LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai
Research Paper
Encoding a J-Domain Protein Controls Heading Date in Rice
Liu Qiao1, #, Qiu Linlin1, #, Hua Yangguang1, Li Jing2, Pang Bo1, Zhai Yufeng1, Wang Dekai1
(College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, China; College of Life Science, University of Chinese Academy of Sciences, Beijing 100049, China; These authors contributed equally to this work)
Heading date is one of the most important agronomic traits of rice, which critically affects rice ecogeographical adaptation, yield and quality. In this study, a() mutant was screened from the60Co-γ irradiation mutant library. Thedelayed heading date in rice under both short day and long day conditions. Map-based cloning combined with Mutmap strategy was adopted to isolate the causalgene. Thegene encodes a DNA_J domain protein, which was ubiquitously expressed in various plant organs, and dominant expressed in stems and leaves. Subcellular localization analysis showed that LHD3 was localized to nucleus, indicating that LHD3 may interact with other elements to regulate the expression of flowering genes. The transcriptions of the heading activators,andsignificantly decreased in themutant, suggesting thatmay control the heading date through the/photoperiodic flowering pathway. The variation and haplotype analyses of the genomic region ofshowed that there were 7 haplotypes in theregion from 4 702 accessions. The haplotypes ofcan be divided into two classes: class a and class b, and the heading dates of these two classes were significantly different. Further study showed that two single nucleotide polymorphisms (SNPs), SNP10 (G2100C) in Hap II and SNP3 (C861T) in Hap VII, may be the functional sites causing early and late heading in accessions. Nucleotide diversity analysis showedhad been selected in thepopulation, rather than in thepopulation. Therefore, the present study sheds light on the regulation ofon heading date in rice and suggests thatis a novel promising new target for rice molecular design and breeding improvement.
rice;; heading stage; J-domain protein; photoperiod flowering pathway
Heading date (also called flowering time) is one of the most important agronomic traits in rice, which critically affects rice ecogeographical adaptation, yield and quality (Xing and Zhang, 2010; Yan et al, 2013). An appropriate heading date of rice will benefit vegetative growth, promote the accumulation of carbohydrates to the greatest extent and give full play to the maximum yield potential of rice (Jung and Müller, 2009; Koo et al, 2013). For example, early heading leads to limited vegetative growth during the growing season, and causes insufficient grain filling, and finally induces yield reduction in rice. Nevertheless, late heading during the growing season prolongs reproductive growth, which will be affected by lower temperature, and ultimately decreases rice yield (Andrés and Coupland, 2012). In addition, late heading of rice will delay the planting of subsequent crops and cause greater economic losses.
Rice is a short-day (SD) plant, whose flowering is generally promoted by SD conditions and inhibited by long-day (LD) conditions (Hayama et al, 2003). The heading date of rice is coordinated by endogenous and environmental factors, such as photoperiod, temperature, hormone and nutrient, of which the photoperiod pathway is well known and considered as a crucial factor (Roberts et al, 2018; Senguttuvel et al, 2021; Zhou et al, 2021). Up to now, numerous heading date- regulating genes have been functionally characterized in rice, and most of these genes regulate heading date by affecting the expression of two florigen genes:() and(). Therefore, heading date genes are generally grouped into two flowering signaling pathways:-mediated conservative evolutionarypathway andcentered monocotyledon specificpathway (Tamaki et al, 2007; Sun et al, 2014). In the/pathway, which is conserved in rice and,[an ortholog of()] receives signals fromand promotes/expression under SD, while it suppresses/expression by inhibitingtranscription under LD (Yano et al, 2000; Tsuji et al, 2011; Nemoto et al, 2016). In the/pathway,integrates various upstream regulator signals and promotes/under both LD and SD (Doi et al, 2004; Tsuji et al, 2011). The expression ofis up- regulated by several upstream positive regulators, such as(Lee et al, 2004),/(Matsubara et al, 2008; Wu et al, 2008),(Matsubara et al, 2011),(Gao et al, 2013),/(Matsubara et al, 2012; Zhao et al, 2012), and(Kim et al, 2007; Bian et al, 2011), while several negative regulators, such as(Peng et al, 2007),(Xue et al, 2008),(Yan et al, 2011),(Li et al, 2022),(Zhang et al, 2019),and(Chai et al, 2021), repress heading by preventingexpression.
The J proteins, also named as heat shock protein 40 kDa (HSP40s) or DNA_J proteins, are invariably associated with HSP70s and other chaperones to form ubiquitous types of molecular chaperone complex, which plays various functions in the growth and development process, as well as in response to biotic and abiotic stresses (Bukau et al, 2006; Kampinga and Craig, 2010; Craig and Marszalek, 2017). More than 100 J-domain proteinshave been found in thegenome and 115 putative J-domain proteins in rice (Lin et al, 2001; Rajan and D’Silva, 2009; Luo et al, 2019). Moreover, the biological functions of many J-domain proteins have been characterized. For example, riceencoding a DnaJ domain protein, is required for thermotolerance of pollen tubes in(Yang et al, 2009). AtJ3 regulates flowering time via interacting directly with short vegetative phase and binding to regulatory sequences of SOC1 and FT (Shen et al, 2011)., AtJ11 and AtJ20regulate stabilization of photosystem II (PSII) complexes under high light stress (Chen et al, 2010). GFA2protein inmitochondria is related to plant development (Christensen et al, 2002). AtJ1plays a crucial role in the regulation of seedling growth, flowering time and abscisic acid response (Park and Kim, 2014). Furthermore, DNAJ1 and DNAJ2 can form a complex with SUVH1 and SUVH3, and bind to methylated DNA sequences in promoters and enhance gene transcription in(Harris et al, 2018)., encoding an endoplasmic reticulum (ER)-resident J-protein, regulates ER quality in rice endosperm (Ohta and Takaiwa, 2020). AtDjC5 is involved in plant basal thermotolerance by aiding in ER stress response (Shen et al, 2022). Furthermore, farnesylatedAtJ2 and AtJ3 are involved in small RNA-mediated gene regulation through association with AGO1 in membrane compartments (Sjögren et al, 2018).
Although some studies have reported that plant DNA_J proteins participate in the growth and development of plants, their biological function and molecular mechanism during flowering have not yet been fully clarified. Furthermore, the DNA_J proteins in rice have not yet been found to regulate heading date. In this study, we characterized a()mutant, and isolated its causalgene based on the map-based combinewith Mutmap method., encoding a DNA_J domain protein, regulates the heading date in rice through the/pathway in rice under both LD and SD conditions. The results will enrich the molecular network of rice flowering regulation and provide genetic resources for molecular design and breeding.
RESULTS
lhd3 mutant exhibits late heading under both SD and LD conditions
Themutant was isolated from the60Co-γ irradiationmutant M2populations of Zhonghua 11 (ZH11) cultivar(ssp.).displayed late headingphenotype under natural long-day (NLD) in Hangzhou, Zhejiang Province, China (Fig. 1-A). Further analysis indicated thatshowed late heading under both NLD and natural short-day (NSD) conditions, and the heading date ofwas approximately 7.2 and 10.0 d later than that of wild type (WT) under NSD and NLD, respectively (Fig. 1-B). However, the grain length and grain width ofwere significantly lower than those of WT. As a result, the 1000-grain weight ofwas reduced by 5.86% compared with WT (Fig. 1-C to -G). In addition, the plant height and panicle length ofwere increased by 8.90% and18.32% relative to WT, respectively (Fig. 1-H and -I). The detail data of agronomic characters ofand WT were showed in Table S1. These results indicated thatpositively regulated heading date, grain size and grain weight, while negatively regulated plant height and panicle length in rice.
