金属簇X(X=Pt-Au,Au-Au)负载在(3×2)TiO2(110)完整表面上的覆盖度效应
赵伟娜 林华香 李 奕 章永凡 黄 昕 陈文凯,,*
(1福州大学化学化工学院,福州350116;2福州大学省部共建光催化国家重点实验室培育基地,福州350002)
金属簇X(X=Pt-Au,Au-Au)负载在(3×2)TiO2(110)完整表面上的覆盖度效应
赵伟娜1林华香2李 奕1章永凡1黄 昕1陈文凯1,2,*
(1福州大学化学化工学院,福州350116;2福州大学省部共建光催化国家重点实验室培育基地,福州350002)
采用自旋极化密度泛函和广义梯度近似的方法并结合周期平板模型,探讨了不同覆盖度(θ)下双金属簇X(X=Pt-Au,Au-Au)在(3×2)TiO2(110)完整表面上的吸附行为.另外,在本文给出的所有覆盖度模式下(θ= 1/6-1 ML),我们仅研究其基态构型.计算结果表明:当θ<1/2 ML时,金属簇X在TiO2(110)表面上吸附能随覆盖度的增加而增加;当θ>1/2 ML时,除了饱和覆盖度下,吸附能随覆盖度的增加而减小;当θ=1/2 ML时,吸附能最大.即使Pt-Au/TiO2体系的吸附能比Au-Au/TiO2体系的小,但相对于Au-Au簇,Pt-Au簇更容易在TiO2(110)表面上形成双金属单分子层.在半覆盖和全覆盖下,X簇的峰与TiO2的峰在-3.0 eV到费米能级之间产生明显重叠,表明簇与底物之间存在化学作用.且当覆盖度小时,X-TiO2相互作用是成簇的主要因素;随着覆盖度的增大,X-X原子间相互作用就逐渐变成了成簇的主要动力.
密度泛函理论;TiO2(110)表面;双金属簇
1 Introduction
Oxide-supported bimetallic clusters have been the subject of enormous attention in recent decades due to the improvement of catalytic properties1-4and the change of surface plasma band energy5,6relative to the separate metals.Nowadays,it is of an appealing challenge and great interest for material scientists to investigate the intrinsic nature of such interfaces.7-15
Owing to the wide range of surface-related applications,titanium dioxide has evolved into one of the best investigated oxide materials and generally used to model TiO2catalytic properties under ultra-high vacuum(UHV)conditions in surface science.16-20TiO2,existing in three main crystalline forms(rutile, anatase,and brookite),is known to have the tetragonal P42/ mmm space group21in the rutile phase.From a theoretical point of view together with an experimental one,the rutile TiO2(110) surface is taken to serve as a substrate of great interest due to its highest stability,22,23though below 1100 K.On the other hand,much intensive research has been undertaken in the field of Pt-based bimetallic nanoparticles because of their better catalytic properties than their monometallic counterparts.24,25As we know,Au is one of only two transition metals more electronegative than Pt,so the incorporation of Au into Pt clusters can be expected to have unique effects on physical and chemical properties of Pt.Then tremendous recent efforts have been devoted to the investigation for Pt-Au bimetallic cluster over the past few years.25-28Such a linkage between titanium dioxide and Pt-Au bimetallic cluster,if established,is of huge incentive.
Experimentally,the past decade has seen considerable efforts in the development of Pt-Au/TiO2system.29-31Tenney et al.29have investigated the growth,composition of the top surface layer,and chemical activity of bimetallic Pt-Au clusters on TiO2(110)via scanning tunneling microscopy(STM)and low energy ion scattering(LEIS).Park and co-workers30have reported that Pt,Au,and Au-Pt clusters were grown on TiO2(110)at room temperature and found that bimetallic clusters were formed from the nucleation of Au at existing Pt clusters. Wen et al.31have synthesized a novel metal/semiconductor bimetallic nanoparticle(noted as f-TiO2-Au/Pt NPs)and this nanostructure was expected as a promising catalyst in electrochemical and photoelectrochemical applications.However,to our knowledge,the number of theoretical works addressing the Pt-Au/TiO2(110)system is surprisingly scarce.Boronat and Corma32have studied the catalytic activity of Pt-Au/TiO2catalysts toward hydrogenation,and found that H2dissociation on Au-Pt nanoparticles was considerably faster than that on Au. Although the Au/Pt metal-enhanced catalytic activity of rutile TiO2has been reported from both theoretical and experimental points of view,the electronic properties of such systems as the originating factor are still far from being well-understood. Hence,it is intriguing that more theoretical investigations on the Pt-Au/TiO2(110)system are compulsory.
