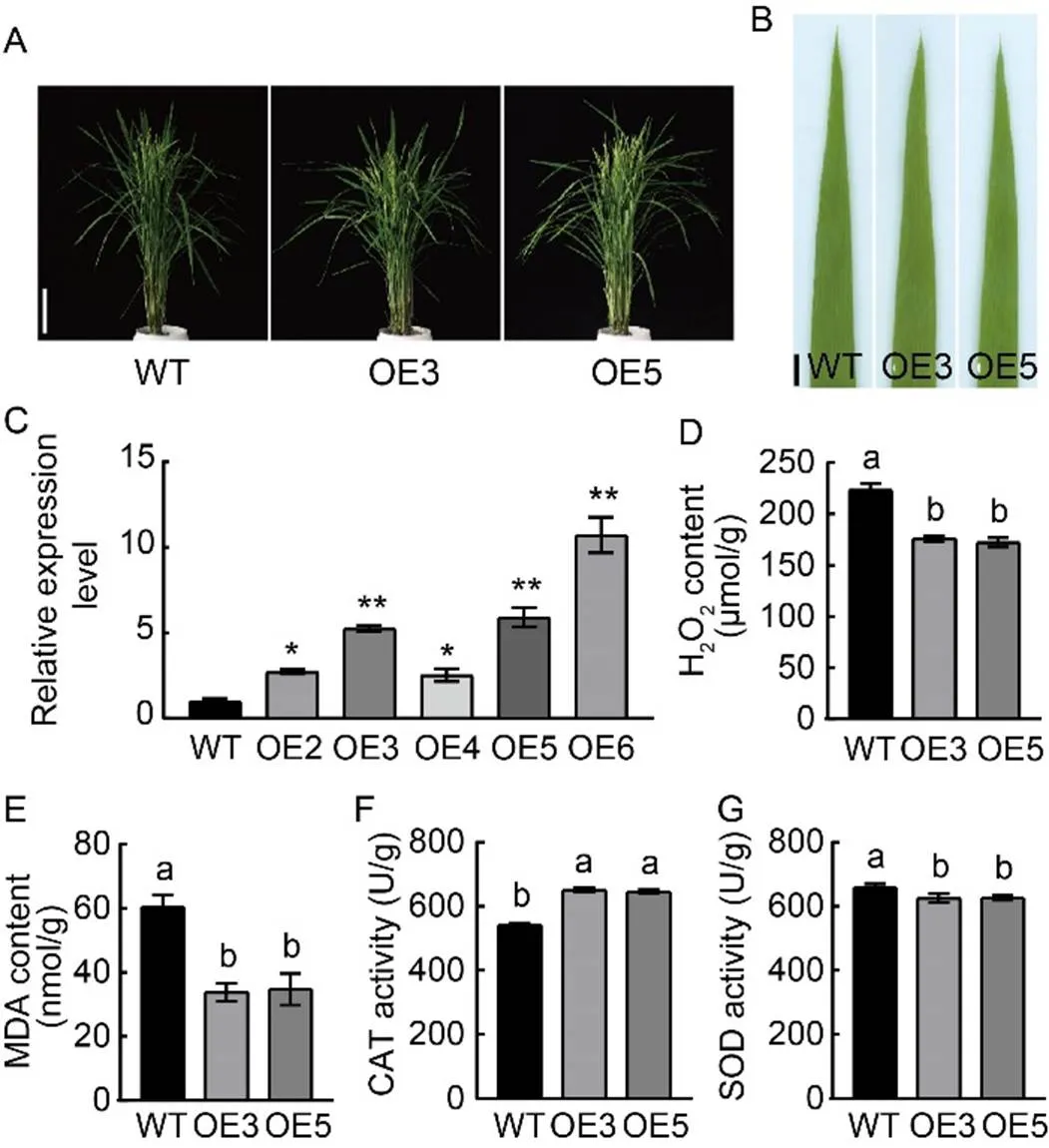
Fig. 3. Characterization of over-expression lines at the heading stage.
A, Phenotypes of wild type (WT) Nipponbare and over-expression lines OE3 and OE5. Scale bar, 15 cm.
B, Leaf phenotypes of WT and over-expression lines OE3 and OE5. Scale bar, 1 cm.
C, Relative expression ofin WT and over-expression lines. * and ** indicate significant differences at< 0.05 and< 0.01 by the Student’s-test, respectively. The first leaves from the top were sampled at the tillering stage.gene was used as a reference gene.
D‒G, Contents of hydrogen peroxide (H2O2) (D) and malondialdehyde (MDA) (E) as well as activities of catalase (CAT) (F) and superoxide dismutase (SOD) (G) in WT and over-expression lines (OE3 and OE5).
Data are Mean ± SD (= 3). Different lowercase letters above the bars indicate significant differences by the Duncan’s multiple test (< 0.05).
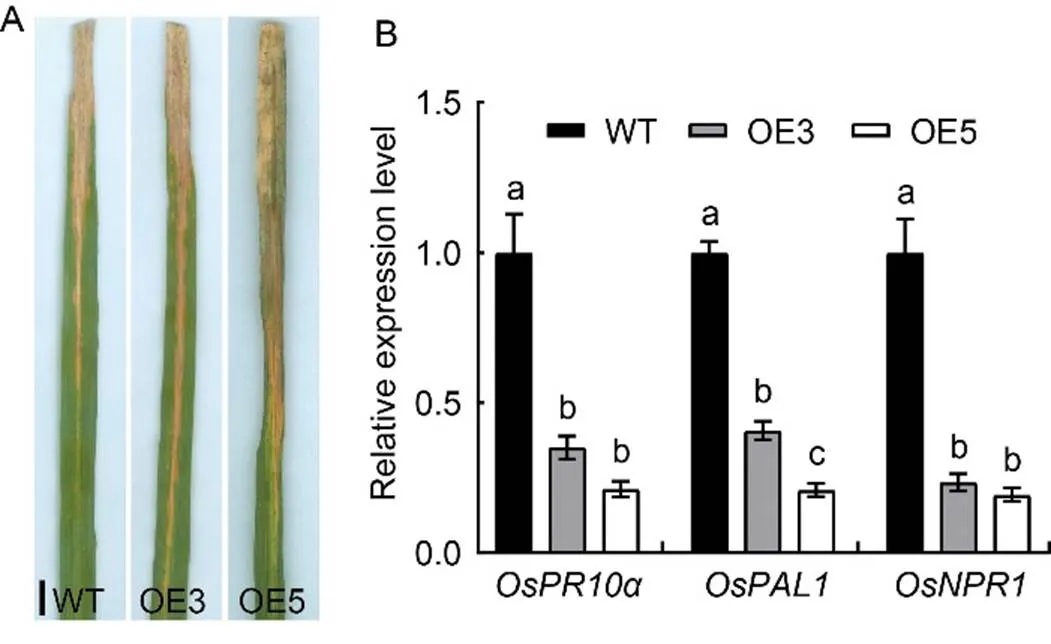
Fig. 4. Responses ofover-expression to bacterial blight pathogen race PXO280.