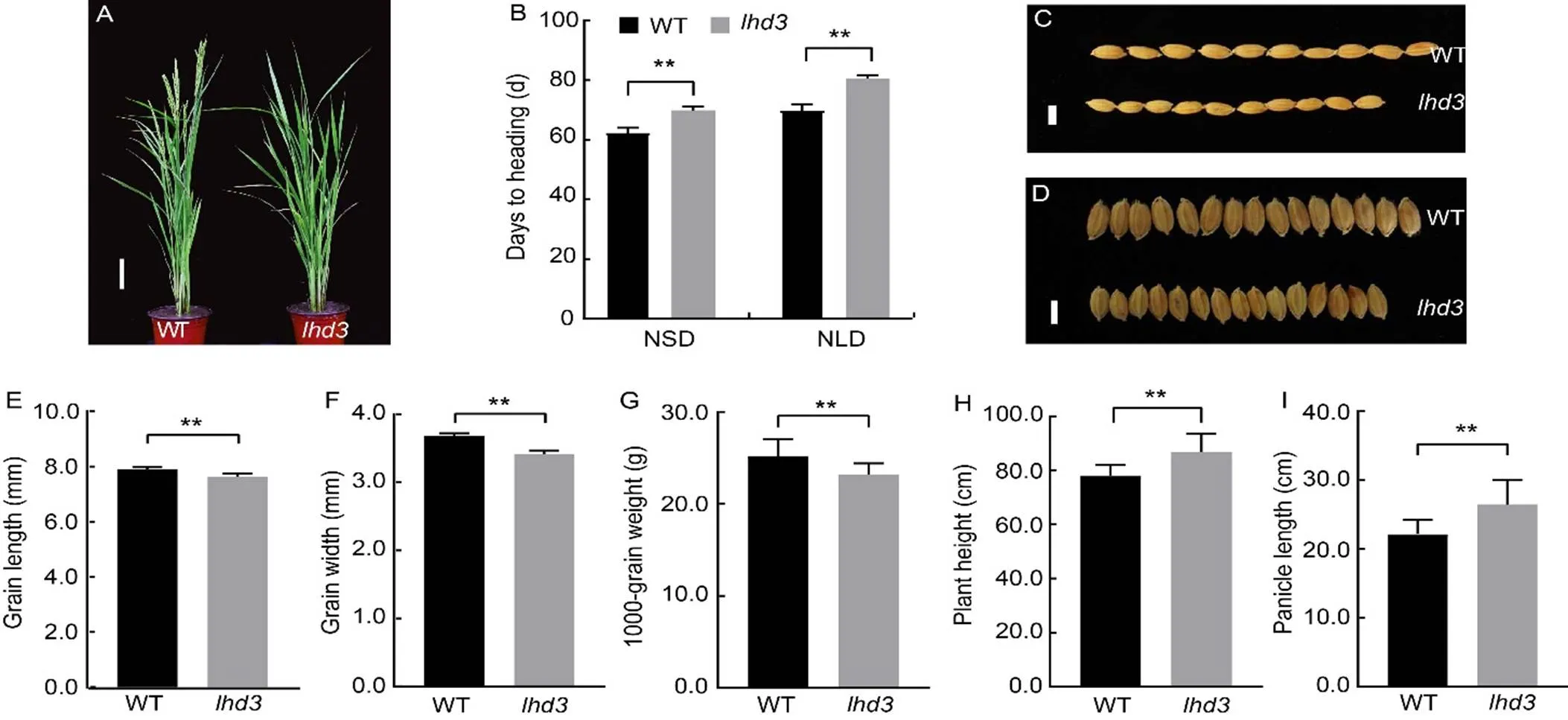
Fig. 1. Phenotypes and agronomic traits of
A, Phenotypes of wild type (WT) and mutant () rice at the heading stage. Scale bar, 10 cm.
B‒I, Heading dates (B), grain lengths (C and E), grain widths (D and F), 1000-grain weights (G), plant heights (H) and panicle lengths (I) of WT and. Scale bars, 1 mm in C and D. NSD, Natural short-day; NLD, Natural long-day.
Values are Mean ± SD (= 10 in B, E, F, H and I;= 3 in G). **,< 0.01 compared with WT by the Student’s-test.
LHD3 encodes a J-domain protein
To determine the genetic control offor heading date, an F2population was generated by crossingwith ZH11 (WT). The heading date of the F1plants was similar to WT, while the F2plants displayed two types of phenotypes: WT type and late heading, and the ratio of WT type tofit into 3:1 (Table S2), suggesting that the late heading date mutant was controlled by a single recessive gene.
Themutation was initially mapped by the F2population crossed byandNipponbare. In the F2population, the ratio of late heading and WT phenotype was also fit into 3:1 (Table S3), which confirmed that the single recessive mutation determined the late heading phenotype ofFirst,was assigned to the long arm of rice chromosome 3 according to the bulked segregant analysis (BSA) method. Then,was further located between two molecular markers of ZNC-6 and ZNC-9, and the genetic distance was 7.3 Mb. New InDel (insertion/deletion) markers were developed to fine mappingaccording to the polymorphic information between Nipponbare and ZH11. A total of five pairs of new developed InDel markers had polymorphism between the two parents. Using three InDel markers, thelocus was further mapped to a 1.8-Mb region between ZNC-24 and ZNC-26 markers. Thelocus was then further narrowed to 480 kb using a new developed InDel marker ZNC-31 (Fig. 2-A). A total of 65 putative genes, including 39 functional genes and 26 transposons/ retrotransposons, were found in this region according to the MSU 7 rice genome annotation system (http:// rice.uga.edu/). No cloned genes or mapped loci related to heading date were found in this region (Table S4).