Aiming to further explore the framework of bimetallic cluster/TiO2(110),in this work the adsorptive mechanism of Pt-Au bimetallic cluster supported on the defect-free TiO2(110)surface at different coverages has been investigated,using firstprinciples density functional theory(DFT)calculations performed under periodic supercell conditions.Additionally,Au-Au/TiO2(110)system is also present for comparison and Pt-Pt/ TiO2(110)will be discussed in our next work.The results obtained here can hopefully shed light on further designing TiO2-based catalysts with high-performance and thus are of general interest to chemistry.
2 Computational details
All the spin-polarized density functional theory(DFT)33-36calculations were performed utilizing the Perdew-Wang (PW91)exchange-correlation functional37as implemented in the Dmol3software,38,39in which the physical wave functions were expanded in terms of accurate numerical basis sets.The generalized gradient approximation(GGA)40was chosen here to describe the effects of exchange and correlation,because it is better than the local density approximation(LDA)for metal oxide system41and the energetics of molecular adsorption.42,43The double-numeric quality basis set with polarization functions(DNP)was utilized.38,44,45In addition to this,the use of numerical basis sets minimizes the basis set superposition error (BSSE).46During the computation,the inner electrons of Pt and Au atoms were kept frozen and substituted by an effective core potential(ECP).Brillouin-zone integrations were performed using 2×3×1 Monkhorst-Pack grid47and a Fermi smearing of 0.005 Hartree.The tolerances of energy,gradient,and displacement convergence were 2×10-5Hartree,4×10-2Hartree·nm-1, and 5×10-4nm,respectively.
Fernandezʹs studies48and many other literature49-52showed that nine atomic layersʹslab was enough to simulate the rutile (110)surface.Thus,in this work,a periodic slab containing nine atomic layers was adopted by a(3×2)supercell.Additionally,this supercell model was successfully used to study the Au adsorption on the defect-free rutile TiO2(110)surface at different coverages by Lopez and Nørskov.53Of particular significance is that a slab approach54with spin polarization provided can avoid problems related to the embedding or to the basis set superposition error.The side and top views of this system were shown in Fig.1.All the atoms in this whole system(adsorbate/ substrate)were allowed to relax and a vacuum region of 1.3 nm has been employed to prevent any noticeable interaction between two successive slabs.During the calculation,the(3×2) supercell,the vacuum layer thickness as well as the numbers of layers were judged to be adequate to yield good convergence of energies and properties,indicating that our computational approach was reasonable.
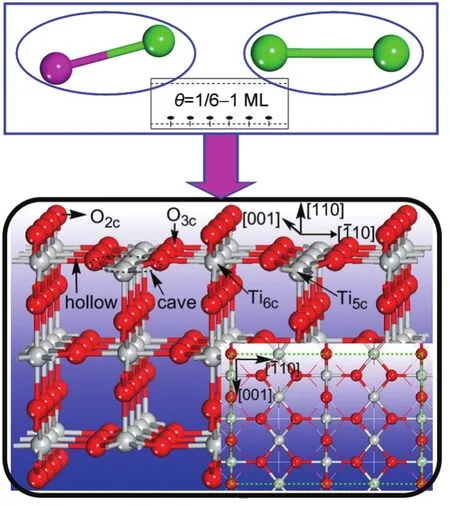
Fig.1 Defect-free(3×2)TiO2(110)supercell model utilized to study the adsorption of bimetallic clusters(Pt-Au andAu-Au)on the TiO2(110)surface at six fixed coverage levelsThe letters are used for identification purposes.
To estimate coverage effect,herein,the coverage of X(X= Pt-Au,Au-Au)clusters on TiO2(110)is defined as the number of X with respect to the number of Ti5cexposed.For example, there are six Ti5catoms exposed on the defect-free(3×2)TiO2(110)surface cell,so the resulting coverage is 1/6,2/6,3/6,4/6, 5/6,and 1 monolayer(ML),respectively.To calculate the stabilities of adsorbed bimetallic clusters(Pt-Au and Au-Au),the average adsorption energy per cluster is considered:

where E(Xn/TiO2),E(TiO2),and E(X)refer to the energies of the total adsorbed system,the bare TiO2(110)surface,and the corresponding free X(X=Pt-Au,Au-Au)cluster in gas phase, respectively.Additionally,n represents the number of adsorbed X cluster.