A, Lesion length in reaction to bacterial blight race PXO280. Scale bar, 1 cm.
B, Relative expression of defense genes in Nipponbare (Wild type, WT) and over-expression lines (OE3 and OE5) inoculated with bacterial blight race PXO280. The first leaves from the top sampled at the tillering stage were used for the gene expression analysis.gene was used as a reference gene. Data are Mean ± SD (= 3). Different lowercase letters above the bars indicate significant differences by the Duncan’s multiple test (< 0.05).
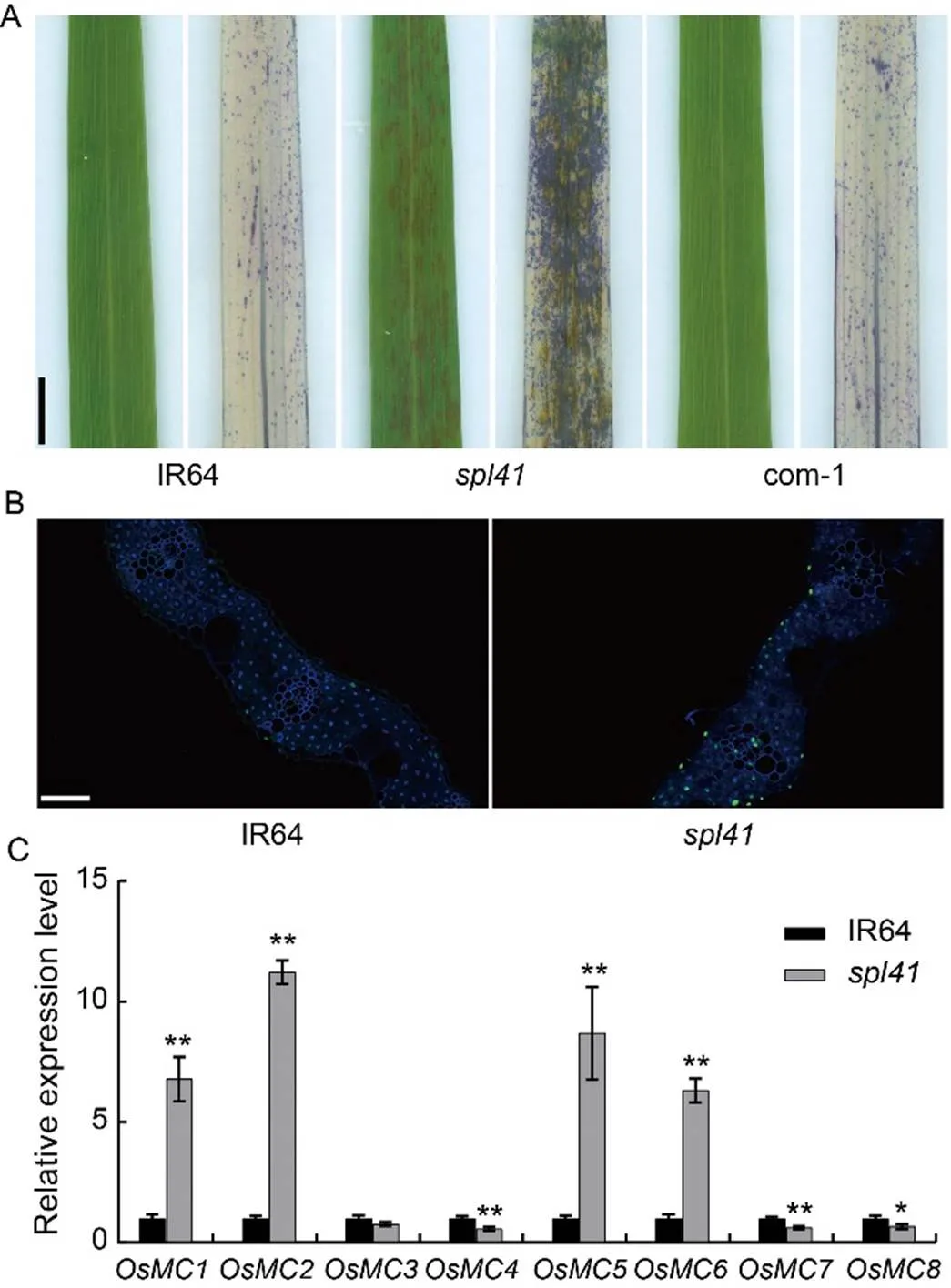
Fig. 5. Histochemical and gene expression analysis of cell death.
A, Trypan blue staining of IR64 (left, before staining; right, after staining),(left, before staining; right, after staining) and complementary plants (left, before staining; right, after staining) at the late-tillering stage. Scale bar, 1 cm.
B, TDT-mediated dUTP Nick End Labeling (TUNEL) assay of IR64 andleaves, blue fluorescence represents 4’,6-diamino-phenylindole (DAPI) staining, green fluorescence represents positive TUNEL signal. Scale bar, 50 µm.
C, Relative expression levels of metascaspase (MC) genes by qRT- PCR. The first leaves from the top sampled at the tillering stage were used for the gene expression analysis.gene was used as a reference gene. Data are Mean ± SD (= 3). * and ** represent significant differences at< 0.05 and< 0.01 by the Student’s-test, respectively.