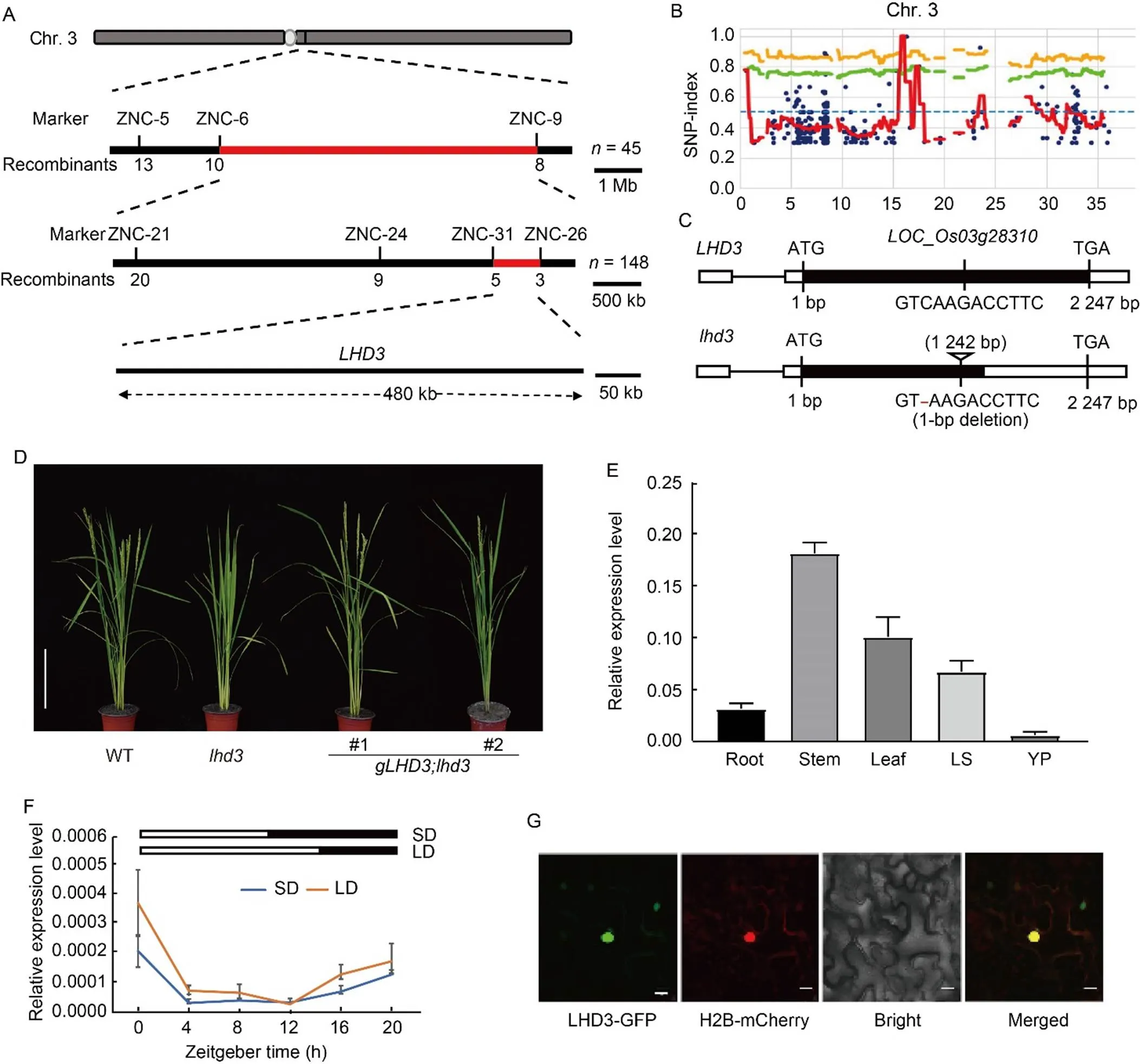
Fig. 2. Cloning and expression analysis of.
A, Molecular mapping of. The numbers below the lines indicate the number of recombinants.
B, Distribution of SNP-index between bulked poolsand Zhonghua 11 on rice chromosome 3 (Chr. 3). Blue dot, Variant; Orange line, p99; Green line, p95; Red line, SNP-index.
C, Schematic diagrams ofand mutated gene. The 1-bp deletion at 1 242 bp ofleads to premature termination of the encoded amino acid of LOC_Os03g28310.
D, Phenotypes of wild type (WT), mutantand its complementary lines. #1 and #2 represent positive transgenic lines from the complementation test. Scale bar, 20 cm.
E, Expression ofin different organs by qRT-PCR analysis. LS, Leaf sheath; YP, Young panicle. Samples were harvested at natural long-day condition. The ricegene was used as an internal control. Values are Mean ± SD of three independent experiments and three biological replicates.
F, Diurnal expression ofunder controlled short-day (SD) and long-day (LD) conditionsThe ricegene was used as an internal control. Values are Mean ± SD of three independent experiments and two biological replicates.
G, Subcellular localization of LHD3 inmesophyll cells. H2B-mCherry is a nucleus marker. Scale bars, 20 μm.
To further identify thegene, the genomic DNA of the late heading plants from F2generation crossed byand ZH11 were pooled and applied for whole genome resequencing. A total of 6.3 G and 10.3 G sequencing data from ZH11 and pooledwere obtained, respectively. There were 428 homozygous SNPs and 439 homozygous InDels between ZH11 and pooled F2plants withphenotypes. Thewas also identified using the MutMap method (Abe et al, 2012). The SNP/InDel ratio in the pooled F2plants were calculated, and several SNP/InDel markers were found closely linked tolocus on the rice chromosome 3 (Figs. 2-B and S1). Among them, only two homozygous InDels (16 295 088 and 16 659 651) were detected within the 480 bp candidate region of. InDel (16 295 088) showed a SNP/InDel ratio of 1 (Table S5), and InDel (16 295 088) contained a 1-bp deletion at 1 242 bp region of, which encodes a J-domain protein. No any other SNP/InDel was found in the candidate region compared with pooledwith ZH11 based on the resequenced sequences. The 1-bp deletion was confirmed by sequencing of the mutant region of, and led to premature termination of the encoded amino acid of LOC_Os03g28310 (Fig. 2-C). Using SMART (http://smart.embl-heidelberg.de/) and NCBI CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/) websites to predict the domain of the LHD3 protein online, it was found that the LHD3 protein has a J-domain near the N-terminal and a conserved DUF3444 domain near the C-terminal. These results suggested thatis a candidate gene for. A phylogenetic tree was constructed using ortholog proteins from wild rice, wheat, barley, rice, maize,and, which showed that LHD3is not an ortholog of AtJ2andAtJ3 in(Fig. S2).
Genetic complementation test was performed to confirm whether the deletion inwas responsible forphenotypesThe genomic fragment of() was transformed into themutantand six positive transgenic lines were generated. Thecomplementary lines were able to fully rescue the extremely late heading phenotypes of(Fig. 2-D). Furthermore, complementary plants fromtransgenic also recovered to WT in plant height and other morphological traits (Table S6). Therefore, the genomic complementation experiment confirmed that thegene was,indicating thatpositively regulates flowering time in rice.
Expression pattern of LHD3 and subcellular localization of LHD3
To investigate the spatial and diurnal expression pattern of, transcript levels ofwere detected by qRT-PCR. Total RNA was isolated from roots, stems, leaf sheaths and leaves at the vegetative stage and young panicles (5 mm) at the reproductive stage under NLD. As shown in Fig. 2-E,was constitutively expressed in all the detected organs and accumulated preferentially in stems and leaves. Then, the diurnal expression pattern ofwas also performed. The results showed that thetranscripts increased during the night period and peaked at zeitgeber time (ZT) of 0, subsequently reduced to the lowest level at ZT12 under LD and at ZT4 under SD (Fig. 2-F). The express pattern ofwas generally similar between SD and LD conditions.