3 Results and discussion
3.1 Bulk and surface structures of rutile
The optimized lattice parameters for bulk structure are as follows:a=0.4594 nm and c=0.2959 nm.As shown in Table 1,the results are all within 0.17%for rutile compared to experimental values,55and in some cases the results are even better than those obtained by B3LYP method.56With respect to the bulk structure,the six-fold coordinated titanium atoms and in-planeoxygen atoms in the defect-free(3×2)TiO2(110)surface exhibit outward relaxation along the[110]direction,whereas the surface five-fold coordinated titaniums and bridging oxygens relax inwards,yielding a slight puckered appearance in the relaxed surface which is in accordance with other studies.21Using the above-depicted supercell models,six adsorption sites on the TiO2(110)surface are taken into account here,as described in Fig.1,i.e.,O2c,O3c,Ti5c,Ti6c,cave and hollow.In position O2cthe X clusters(X=Pt-Au,Au-Au)interacts with the protruded oxygen atoms in the surface,while O3csite corresponds to adsorption on top of in-plane oxygen atoms,with both the Pt-end and Au-end of the cluster closer to the surface,respectively.Position Ti5cdenotes that the adsorbed Pd atoms are located on top 5-fold coordinated titanium atoms and Ti6cis on top of 6-fold coordinated titanium atoms.Cave sites denote that the X clusters locate along to the site bridging two 5-fold coordinated titanium atoms and two 3-fold oxygens,while hollow sites express that the X clusters is on the site bridging two 6-fold titanium atomsand two 3-fold oxygens.

Table 1 Structural parameters of bulk rutile:comparing this work with other experimental and theoretical results
3.2 Coverage effect on the ground-state structure and energy
The adsorption of per bimetallic cluster(Pt-Au and Au-Au) on the above-depicted supercell model was first investigated by performing calculations in which the bimetallic cluster occupies all the six adsorption sites as shown in Fig.1.Based on thermodynamic favorability,the most stable structure for single-molecule adsorption is determined from several possible structural candidates(Fig.2(a)and Fig.3(a)).Many more structures are obtained but we do not show them here for clarity. Then we turn to study the certain fixed coverage patterns(θ= 1/6-1 ML)where the bimetallic clusters only locate on the most favorable site.Notice that only the ground-state configurations will be discussed in this work.
The ground-state structures corresponding to the given coverage patterns(θ=1/6-1 ML)for Pt-Au and Au-Au clusters adsorbed on the defect-free TiO2(110)surface are determined,respectively(Figs.2 and 3).For the Pt-Au cluster at the adsorption coverage of 1/6 ML,Fig.2(a)shows that the Pt atom is bonded to a protruded oxygen atom and a 5-fold coordinated titanium atom,while Au atom also binds to the Ti5catom with the Pt-Au bond parallel to the[1ˉ10]direction.With the increase of coverage,the degree of surface buckling is strengthen, whereas there is no interaction between Au atoms and the surface(as shown in Fig.2(b-f)).The Pt-Au cluster is bonded to the surface symmetrically except for Fig.2(b,e)in which there is some noticeable interaction between two adjacent Pt-Au clusters.For the additions of Au-Au clusters onto TiO2(110) surface,the as-formed surface structure remains practically unaltered from the lower to the saturated coverage with the Au-Au dimer symmetrical.