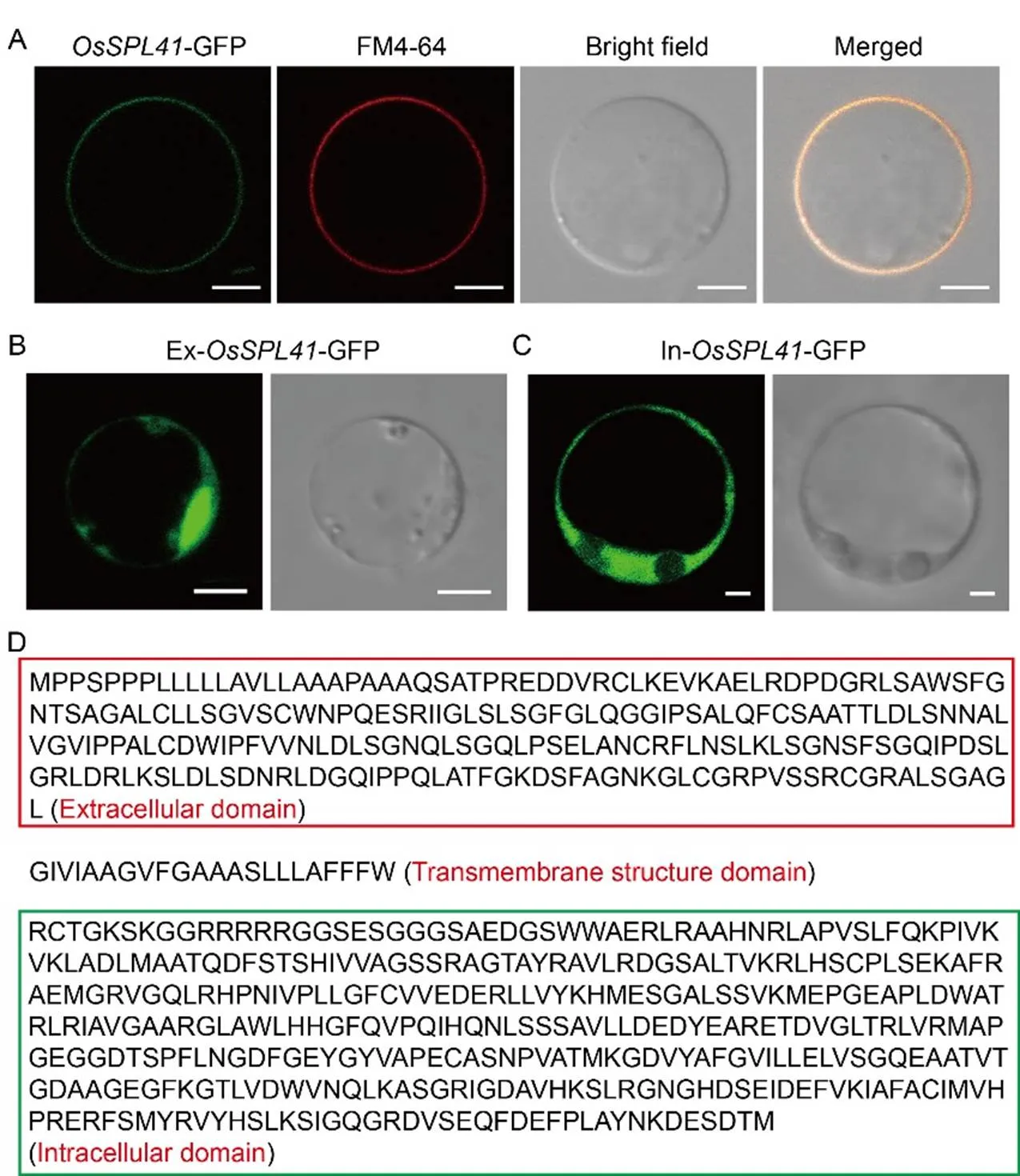
Fig. 6. Subcellular localization of OsSPL41.
A, Subcellular localization of OsSPL41-GFP fusion proteins. FM4-64, Cell membrane specific dye. Scale bar, 5 µm.
B, Subcellular localization of extracellular domain. Scale bar, 5 µm.
C, Subcellular localization of intracellular domain. Scale bar, 2 µm.
D, Structural identification of OsSPL41 amino acid sequence. Red and green boxes indicate the extracellular and intracellular domains, respectively.
OsSPL41 localizes to plasma membrane and is widely distributed
OsSPL41 possesses a typical transmembrane domain and is likely localized to the plasma membrane (Zhu et al, 2020). To validate it, we introduced the construct p1132-OsSPL41-sub into rice protoplasts and stained with the membrane specific dye FM4-64. The results showed that the OsSPL41-GFP fusion proteins localized to the plasma membrane (Fig. 6-A). In contrast, it was no longer localized to the plasma membrane when only the extracellular domain or the intracellular domain was retained, indicating that the transmembrane domain was critical to subcellular localization of OsSPL41 in rice (Fig. 6-B to -D). To determine the spatial-temporal expression pattern of, we performed the β-glucuronidase (GUS) transient expression assay on various tissues of Nipponbare and qRT-PCR on different tissues of IR64 at the heading stage. In the transient assay, GUS signals were detected in all the examined tissues including roots, stems, leaves, sheaths and spikelets (Fig. 7-A). For qRT-PCR, the expression ofwas also observed in all the tested tissues similar to the GUS transient assay, but with the highest relative expression in the sheaths (Fig. 7-B). The results demonstrated thatwas constitutively expressed in rice.