To determine the subcellular location of LHD3 in rice, a subcellular location experiment was performed based on the transient expression assay. The full-lengthopen reading frame ofwas fusion to the C- terminus of green fluorescent protein (GFP) in the same reading frame derived by CaMV35S promoter and generated LHD3-GFPfusion construct. TheLHD3- GFPconstruct was then transformed intomesophyll cells. As expected, the fluorescence signals of LHD3-GFP and H2B-mCherry were co-located in the nucleus, which confirmed that the LHD3 protein was located to the nucleus (Fig. 2-G).
LHD3 regulates heading date gene expression in rice
To investigate the flowering pathway ofin rice, the transcription levels of genes related to flowering time were detected in WT andby qRT-PCR under SD and LD conditions. First, the expression levels of two florigen genes,and, were almost undetectable incompared with WT under both SD and LD conditions (Fig. 3-A to -D). Then,transcripts showed a significant decrease incompared with WT under both LD and SD conditions (Fig. 3-E and -F). However, the transcriptional level ofwas slightly increased inunder SD compared with WT, and there was no significant difference betweenand WT under LD conditions (Fig. 3-G and -H). In addition, the transcripts of upstream regulator genes of, such as,and, were slightly decreased incompared with WT under both LD and SD conditions (Fig. S3). The/andtranscripts showed no significant differences betweenand WT under SD conditions. The results indicated thatwas involved in/flowering pathway by promotingexpression under both LD and SD conditions.
LHD3 natural variation and haplotype analyses
To further investigate the relationship between natural variation inand flowering time, the SNPs and InDels of thegenomic region ofwere analyzed. Variation analysis showed that 31 variations, including 27 SNPs and 4 InDels, were found in thegenomic region ofin 4 702 rice accessions, including2 748ssp., 1 502ssp.,269ssp.and 183 intermediate. Subsequently, there were 4, 21 and 6 variations in 5ʹ-untranslated regions (UTR), coding sequences (CDS) and 3ʹ-UTR, respectively.
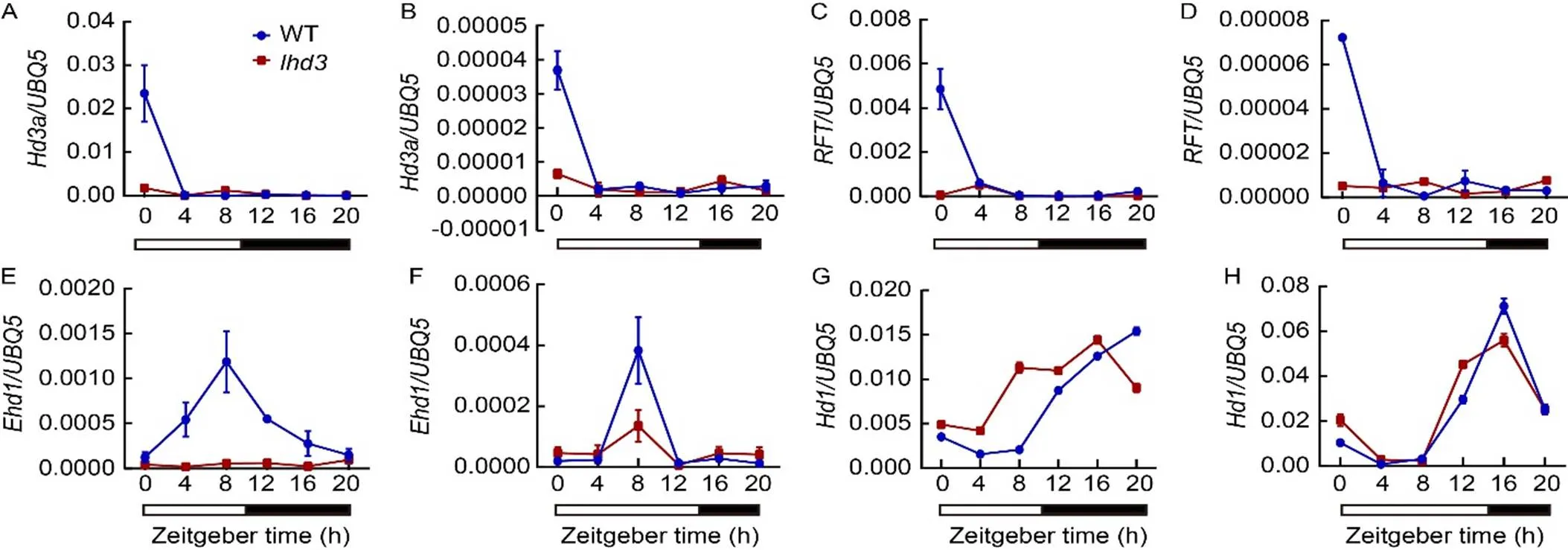
Fig. 3. Rhythmic expression pattern of flowering genes in wild type (WT) and mutant.
A, C, E and G showed short-day conditions. B, D, F and H showed long-day conditions.
The open and filled bars at the bottom represent the light and dark periods, respectively. The rice() gene was used as an internal control. Values are Mean ± SD of three independent experiments and three biological replicates.
After removing intron variations, heterozygous variations and synonymous variations, the remaining 10 SNP variations with amino acid changes were obtained, and a total of 7 haplotypes were identified (Fig. 4-A and -B; Table S7). Phylogenetic analysis showed that the haplotypes ofgene were divided into two classes: class a (Hap I, Hap II and Hap VI) and class b (Hap III, Hap IV, Hap V and Hap VII). Hap I and Hap III contained 1 329 and 2 602 accessions, respectively, which had more accessions than the other haplotypes (Fig. 4-B). According to the subspecies distribution of 7 haplotypes, Hap I contained 53.7% of, 30.1% ofand 8.4% of, while most of the Hap II and Hap VI accessions were identified in, accounting for 95.7% and 94.2%, respectively. Also, most of the Hap III accessions were identified in,and, accounting for 84.6%, 6.9% and 6.0%, respectively. Most of the Hap V and Hap VII accessions were mainly found in, accounting for 89.2% and 100%, respectively, while most of the Hap IV was mainly in, accounting for 95.6% (Fig. 4-B and -C).
Further statistical analysis showed that the heading date of the seven haplotypes ranged from 76.3 ± 15.2 dto 129.7 ± 24.1 d, and there were significant differences among the haplotypes (Fig. 4-D). Interestingly, the heading date of class a was significantly earlier than that of class b. Sequence analysis showed that there were five SNP differences between class a and class b (SNP2: T729G, SNP4: G1066A, SNP5: C1077T, SNP8: A1967C, SNP9: A2014G), suggesting that these five SNPs were the key sites for regulating heading date. In addition, the heading date of Hap II belonged to class a and dominated in, was significantly earlier than that of the other haplotypes. Sequence analysis showed that there was only one SNP10 (G2100C) difference between Hap I and Hap II, and the heading date of Hap II was 14.7 d earlier than that of Hap I, suggesting that the SNP10 (G2100C) variation was the key site, leading to significantly earlier heading date. Furthermore, the heading date of Hap VII belonged to class b and dominated in, was significantly later than that of the other haplotypes. Sequence analysis showed that there was only one SNP3 (C861T) difference between Hap V and Hap VII, while the heading date of Hap VII (129.7 ± 24.1 d) was 27.2 d later than that of Hap V (102.5 ± 19.6 d) (Fig. 4-D), suggesting that the SNP3 (C861T) of Hap VII may be the key siteregulating the delay of heading date.
To investigate the nucleotide diversity ofin wild and cultivated rice, the nucleotide polymorphism () across a 20-kb upstream and downstream genomicregion flankingin all rice was estimated using ECOGEMS (https://venyao.xyz/ECOGEMS/), which included 1 612 cultivated rice accessions and 446accessions. The nucleotide diversity ofwas strongly reduced incompared with wild rice (Fig. 4-E and -F). The ratio of the genetic diversityπtoπwas 17.201 (Fig. 4-F), which was strongly higher than the genome-wide threshold of selection signals (WC> 3) (Huang et al, 2012). These results suggested thathad strong selection signals in thepopulation, rather than in thepopulation.
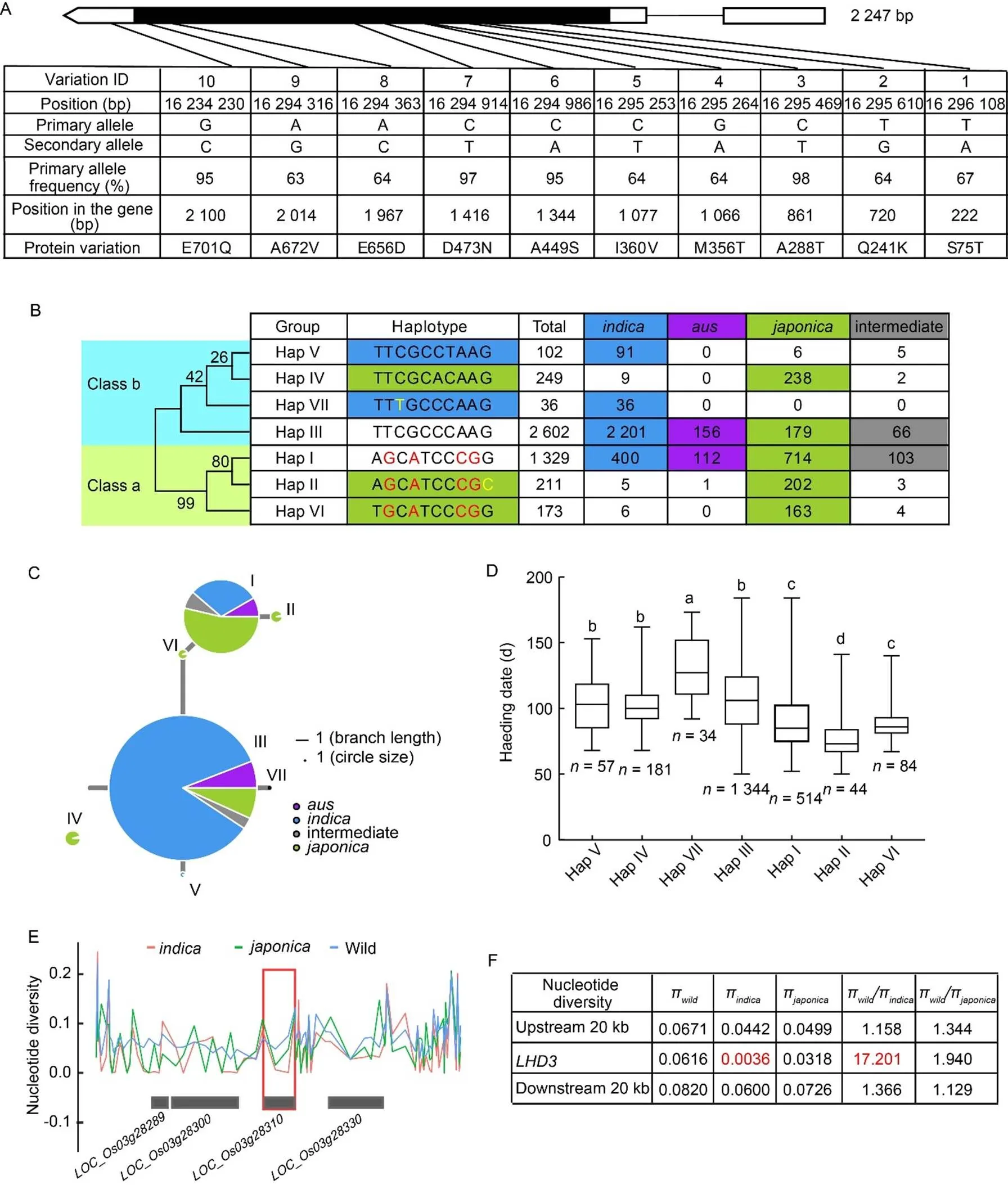
Fig. 4. Haplotype and nucleotide diversity analysis of.
A, Natural variation ingenomic region revealed 7 haplotypes in 4 702 rice accessions (ssp.,ssp.,ssp., and intermediate).
B,Haplotype analysis of. The neighbor-joining phylogenetic tree was constructed using MEGA 11 software with 1 000 bootstrap replicates (left).
C, Haplotype network analysis ofin 4 702 rice accessions. Allele frequencies are proportional to the size of circles.
D, Box diagram of heading date in seven haplotypes. Different lowercase letters represent significant difference at 5% level according to the Duncan’s multiple range.
E, Nucleotide diversity ofin wild,andrice. Red box marked the position of.
F, Nucleotide diversity, the ratios ofπtoπandπtoπsurrounding.
DISCUSSION
As one of the most important agronomic traits in rice, heading date affects rice adaptation to diverse cultivation regions, cropping seasons, maturity, grain yield and quality (Song et al, 2015). Numerous investigations have been conducted to map and characterize 734 heading date genes/QTLs that have been documented in the Gramene database (http://archive.gramene.org/qtl/,accessed on 30 December, 2022). However, few genes/ QTLs have been mapped and cloned (Cho et al, 2017). In this study,exhibited a late heading phenotype under both SD and LD conditions. In addition, there were significant differences in plant height, panicle length, grain type, 1000-grain weight, and other related agronomic traits incompared with WT. The results indicated thatpositively regulated the heading date regardless of the photoperiod. In the photoperiodic flowering pathway, the two pathways/and/undertake main floral signal pathway in rice (Sun et al, 2014; Hori et al, 2016).positively regulates the expression ofandunder both SD and LD conditions (Doi et al, 2004)Under SD conditions,can promote rice flowering by directly regulating the expression ofandindependently of, while under LD conditions,can only promote rice flowering by regulatingexpression becauseexpression is inhibited (Galbiati et al, 2016; Wei et al, 2016). In our study, we found that the expression levels of flowering genes in/pathway showed no obvious change, while the expression levels of genes in/pathway were decreased significantly, indicating thatpositively regulated the heading date through/pathway
The J-domain proteins have been identified in different organisms where they perform crucial functionsin growth, development and stress response (Tamadaddi et al, 2021, 2022). The phylogenetic analysis offrom different species revealed thatwas not an ortholog ofand. Considering thatis positive for the flowering through the/pathway, it might promote rice flowering in a specific way compared with.encodes a DNA_J protein J3, which can not only regulate H+- ATPase on the plasma membrane (Yang et al, 2010), but also promote flowering by directly antagonizing short vegetative phase activity in suppressingandtranscription in(Shen et al, 2011). These reports demonstrated that J-domain proteins play an indispensable role in mediating plant development through interactions with other transcriptional factors other than HSP70. LHD3 was located to the nucleus, andwas constitutively expressed in all the detected organs and accumulated preferentially in stems and leaves, hinting thatmight integrate the flowering pathway through interactions with other transcriptional factors, and providing a direction for our future research.