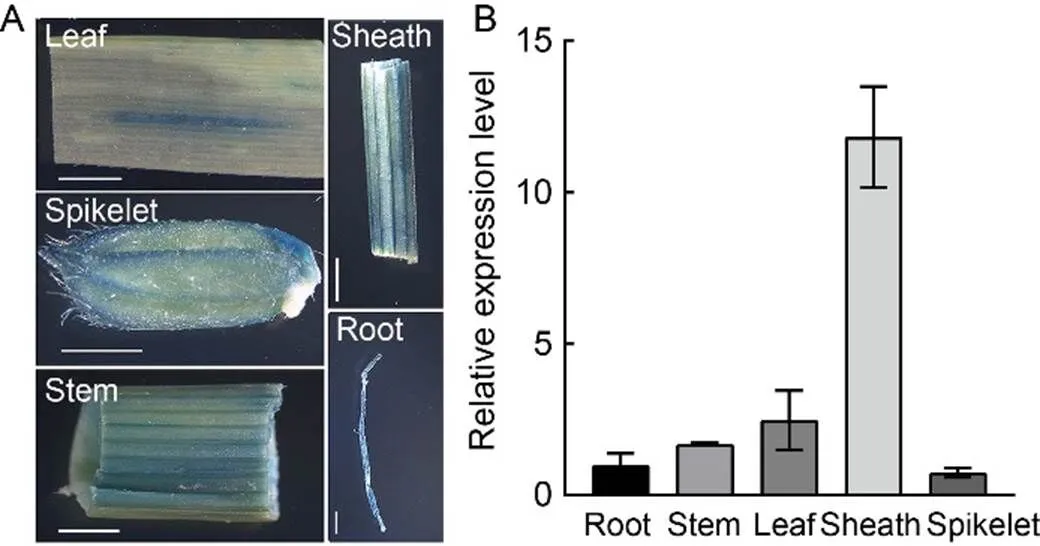
Fig. 7. Spatial-temporal expression pattern of.
A, β-glucuronidase (GUS) transient expression driven bypromoter in root, stem, leaf, sheath and spikelet of Nipponbare. Scale bar, 0.2 cm.
B, Relative expression levels ofin roots, stems, leaves, sheaths and spikelets of IR64 at the heading stage.gene was used as a reference gene. Data are Mean ± SD (= 3).
Enhanced resistance is associated with activation of SA signaling pathway in spl41
Spotted-leaf mutants normally exhibit enhanceddisease resistance with up-regulated expression of defense genes (Zhang et al, 2018). To characterize it, we evaluated the disease responses of WT IR64,and the com- plementary lines to three races (PXO280, PXO339 and NX42) ofat the tillering stage. The results showed that the lesion lengths of WT and the complementary lines inoculated with the three races were similar but significantly longer than that of(Fig. 8-A to -B), indicating thatgained a higher level of resistance to the races than WT. To confirm whether this elevated resistance was associated with the expression of defense genes, we determined the expression of a set defense response- related genes. The results exhibited that the relative expression levels of,,,,,, andinwere all up-regulated significantly compared with WT (Fig. 8-C). To further determine whether the enhanced resistance was associated with the internal level of plant hormones, we measured the contents of SA andjasmonic acid (JA) in WT and. The results indicated that the level of SA inwas markedly increased, in contrast, the level of JA was prominently decreased incompared with WT (Fig. 8-D), indicating a typical antagonistic function between the SA andJA pathways for OsSPL41-mediated resistance to. Our results demonstrated that the enhanced resistance oftowas associated with the activation of defense response genes and the activation of SA signaling pathway in rice.
DISCUSSION
In the present study, we identified and characterized a novel loss-of-function mutation resulted from an 8-bp insertion by diepoxybutane mutagenesis of an eliterice variety IR64. The mutationwas genetically recessive and stable, and introduction of the WT alleleinto the mutant-derived calli by functional complementation was able to restore the mutant both phenotypically and physiologically to the WT level. Because of this, we did not perform the other strategies such as RNAi or CRISPR/Cas9 for further validation. Like many spotted-leaf mutants, the main characteristic ofwas the formation of HR-like purple-brown lesions on the leaves without foreign pathogen invasion. The formation oflesions is also light-dependent (Li et al, 2015), but it has not yet been determined whether the lesion formation is affected by other abiotic stresses such as temperature (Yamanouchi et al, 2002), humidity (Jambunathan et al, 2001) and chemical wounding (Campbell and Ronald, 2005). In addition, how light plays a role inlesion initiation also remains as a mystic (Li et al, 2015). It is known that, in many cases, the necrotic or HR-like lesions are directly associated with the accumulation of a certain type of ROS and the disruption of ROS scavenging network (Sathe et al, 2019). In fact, the accumulation of H2O2is most often observed in many spotted-leaf or lesion mimic mutants, while in our case, the accumulation of both H2O2and O2·̄ was observed and could be the direct cause for lesion formation (Li et al, 2015) and programed cell death as evidenced by markedly up-regulated expression of pathogenesis-related genes such asand,and as well as the up/down regulated expression of multiple metacaspase genes in.