Thegene in this study is a new gene locus affecting the heading date of rice. So far, there has no report on the regulation of rice flowering time by the DNA_J gene. The variation and haplotype analyses showed 7 haplotypes in theregion from 4 702 accessions, and the haplotypes ofcan be divided into two classes: class a and class b. In addition, the heading date of class a was significantly earlier than that of class b (Fig. 4-D), suggesting class a and class b may have different origins. SNP10 (G2100C) in Hap II and SNP3 (C861T) in Hap VII showed significant earlier and later heading than the other haplotypes, respectively, suggesting that these two SNPs may have a function in controlling heading date and can be used in molecular breeding. The nucleotide diversity analysis showed thathad strong selection signals in thepopulation rather than in thepopulation (Fig. 4-E and -F). Similar results were also found in other HSP40genes, such as(Wang et al, 2022), suggesting that the HSP40 gene family may play important roles in environmental adaptation and frequently be selected. Further studies on the function of thegene can enrich the regulation pathway of photoperiod flowering in rice and provide theoretical basis and gene resources for molecular breeding. In addition to the multiple functions at the heading date,expression may also be regulated by a variety of abiotic stresses, which means thatcan be involved in the regulation of the heading date mediated by abiotic stress, which is worthy of further studying.
METHODS
Rice materials and growth conditions
Themutant was initially screened from a rice (ssp.cv Zhonghua 11)60Co-γ irradiated mutant library. The WT and mutant plants were grown in transgenic experimental fields under NLD conditions at Hangzhou, Zhejiang Province, China (120.42º E, 30.32º N) and NSD conditions in Lingshui, Hainan Province, China (110.05º E, 18.51º N), respectively. Days to heading were scored when the first panicle bolted after planting. The other agronomic traits of WT andwere characterized under NLD conditions in Hangzhou, Zhejiang Province, China.
The plants were also grown in controlled growth chambers under SD (10 h light/14 h dark at 28 ºC) and LD (14 h light/10 h dark at 28 ºC) with a relative humidity of 70%. The light intensity was 800 mmol/(m2·s).
Phenotype investigation and genetic analysis
The F1population was obtained by crossingwith ZH11, and the heading date of the F1plants was investigated. Then, the F1seeds were harvested per plant, and planted to generate the F2segregation population. The numbers of WT andplants were counted and subjected to a-square test.
At the maturity stage, 10 WT and 10plants were randomly selected for the measurements of plant height and tiller number per plant. The panicle length and the grain number from the main panicle were also measured. The 1000-grain weight was calculated from full-filled grains at the maturity stage and weighted with a balance. The grain length and width were determined with a vernier caliper. All data were treated with the SPSS 26.0 software.
Mapping and cloning of LHD3
For molecular mapping of, the F1plants were generated by crossingwith Nipponbare. Self-pollinated F1seeds were harvested from individual plants to generate an F2population. Inthe F2population, homologous individuals with the mutant phenotype were selected for gene mapping. Based on the BSA method (Rogers and Bendich, 1985), the genome DNA extracted from leaves of 10 WT and 10was mixed in an equal ratio, and then formed WT andDNA mutant pools. A total of 183 pairs of simple sequence repeat primers evenly distributed on 12 rice chromosomes and polymorphic between the two parents were selected for BSA. Then, InDel markers were developed for fine mapping according to the sequence polymorphism between the two parents. The newly developed InDel markers were shown in Table S8.
Fifty homologous individuals with mutant phenotypes were harvested from the F2population crossed betweenand its WT, and their DNAs were extracted and pooled in the same ratio for the genome resequencing using the Illumina HiSeq 2500platform at Berry Genomics Co., Ltd, Beijing, China. Whole-genome resequencing, InDel and SNP were analyzed as described previously (Fang et al, 2016). The MutMap analysis was performed according to previously described (Abe et al, 2012).
Protein sequences of LHD3 and its homologs were retrieved from the NCBI database using BLASTp program (https://blast. ncbi.nlm.nih.gov/Blast.cgi) (accessed on 30 December, 2022) and aligned using the Clustal X 1.83 software with the default parameters. Phylogenetic tree was constructed using the MEGA 11 software (http://wwwmegasoftwarenet/megaphp) employing the neighbor-joining method with 1 000 bootstrap replicates.
Vector construction and rice transformation
The primers LHD3-G3-BamHI and LHD3-G5-HindIIIwere used to amplify the genomic sequence of,which contained a 2 049-bp 5ʹ-flanking region, the whole gene 2 231-bp region and a 1 226-bp 3ʹ-flanking region. The whole genomic sequence was then inserted into the pCAMBIA1300 vector to generate therecombinant construct, and introduced into theEHA105. The primers used for constructing were listed in Table S8.was transformed intocalli using the-mediated method as described (Hiei et al, 1994).
Subcellular localization of LHD3
To generate the LHD3-GFP fusion protein, theCDS(1 536 bp) was amplified and cloned into the entry vector pCR®8/GW⁄TOPO®TA (Invitrogen Co., Ltd, USA). After sequencing and confirming that there was no mutation, the CDS ofwas recombinant in the transient expression vectorGFP-N-BIN using the Gateway™ LR Clonase™ II Kit (Invitrogen Co., Ltd, USA). The LHD3-GFP plasmids were introduced into(strain GV3101) and infiltrated intomesophyll cells by acetosyringone for transient expression. Confocal imaging analysis was visualized using a laser scanning microscopy (LSM 700; Carl Zeiss, Germany).
Diurnal rhythmic expression of LHD3
The 30-day-old plants were grown in an incubator and harvested every 4 h within 1 d (6-time points) with three biological replicates. Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized using the MMLV Reverse Transcriptase (Promega Co., Ltd, USA). qRT-PCR assays were performed using a TORO Green®qPCR Master Mix (QST-100) kit (TOROIVD Co., Ltd, Shanghai, China), and() was applied as an internal control.
RNA extraction and qRT-PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen Co., Ltd, USA) according to the manufacturer’s manual. Complementary DNA (cDNA) was synthesized using the MMLV Reverse Transcriptase (Promega, USA) with oligo(dT)18primer. qRT- PCR was performed using a THUNDERBIRD qPCR Mix Kit (Toyobo Co., Ltd, Japan) on an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Waltham, CA, USA) with three biological repeats. The ricegene was used as an internal control. Primers for qRT-PCR were list in Table S8.
Variation and haplotype analysis
To determine the variation and haplotype information of thelocus in rice, the genomic region ofwas analyzed according to RiceVarMap2 (http://ricevarmap.ncpgr.cn/). The haplotype network analysis ofwas performed using the online R package in RiceVarMap2 (Zhao et al, 2021).
ACKNOWLEDGEMENTS
This study was funded by the National Basic Research Program of China (Grant No. 2016YFD0100401), National Natural Science Foundation of China (Grant No. 31571742), and Scientific Research Initiation Fund of Zhejiang University of Science and Technology, China (Grant No. 19042142-Y).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Distribution of SNP-index across 12 rice chromosomes.
Fig. S2. Phylogenetic tree showing relationship between LHD3 homologs in monocots and dicots.
Fig. S3. Rhythmic expression pattern of other flowering genes in wild type (WT) and mutant.
Table S1. Agronomic traits ofand wild type (WT).
Table S2. Segregation of phenotype in F2generation crossed byand Zhonghua 11.
Table S3. Segregation of phenotype in F2generation crossed byandNipponbare
Table S4. Putative genes in candidate region of
Table S5. SNP-index in candidate region of.
Table S6. Agronomic traits in positive complementary transgenic lines.
Table S7. Haplotype information oflocus.
Table S8. Primers used in this study.
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M, Innan H, Cano L, Kamoun S, Terauchi R. 2012. Genome sequencing reveals agronomically important loci in rice using MutMap., 30(2): 174–178.
Andrés F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues., 13(9): 627–639.
Bian X F, Liu X, Zhao Z G, Jiang L, Gao H, Zhang Y H, Zheng M, Chen L M, Liu S J, Zhai H Q, Wan J M. 2011. Heading date gene,controlled late flowering inSteud. by down-regulating., 30(12): 2243–2254.
Bukau B, Weissman J, Horwich A. 2006. Molecular chaperones and protein quality control., 125(3): 443–451.
Chai J T, Zhu S S, Li C N, Wang C M, Cai M H, Zheng X M, Zhou L, Zhang H, Sheng P K, Wu M M, Jin X, Cheng Z J, Zhang X, Lei C L, Ren Y L, Lin Q B, Zhou S R, Guo X P, Wang J, Zhao Z C, Wan J M. 2021.interacts withto regulate rice heading date by finely modulatingexpression., 19(2): 300–310.
Chen K M, Holmström M, Raksajit W, Suorsa M, Piippo M, Aro E M. 2010. Small chloroplast-targeted DnaJ proteins are involved in optimization of photosynthetic reactions in., 10: 43.
Cho L H, Yoon J, An G. 2017. The control of flowering time by environmental factors., 90(4): 708–719.
Christensen C A, Gorsich S W, Brown R H, Jones L G, Brown J, Shaw J M, Drews G N. 2002. Mitochondrial GFA2 is required for synergid cell death in., 14(9): 2215–2232.
Craig E A, Marszalek J. 2017. How do J-proteins get Hsp70 to do so many different things?, 42(5): 355–368.
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. 2004., a B-type response regulator in rice, confers short-day promotion of flowering and controlsgene expression independently of., 18(8): 926–936.
Fang N, Xu R, Huang L J, Zhang B L, Duan P G, Li N, Luo Y H, Li Y H. 2016.controls grain size, grain number and grain yield in rice., 9(1): 64.
Galbiati F, Chiozzotto R, Locatelli F, Spada A, Genga A, Fornara F. 2016.,andintegrate photoperiodic and drought stress signals to delay the floral transition in rice., 39(9): 1982–1993.
Gao H, Zheng X M, Fei G L, Chen J, Jin M N, Ren Y L, Wu W X, Zhou K N, Sheng P K, Zhou F, Jiang L, Wang J, Zhang X, Guo X P, Wang J L, Cheng Z J, Wu C Y, Wang H Y, Wan J M. 2013.encodes a novel and-genus-specific regulator of photoperiodic flowering in rice., 9(2): e1003281.
Harris C J, Scheibe M, Wongpalee S P, Liu W L, Cornett E M, Vaughan R M, Li X Q, Chen W, Xue Y, Zhong Z H, Yen L D, Barshop W D, Rayatpisheh S, Gallego-Bartolome J, Groth M, Wang Z H, Wohlschlegel J A, Du J M, Rothbart S B, Butter F, Jacobsen S E. 2018. A DNA methylation reader complex that enhances gene transcription., 362: 1182–1186.
Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K. 2003. Adaptation of photoperiodic control pathways produces short- day flowering in rice., 422: 719–722.
Hiei Y, Ohta S, Komari T, Kumashiro T. 1994. Efficient transformationof rice (L.) mediated byand sequence analysis of the boundaries of the T-DNA., 6(2): 271–282.
Hori K, Matsubara K, Yano M. 2016. Genetic control of flowering time in rice: Integration of Mendelian genetics and genomics., 129(12): 2241–2252.
Huang X H, Kurata N, Wei X H, Wang Z X, Wang A H, Zhao Q, Zhao Y, Liu K Y, Lu H Y, Li W J, Guo Y L, Lu Y Q, Zhou C C, Fan D L, Weng Q J, Zhu C R, Huang T, Zhang L, Wang Y C, Feng L, Furuumi H, Kubo T, Miyabayashi T, Yuan X P, Xu Q, Dong G J, Zhan Q L, Li C Y, Fujiyama A, Toyoda A, Lu T T, Feng Q, Qian Q, Li J Y, Han B. 2012. A map of rice genome variation reveals the origin of cultivated rice., 490: 497–501.
Jung C, Müller A E. 2009. Flowering time control and applications in plant breeding., 14(10): 563–573.
Kampinga H H, Craig E A. 2010. The HSP70 chaperone machinery: J proteins as drivers of functional specificity., 11(8): 579–592.
Kim S L, Lee S, Kim H J, Nam H G, An G. 2007.is a short-day flowering promoter that functions upstream of,, and., 145(4): 1484–1494.
Koo B H, Yoo S C, Park J W, Kwon C T, Lee B D, An G, Zhang Z Y, Li J J, Li Z C, Paek N C. 2013. Natural variation inregulates heading date and contributes to rice cultivation at a wide range of latitudes., 6(6): 1877–1888.
Lee S, Kim J, Han J J, Han M J, An G. 2004. Functional analyses of the flowering time gene, the putative/(/)ortholog in rice., 38(5): 754–764.
Li X F, Tian X J, He M L, Liu X X, Li Z Y, Tang J Q, Mei E Y, Xu M, Liu Y X, Wang Z Y, Guan Q J, Meng W, Fang J, Zhang J, Bu Q Y. 2022.delays flowering by suppressingexpression in rice., 64(7): 1352–1363.
Lin B L, Wang J S, Liu H C, Chen R W, Meyer Y, Barakat A, Delseny M. 2001. Genomic analysis of the Hsp70 superfamily in., 6(3): 201–208.
Luo Y, Fang B H, Wang W P, Yang Y, Rao L Q, Zhang C. 2019. Genome-wide analysis of the rice J-protein family: Identification, genomic organization, and expression profiles under multiple stresses., 9(10): 358.
Matsubara K, Yamanouchi U, Wang Z X, Minobe Y, Izawa T, Yano M. 2008., a rice ortholog of the maizegene, promotes flowering by up-regulating., 148(3): 1425–1435.
Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang Z X, Minobe Y, Yano M. 2011., encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering., 66(4): 603–612.
Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, Yano M. 2012. Natural variation in, a homolog ofthat is involved in rice photoperiodic flowering., 53(4): 709–716.
Nemoto Y, Nonoue Y, Yano M, Izawa T. 2016., a CONSTANS ortholog in rice, functions as anrepressor through interaction with monocot-specific CCT-domain protein Ghd7., 86(3): 221–233.
Ohta M, Takaiwa F. 2020.is an ER-resident J-protein involved in ER quality control in rice endosperm., 245: 153109.
Park M Y, Kim S Y. 2014. TheJ protein AtJ1 is essential for seedling growth, flowering time control and ABA response., 55(12): 2152–2163.
Peng L T, Shi Z Y, Li L, Shen G Z, Zhang J L. 2007. Ectopic expression ofin rice repressesby binding on its promoter., 360(1): 251–256.
Rajan V B V, D’Silva P. 2009.J-class heat shock proteins: Cellular stress sensors., 9(4): 433–446.
Roberts J A, Evan D, Mcmanus M T, Rose J. 2018. Photoreceptors and light signaling pathways in plants., 10(21): 107–131.
Rogers S O, Bendich A J. 1985. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues., 5(2): 69–76.
Senguttuvel P, Sravanraju N, Jaldhani V, Divya B, Beulah P, Nagaraju P, Manasa Y, Prasad A S H, Brajendra P, Gireesh C, Anantha M S, Suneetha K, Sundaram R M, Madhav M S, Tuti M D, Subbarao L V, Neeraja C N, Bhadana V P, Rao P R, Voleti S R,Subrahmanyam D. 2021. Evaluation of genotype by environment interaction and adaptability in lowland irrigated rice hybrids for grain yield under high temperature., 11(1): 15825.
Shen L S, Kang Y G G, Liu L, Yu H. 2011. The J-domain protein J3 mediates the integration of flowering signals in., 23(2): 499–514.
Shen T T, Wang L, Shang C H, Zhen Y C, Fang Y L, Wei L L, Zhou T, Bai J T, Li B. 2022. TheJ-protein AtDjC5 facilitates thermotolerance likely by aiding in the ER stress response., 23(21): 13134.
Sjögren L, Floris M, Barghetti A, Völlmy F, Linding R E, Brodersen P. 2018. Farnesylated heat shock protein 40 is a component of membrane-bound RISC in., 293(43): 16608–16622.
Song Y H, Shim J S, Kinmonth-Schultz H A, Imaizumi T. 2015. Photoperiodic flowering: Time measurement mechanisms in leaves., 66: 441–464.
Sun C H, Chen D, Fang J, Wang P R, Deng X J, Chu C C. 2014. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways., 5(12): 889–898.
Tamadaddi C, Sagar V, Verma A K, Afsal F, Sahi C. 2021. Expansion of the evolutionarily conserved network of J-domain proteins in themitochondrial import complex., 105(4): 385–403.
Tamadaddi C, Verma A K, Zambare V, Vairagkar A, Diwan D, Sahi C. 2022. J-like protein family of: The enigmatic cousins of J-domain proteins., 41(6): 1343–1355.
Tamaki S, Matsuo S, Wong H L, Yokoi S, Shimamoto K. 2007. Hd3a protein is a mobile flowering signal in rice., 316: 1033–1036.
Tsuji H, Taoka K I, Shimamoto K. 2011. Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation., 14(1): 45–52.
Wang F H, Tang Z B, Wang Y, Fu J, Yang W B, Wang S X, Wang Y T, Bai T, Huang Z B, Yin H Q, Wang Z F. 2022.encoding heat shock proteinregulates leaf size in rice., 23(8): 4446.
Wei F J, Tsai Y C, Wu H P, Huang L T, Chen Y C, Chen Y F, Wu C C, Tseng Y T, Hsing Y I C. 2016. Bothandare important for artificial selection of flowering time in cultivated rice., 242: 187–194.
Wu C Y, You C J, Li C S, Long T, Chen G X, Byrne M E, Zhang Q F. 2008., encoding a Cys2/His2-type zinc finger transcriptionfactor, acts as a master switch from vegetative to floral development in rice., 105(35): 12915–12920.
Xing Y Z, Zhang Q F. 2010. Genetic and molecular bases of rice yield., 61: 421–442.
Xue W Y, Xing Y Z, Weng X Y, Zhao Y, Tang W J, Wang L, Zhou H J, Yu S B, Xu C G, Li X H, Zhang Q F. 2008. Natural variation inis an important regulator of heading date and yield potential in rice., 40(6): 761–767.
Yan W H, Wang P, Chen H X, Zhou H J, Li Q P, Wang C R, Ding Z H, Zhang Y S, Yu S B, Xing Y Z, Zhang Q F. 2011. A major QTL,, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice., 4(2): 319–330.
Yan W H, Liu H Y, Zhou X C, Li Q P, Zhang J, Lu L, Liu T M, Liu H J, Zhang C J, Zhang Z Y, Shen G J, Yao W, Chen H X, Yu S B, Xie W B, Xing Y Z. 2013. Natural variation inplays an important role in grain yield and adaptation in rice., 23(7): 969–971.
Yang K Z, Xia C, Liu X L, Dou X Y, Wang W, Chen L Q, Zhang X Q, Xie L F, He L Y, Ma X, Ye D. 2009. A mutation in, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in., 57(5): 870–882.
Yang Y Q, Qin Y X, Xie C G, Zhao F Y, Zhao J F, Liu D F, Chen S Y, Fuglsang A T, Palmgren M G, Schumaker K S, Deng X W, Guo Y. 2010. Thechaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase., 22(4): 1313–1332.
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T. 2000., a major photoperiod sensitivity quantitative trait locus in rice, is closely related to theflowering time gene., 12(12): 2473–2484.
Zhang H, Zhu S S, Liu T Z, Wang C M, Cheng Z J, Zhang X, Chen L P, Sheng P K, Cai M H, Li C N, Wang J C, Zhang Z, Chai J T, Zhou L, Lei C L, Guo X P, Wang J L, Wang J, Jiang L, Wu C Y, Wan J M. 2019.interacts with, delays flowering time and enhances yield in rice., 17(2): 531–539.
Zhao H, Li J C, Yang L, Qin G, Xia C J, Xu X B, Su Y M, Liu Y M, Ming L C, Chen L L, Xiong L Z, Xie W B. 2021. An inferred functional impact map of genetic variants in rice., 14(9): 1584–1599.
Zhao J M, Huang X, Ouyang X H, Chen W L, Du A P, Zhu L, Wang S G, Deng X W, Li S G. 2012., an ortholog of, regulates rice circadian rhythm and photoperiodic flowering., 7(8): e43705.
Zhou S R, Zhu S S, Cui S, Hou H G, Wu H Q, Hao B Y, Cai L, Xu Z, Liu L L, Jiang L, Wang H Y, Wan J M. 2021. Transcriptional and post-transcriptional regulation of heading date in rice., 230(3): 943–956.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.03.015
9 January 2023;
30 March 2023
Wang Dekai (wangdk@zstu.edu.cn)
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa
- Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth