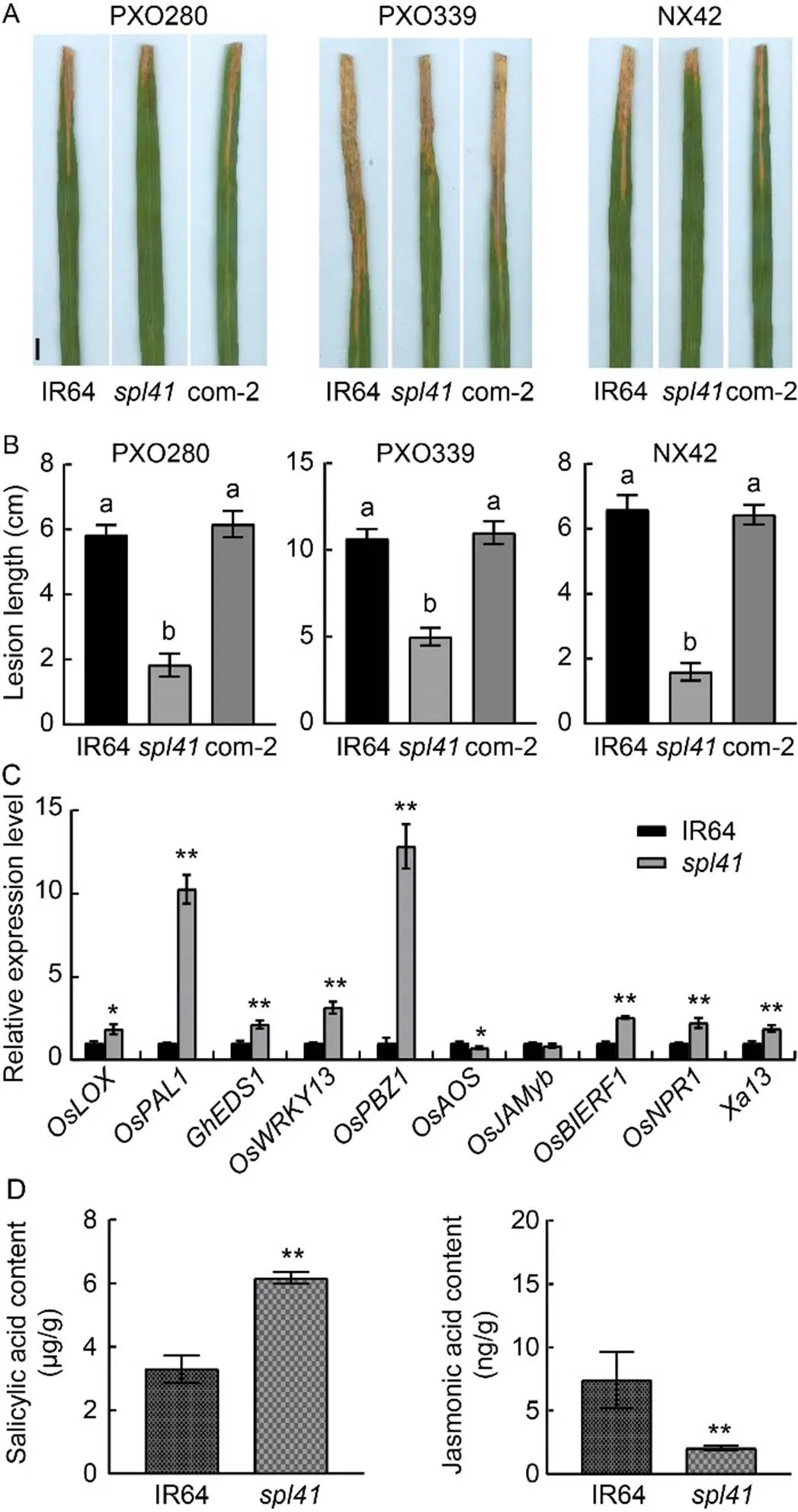
Fig. 8. Disease evaluation, gene expression and hormone levels in IR64 and.
A, Phenotypes of IR64 (wild type),and complementary line (com-2) inoculated with bacterial blight races PXO280, PXO339 and NX42 at 14 d after inoculation. Scale bar, 1 cm.
B, Lesion lengths of IR64,and complementary line (com-2) inoculated with bacterial blight races PXO280, PXO339 and NX42 at 14 d after inoculation.
C, Relative expression levels of defense genes in IR64 and. The first leaves from the top sampled at the tillering stage were used for the gene expression analysis.gene was used as a reference gene.
D, Hormone levels in IR64 and.
Data are Mean ± SD (= 3). Different lowercase letters above the bars indicate significant differences by the Duncan’s test (< 0.05). * and ** represent significant differences at< 0.05 and< 0.01 by the Student’s-test, respectively.
Different mutagens produce different types of genetic lesions. Diepoxybutane is predicted to generate point mutations and small deletions around one kilobase (Recio et al, 2001). Ethyl methane suphonate creates point mutations with G/C to A/T transitions randomly distributed in the genome (Greene et al, 2003). Deletions from one to several base pairs might not be very often observed in ethyl methane suphonate mutagenesis (Shi et al, 2000). In the present study, we would consider that the 8-bp insertion by diepoxybutane mutagenesis might be a rare event in contrast to point mutation detected in. Nevertheless, the insertion mutation inled to the loss-of-function ofthat negatively regulated disease resistance to.
encodes a putative LRR-RLK with a typical extracellular domain, a transmembrane domain and an intracellular domain (Wang et al, 2008). RLKs-mediated immunity firstly occurs with the perception of pathogen associated molecular patterns by the host’s cell surface- localized pattern recognition receptors (PRRs) including RLKs (Wu and Zhou, 2013; Macho and Zipfel, 2014). Since OsSPL41 belongs to a putative LRR-RLK type of PRRs, it is expected that the extracellular domain is required for potential ligand binding (Zipfel et al, 2006). Therefore, on one hand, the identification of potential ligands for OsSPL41 becomes important in elucidation of OsSPL41-mediated immunity in the coming future. On the other hand, the insertion occurred in the intracellular domain, generating a 19 amino acid substitution immediately after the insertion site and terminated at position 310 bp, leading to a putative truncated protein supposedly defective in the kinase function. Because the intracellular kinase domain involves in signal transduction to activate the downstream signaling cascade (Macho and Zipfel, 2014), it provides us immediately in search for OsSPL41-interacting factors/proteins that are required for downstream intracellular signaling (Lu et al, 2010; Zhang et al, 2010; Yamaguchi et al, 2013).
Most spotted-leaf mutants exhibit various levels of enhanced resistance to bacterial and fungal pathogens (Zhu et al, 2020). The enhanced disease resistance is generally associated with the up-regulation of multiple defense response genes and elevated levels of phyto- hormones (He et al, 2017; Zhang et al, 2018). In the present study, the endogenous level of SA inwas markedly increased while the level of JA was apparently decreased. In addition, the resistance ofto multiple races ofwas significantly enhanced with the up-regulation of multiple pathogenesis- related genes involved in SA signaling pathway and the down-regulation of pathogenesis-related genes in JA signaling pathway. This observation is consistent to the accepted antagonistic relationship between SA and JA (He et al, 2017; Zhang et al, 2018). Thus, we concluded that the OsSPL41-mediated resistance towas associated with the activation of SA signaling pathway in rice.
It has been shown that a large number of genes have pleotropic effects (Qiao et al, 2010). For example, mutations innot only increase resistance to, but also lead to reduction in yield and grain quality (Qiao et al, 2010). The dominant alleleis required for bacterial growth and pollen development (Chu et al, 2006). In the present study, it is worth noting that the expression ofwas significantly activated inat the tillering stage. Unlike the other activated PR genes such asand, which are thought likely to be associated with enhanced disease resistance, the activation of the major geneat the tillering stage ofseems to mimic the need for bacterial growth since IR64 andboth carry the dominantallele (Chu et al, 2006). This is also consistent to the increased susceptibility whenwas over- expressed. Furthermore,, an allele of, is involved in rice leaf angle regulation via positive regulation of brassinosteroid signaling, indicating thatmay play multiple roles in rice (Song et al, 2022). In conclusion, OsSPL41-mediated bacterial disease resistance is not only highly similar to HR with the ROS burst, the subsequent activation of PR genes and the elevated level of SA, but also imposes a huge negative impact on the growth and development in rice. Our current data would facilitate further characterization of OsSPL41-mediated molecular mechanism of disease resistance toin rice.
METHODS
Rice materials
The wild type IR64 and its mutant(previously named as) (Li et al, 2015) were grown in the paddy field under the natural conditions at the China National Rice Research Institute. The transgenic plants including complementary and over-expression lines were grown in the greenhouse.
Agronomic trait evaluation
The agronomic traits including plant height, the number of effective panicles per plant, panicle length, the number of grains per panicle, seed-setting rate and 1000-grain weight of IR64,and complementary lines were evaluated at the maturity stage. One plant per replicate was analyzed, and the means from three replicates were subjected to one-way analysis of variance and Duncan’s multiple test (< 0.05).
Cell death detection
Fresh leaves were taken from IR64,and the comp- lementary lines at the late-tillering stage. Trypan blue staining was performed as described by Yin et al (2000). The pictures were recorded using a HP ScanJet G4010 scanner (HP, Shanghai, China). The TUNEL assay was performed by using a FluoresceinCell Death Detection Kit following the manufacturer’s instructions (Roche, Basel, Switzerland). The methods used for sectioning and fluorescence labeling were described as previously reported (Huang et al, 2007).
Determination of photosynthetic pigments and parameters
Chlorophyll (Chl) a and b contents of IR64,and the complementary lines were measured at the tillering stage as described by Arnon (1949), while the carotenoid contents from the same leaves at the tillering stage were determined following the method described by Wellburn (1994). The photosynthetic parameters including the net photosynthetic rate [, μmol/ (m2·s)], intercellular CO2concentration (, μmol/mol) and transpiration rate [, mol/(m2·s)] were determined as described previously (Huang et al, 2016). The means from three measurements were used for analysis.
Determination of ROS-related parameters
Fresh leaves of IR64,, complementary and over-expression lines as well as Nipponbare (the wild type of over-expression lines) plants were collected at the tillering stage. The contents of MDA and total soluble proteins, as well as the activities of ROS scavenging enzymes including POD, SOD, CAT and APX were determined following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The H2O2content was determined using the H2O2assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The means from three measurements were used for analysis.
Extraction and determination of hormones
The extraction of plant hormones at the tillering stage was described previously (Zhang et al, 2018), and the levels of JA and SA were determined by the Zoonbio Biotechnology Co., Ltd, China.
Disease evaluation
Reaction of the WT IR64, mutantand complementary lines to the bacterial blight pathogen was evaluated using three races (PXO280, PXO339 and NX42) of. The over- expression lines were inoculated only with race PXO280. All rice lines were inoculated at the maximum tillering stage using the leaf clipping method (Kauffman et al, 1973). The lesion length was measured using a transparent plastic ruler at 14 d after inoculation. The means of lesion length from three inoculated leaves were used for analysis.
Vector construction, transformation and qRT-PCR
Total RNA was extracted using a NucleoZOL reagent kit (Machereynagel, Düren, Germany) according to the manufacturer’s instructions. For the functional complementary test, a 8 410-bp genomic sequence ofincluding 3 941 bp promoter region, 2 839 bp genomic sequence and 1 630 bp downstream sequence was amplified by TaKaRa PrimeSTAR®Max Polymerase (TaKaRa Biomedical Technology Co., Ltd, Beijing, China) from IR64 and cloned into a binary vector pCAMBIA1300 to form a new construct, p1300-OsSPL41-com, which was then introduced into embryogenic calli induced frommature seeds using the- mediated transformation method (Hiei and Komari, 2008). For the subcellular location and over-expression, the first strand cDNA was synthesized from 1 µg total RNA by using TaKaRa PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa Biomedical Technology Co., Ltd, Beijing, China). The 1 869 bp full length cDNA ofwas amplified by the TaKaRa PrimeSTAR®Max Polymerase from 1 μL of the first strand cDNA template and inserted in front ofin the plasmid pYBA1132 (Zhang et al, 2019) and after the maizepromoter in the plasmid pCAMBIA1300-UBI, respectively. The resulting constructs, P1132-OsSPL41-suband p1300- UBI, were used as subcellular location vector and over-expression vector, respectively. The P1132-OsSPL41-sub vector was introduced into rice Zhongjian 100 protoplasts and GFP fluorescence signals were detected using a Zeiss lsm710 confocal laser scanning microscope (Carl Zeiss, Inc., Jena, Germany). The over-expression vector p1300-UBI-OsSPL41 was used to transform embryogenic calli induced from Nipponbare mature seeds using the-mediated method (Hiei and Komari, 2008). For the GUS transient assay, a 3-kb promoter ofwas amplified from the plasmid p1300- OsSPL41-comandinserted in front of thereporter in the plasmid pCAMBIA1381Z (Abcam plc., Shanghai, China), and the recombinant plasmid p1381Z-ProOsSPL41-GUS was introduced into the embryogenic calli induced from Nipponbare mature seeds following the-mediated method (Hiei and Komari, 2008). GUS staining was carried out on various organs/tissues of the transgenic plants at different growing stages using the GUS staining kit (Coolaber Science & Technology Co., LTD, Beijing, China) according to the manufacturer’s instructions. Images were photographed using a stereomicroscope (LEICA MC120 HD, Weztlar, Germany). qRT-PCR was performed in total of 20 µL reaction mixture containing 1 µL cDNA template, 10 µL 2× SYBR Green Master Mix enzyme (ABI, Shanghai, China), 2 µL forward and reverse primers, and 7 µL ddH2O, following by pre-denature at 95 ºC for 10 min, and 40 cycles of 95 ºC, 15 s; 60 ºC, 15 s; 72 ºC, 1 min on a Thermal Cycle Dice1 Real Time System (TaKaRa, Dalian, China). Ricegene () was used as an internal control. All assays were biologically repeated at least three times and the means were used for analysis. Primers used for vector construction and qRT-PCR are listed in Table S1.
Phylogenetic analysis
Blast analysis of the protein sequence of OsSPL41 was performed on the NCBI website (http://www.ncbi.nlm.nih.gov/) using the homologues of OsSPL41 from nine plant species including OsSPL41 (XP_015635807.1) from, ObSPL41 (XP_006653547.2) from, ZmSPL41 (XP_ 008669246.1) from, SbSPL41 (XP_002446698.1) from, SiSPL41 (XP_004978090.1) from, CnSPL41(KAG1331335.1) from, EgSPL41 (XP_010912844.2) from, AtSPL41 (NP_174039.1) from, and VvSPL41 (XP_003633815.1) from. DNAMAN v7 software (http://www.lynnon.com/) was used for multiple protein sequence alignment. The phylogenetic tree was constructed based on the neighbor-joining method provided by the MEGA v6 software (http://www.megasoftware.net/).
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 32072049) and the Central Public-Interest Scientific Institution Basal Research Fund, China (Grant No. CPSIBRF-CNRRI-202203). We thank Dr. He Yan at the China National Rice Research Institute for help during the experiments.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Performances of agronomic traits in IR64,and complementary lines.
Fig. S2. Photosynthetic parameters in IR64,and complementary lines.
Fig. S3. Comparison of amino acid sequence of SPL41 in IR64 and.
Fig. S4. Amino acid sequence alignment and phylogenetic analysis of SPL41 homologues.
Table S1. Primers used in this study.
Arase S. 2005. Studies on fungal pathogenicity and host resistance in rice blast disease using a lesion mimic mutant of rice., 71(6): 448–450.
Arnon D I. 1949. Copper enzymes in isolated chloroplasts. polyphenoloxidase in., 24(1): 1–15.
Campbell M A, Ronald P C. 2005. Characterization of four rice mutants with alterations in the defence response pathway., 6(1): 11–21.
Chen P P, Ye S H, Zhao N C, Lu Y T, Liu H J, Yang L, Jin Q S, Zhang X M. 2010. Characteristics and genetic mapping of a lesion mimic mutant(t) inrice variety Zhejing 22., 24(1): 1–6. (in Chinese with English abstract)
Chu Z H, Yuan M, Yao J L, Ge X J, Yuan B, Xu C G, Li X H, Fu B Y, Li Z K, Bennetzen J L, Zhang Q F, Wang S P. 2006. Promoter mutations of an essential gene for pollen development result in disease resistance in rice., 20(10): 1250–1255.
Fan J B, Bai P F, Ning Y S, Wang J Y, Shi X T, Xiong Y H, Zhang K, He F, Zhang C Y, Wang R Y, Meng X Z, Zhou J G, Wang M, Shirsekar G, Park C H, Bellizzi M, Liu W D, Jeon J S, Xia Y, Shan L B, Wang G L. 2018. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice., 23(4): 498–510.
Fujiwara T, Maisonneuve S, Isshiki M, Mizutani M, Chen L T, Wong H L, Kawasaki T, Shimamoto K. 2010. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice., 285(15): 11308–11313.
Greene E A, Codomo C A, Taylor N E, Henikoff J G, Till B J, Reynolds S H, Enns L C, Burtner C, Johnson J E, Odden A R, Comai L, Henikoff S. 2003. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in., 164(2): 731–740.
He X, Jiang J S, Wang C Q, Dehesh K. 2017. ORA59 and EIN3 interaction couples jasmonate-ethylene synergistic action to antagonistic salicylic acid regulation of PDF expression., 59(4): 275–287.
Hiei Y, Komari T. 2008.-mediated transformation of rice using immature embryos or calli induced from mature seed., 3(5): 824–834.
Huang L M, Sun Q W, Qin F J, Li C, Zhao Y, Zhou D X. 2007. Down-regulation of a- related histone deacetylase gene,, induces DNA fragmentation and cell death in rice., 144(3): 1508–1519.
Huang Q N, Yang Y, Shi Y F, Chen J, Wu J L. 2010. Spotted-leaf mutants of rice ()., 17(4): 247–256.
Huang Q N, Shi Y F, Yang Y, Feng B H, Wei Y L, Chen J, Baraoidan M, Leung H, Wu J L. 2011. Characterization and genetic analysis of a light- and temperature-sensitive spotted-leaf mutant in rice., 53(8): 671–681.
Huang Q N, Shi Y F, Zhang X B, Song L X, Feng B H, Wang H M, Xu X, Li X H, Guo D, Wu J L. 2016. Single base substitution inis responsible for premature senescence and death phenotype in rice., 58(1): 12–28.
Jambunathan N, Siani J M, McNellis T W. 2001. A humidity- sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance., 13(10): 2225–2240.
Kauffman H E, Reddy A, Hsieh S, Merca S. 1973. An improved technique for evaluating resistance of rice varieties to., 57: 537–541.
Kim J A, Cho K, Singh R, Jung Y H, Jeong S H, Kim S H, Lee J E, Cho Y S, Agrawal G K, Rakwal R, Tamogami S, Kersten B, Jeon J S, An G, Jwa N S. 2009. Rice(accelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance., 28(5): 431–439.
Li X H, Shi Y F, Zhang X B, Feng B H, Song L X, Wang H M, Xu X, Huang Q N, Guo D, Wu J L. 2015. Identification and gene mapping of a spotted-leaf mutantin rice., 29(5): 447–456. (in Chinese with English abstract)
Liu J L, Park C H, He F, Nagano M, Wang M, Bellizzi M, Zhang K, Zeng X S, Liu W D, Ning Y S, Kawano Y, Wang G L. 2015. The RhoGAP SPIN6 associates with SPL11 and OsRac1 and negatively regulates programmed cell death and innate immunity in rice., 11(2): e1004629.
Lu D P, Wu S J, Gao X Q, Zhang Y L, Shan L B, He P. 2010. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity., 107(1): 496–501.
Macho A P, Zipfel C. 2014. Plant PRRs and the activation of innate immune signaling., 54: 263–272.
Nanda A K, Andrio E, Marino D, Pauly N, Dunand C. 2010. Reactive oxygen species during plant-microorganism early interactions.,52(2): 195–204.
Qiao Y L, Jiang W Z, Lee J, Park B, Choi M S, Piao R H, Woo M O, Roh J H, Han L Z, Paek N C, Seo H S, Koh H J. 2010.encodes a clathrin-associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice ()., 185(1): 258–274.
Recio L, Steen A M, Pluta L J, Meyer K G, Saranko C J. 2001. Mutational spectrum of 1,3-butadiene and metabolites 1, 2-epoxybutene and 1, 2, 3, 4-diepoxybutane to assess mutagenic mechanisms., 135/136: 325–341.
Sathe A P, Su X N, Chen Z, Chen T, Wei X J, Tang S Q, Zhang X B, Wu J L. 2019. Identification and characterization of a spotted-leaf mutantwith enhanced bacterial blight resistance in rice., 12(1): 68.
Shang H H, Li P P, Zhang X B, Xu X, Gong J Y, Yang S H, He Y Q, Wu J L. 2022. The gain-of-function mutation,, positively regulates plant immunity in rice., 23(22): 14168.
Shen X L, Liu H B, Yuan B, Li X H, Xu C G, Wang S P. 2011. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis., 34(2): 179–191.
Shi H, Ishitani M, Kim C, Zhu J K. 2000. Thesalt tolerance geneencodes a putative Na+/H+antiporter., 97(12): 6896–6901.
Shi L, Zhang X B, Shi Y F, Xu X, He Y Q, Shao G S, Huang Q N, Wu J L. 2019. OsCDC48/48E complex is required for plant survival in rice (L.)., 100(1/2): 163–179.
Song Y J, Niu R F, Yu H L, Guo J, Du C H, Zhang Z L, Wei Y, Li J X, Zhang S Q. 2022. OsSLA1 functions in leaf angle regulation by enhancing the interaction between OsBRI1 and OsBAK1 in rice., 110(4): 1111–1127.
Sun C H, Liu L C, Tang J Y, Lin A H, Zhang F T, Fang J, Zhang G F, Chu C C. 2011., encoding a putative coproporphyrinogen III oxidase, is involved in lesion initiation in rice., 38(1): 29–37.
Wang G D, Ellendorff U, Kemp B, Mansfield J W, Forsyth A, Mitchell K, Bastas K, Liu C M, Woods-Tör A, Zipfel C, de Wit P J G M, Jones J D G, Tör M, Thomma B P H J. 2008. A genome-wide functional investigation into the roles of receptor- like proteins in., 147(2): 503–517.
Wang S H, Lim J H, Kim S S, Cho S H, Yoo S C, Koh H J, Sakuraba Y, Paek N C. 2015. Mutation of() impairs abscisic acid-responsive signalling and delays leaf senescence in rice., 66(22): 7045–7059.
Wellburn A R. 1994. The spectral determination of chlorophyllsand, as well as total carotenoids, using various solvents with spectrophotometers of different resolution., 144(3): 307–313.
Wu C J, Bordeos A, Madamba M R S, Baraoidan M, Ramos M, Wang G L, Leach J E, Leung H. 2008. Rice lesion mimic mutants with enhanced resistance to diseases., 279(6): 605–619.
Wu Y, Zhou J M. 2013. Receptor-like kinases in plant innate immunity., 55(12): 1271–1286.
Yamaguchi K, Yamada K, Ishikawa K, Yoshimura S, Hayashi N, Uchihashi K, Ishihama N, Kishi-Kaboshi M, Takahashi A, Tsuge S, Ochiai H, Tada Y, Shimamoto K, Yoshioka H, Kawasaki T. 2013. A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity., 13(3): 347–357.
Yamanouchi U, Yano M, Lin H X, Ashikari M, Yamada K. 2002. A rice spotted leaf gene,, encodes a heat stress transcription factor protein., 99(11): 7530–7535.
Yin Z C, Chen J, Zeng L R, Goh M, Leung H, Khush G S, Wang G L. 2000. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight., 13(8): 869–876.
Yuan Y X, Zhong S H, Li Q, Zhu Z R, Lou Y G, Wang L Y, Wang J J, Wang M Y, Li Q L, Yang D L, He Z H. 2007. Functional analysis of rice NPR1-like genes reveals thatis the rice orthologue conferring disease resistance with enhanced herbivore susceptibility., 5(2): 313–324.
Zeng L R, Qu S H, Bordeos A, Yang C W, Baraoidan M, Yan H Y, Xie Q, Nahm B H, Leung H, Wang G L. 2004., a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity., 16(10): 2795–2808.
Zhang J, Li W, Xiang T T, Liu Z X, Laluk K, Ding X J, Zou Y, Gao M H, Zhang X J, Chen S, Mengiste T, Zhang Y L, Zhou J M. 2010. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by aeffector., 7(4): 290–301.
Zhang X B, Feng B H, Wang H M, Xu X, Shi Y F, He Y, Chen Z, Sathe A P, Shi L, Wu J L. 2018. A substitution mutation inconfers bacterial blight resistance by activating the salicylic acid pathway., 60(2): 160–172.
Zhang Y, Gao Y H, Liang Y B, Dong Y J, Yang X F, Qiu D W. 2019.PevD1, an Alt a 1-like protein, targets cotton PR5-like protein and promotes fungal infection., 70(2): 613–626.
Zhu X B, Ze M, Chern M, Chen X W, Wang J. 2020. Deciphering rice lesion mimic mutants to understand molecular network governing plant immunity and growth., 27(4): 278–288.
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones J D G, Boller T, Felix G. 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts-mediated transformation., 125(4): 749–760.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.02.004
19 December 2022;
21 February2023
Wu Jianli (beishangd@163.com); Gong Junyi (gongjunyi@caas.cn)
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa
- Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth