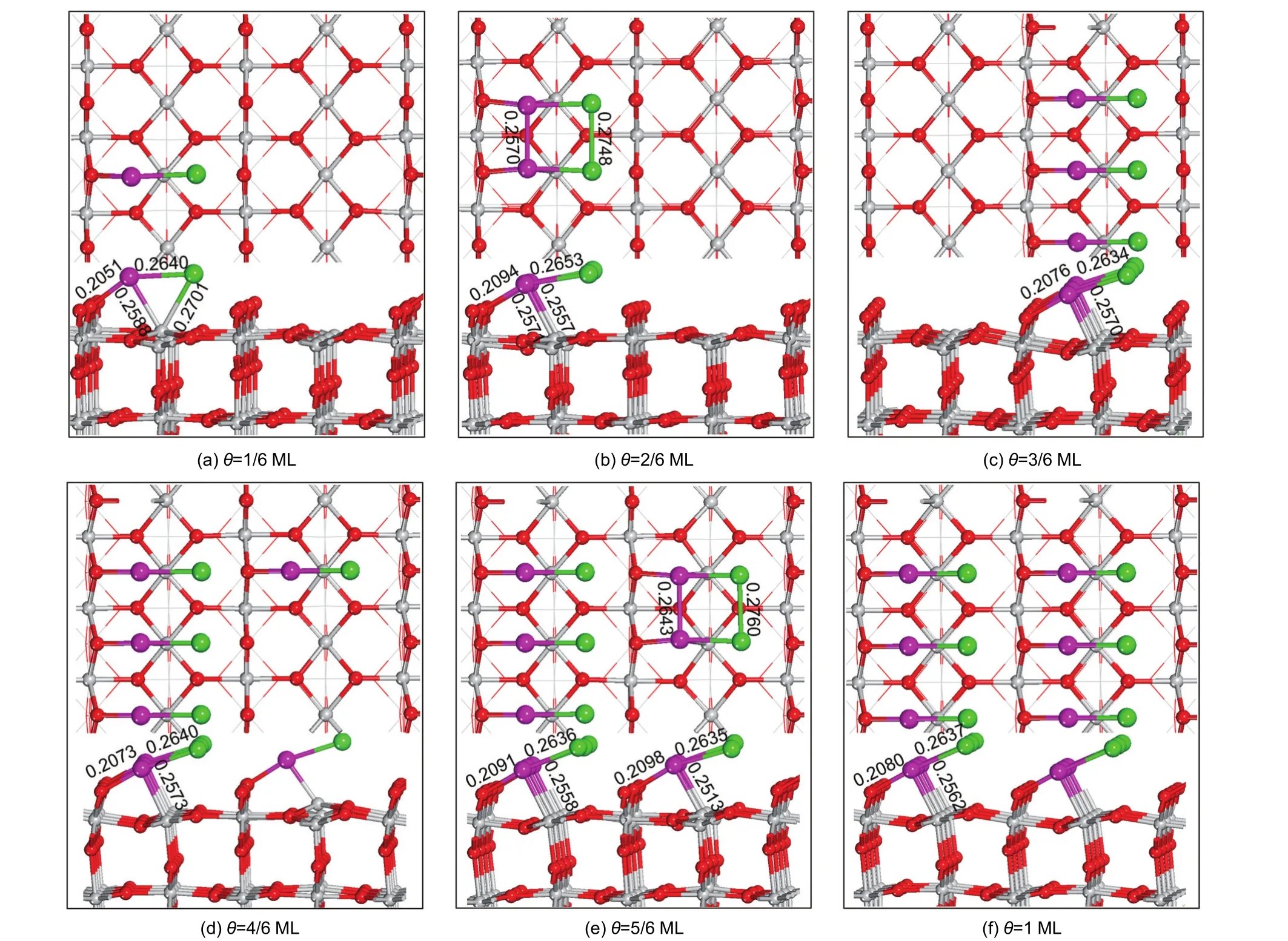
Fig.2 Top and side views for Pt-Au bimetallic clusters on the defect-free(3×2)TiO2(110)surface at six different coverage levelsOnly part of the TiO2(110)surface and structural parameters are shown for display purposes.The bond lengths labeled in the figures are in nm.
Showing a clear resemblance to Pt-Au bimetallic clusters, Au-Au clusters are on the atop site symmetrically above the fivefold-coordinate titanium surface atom with the Au―Au bond along the[1ˉ10]direction.This observation is qualitatively in accordance with the theoretical works reported by Thiên-Nga57and Yang58et al.,and also in agreement with experimental STM images where chains of gold atoms on Ti5csites have been found.59On the basis of single-molecule adsorption(θ=1/ 6 ML),of particular importance is that it turns out to be preferable for the next clusters(Pt-Au and Au-Au)to interact with TiO2(110)surface along the[001]direction rather than the[1ˉ10] direction(Fig.2 and Fig.3).Take Pt-Au bimetallic clusters(θ= 2/6 ML)for example,along the[1ˉ10]direction the adsorption structure has an adsorption energy of 444.33 kJ·mol-1while 530.42 kJ·mol-1is for the[001]direction.Thus the combination way of two adsorbed clusters definitely influences the adsorption energy.
The change trend of the spin state as well as the adsorption energy per adsorbate with increasing coverage for the additions of bimetallic clusters(Pt-Au and Au-Au)onto the TiO2(110) surface is shown in Fig.4.As can been seen,for Pt-Au clusters, the adsorption systems with odd-numbered Au atoms(1/6,3/6, and 5/6 ML)exist in the doublet spin state,while singlet spin state is the ground state for the configurations at the coverage of 2/6,4/6,and 1 ML.Relatively,all the Au-Au/TiO2adsorption structures prefer to remain in the singlet spin state,corroborating that the corresponding ground state with fixed coverage patterns has no unpaired electrons.However,it is important to notice that the defect-free TiO2(110)surface has not any net spin.60Consequently,the resulting spin arises solely from the adsorbed metallic clusters.As for the adsorption energy,it increases as the coverage is less than 3/6 ML and then decreases except for the saturated coverage,while the adsorption energy for the half coverageis higher than that of the saturated coverage.Regarding the addition of Pt-Au(Au-Au)clusters onto TiO2(110),the adsorption energies are 445.86,530.42,581.07, 547.66,549.95,and 573.46 kJ·mol-1(599.94,610.23,649.84, 629.40,619.64,and 632.73 kJ·mol-1),corresponding to that with a coverage of 1/6-1 ML,respectively.Note that the ad-sorption energy for Pt-Au clusters is lower than that for Au-Au clusters,which can be attributed to the disparity in their bonding modes.Moreover,the large adsorption energies at the high coverage show the feasibility of forming a monolayer structure for Pt-Au and Au-Au clusters onto the TiO2(110)surface.In forthcoming discussion we will suggest a possible explanation to the adsorptive mechanisms.

Fig.3 Top and side views forAu-Au clusters on the defect-free(3×2)TiO2(110)surface at six different coverage levelsOnly part of the TiO2(110)surface and structural parameters are shown for display purposes.The bond lengths labeled in the figures are in nm.
3.3 Coverage effect on electronic properties
To gain an insight into the coverage effect on electronic properties of the above-depicted adsorption system,we have reported the total density of states(TDOS)as well as the partial DOS projected on the adsorbate and substrate at different coverage levels,plots(see Figs.5 and 6)were computed using the 3×2 three-layer thick model slab.For comparison,the corresponding DOS of clean surface is also displayed.
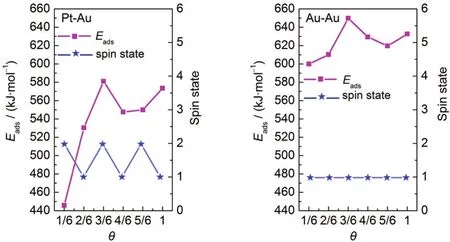
Fig.4 Variation of the adsorption energy per adsorbate(Eads)and spin state with the coverage for the additions of X bimetallic clusters (X=Pt-Au,Au-Au)onto the TiO2(110)surface
A point of interest we have emphasized is the peak around the Fermi level and some characteristic peaks.The valence band of the bare TiO2surface arises from O 2p states,while the conduction band is contributed by the empty Ti 4d states.Notice that,as well-known from the tendency of GGA calculations,61the band gap appears clearly underestimated in comparison with the experimental value(3.0 eV).After adsorption, there is an obvious difference in the electronic structure of the adsorption system at all the coverage levels vis-à-vis the original perfect surface.As can be seen from Figs.5 and 6,the TDOS of the adsorption system is almost dominated by the substrate,while the adsorbate contributions are very small.According to Fig.5,the partial DOS projected on the adsorbate exhibits peaks ranging from-5.0 eV to Fermi level,which can be traced back to the 5d/6s orbital of the Pt-Au bimetallic cluster.Upon closer inspection,the Fermi level crosses from the top of valence band to the bottom of conduction band as the coverage increases,with the Fermi level pinned to the bottom conduction band at the high coverage.For the half monolayer coverage,i.e.,one of the sides of a rutile(110)slab is covered by the bimetallic clusters molecular layer,the peak of the substrate at about 1.5 eV diminishes,yielding the large split between the highest occupied crystal orbital(HOCO)and the lowest unoccupied crystal orbital(LUCO).Above the Fermi level,the contributions from the Pt-Au 5d/6s orbitals are quite weak and featureless while those from the surface molecular orbitals are much more pronounced and lie lower than 1.5 eV. Of particular importance,especially for the half and saturated coverage,is that there is a broadening of Pt-Au peaks overlap with the TiO2state ranging from-3.0 eV to Fermi level,indicating the reasonably strong interaction between the surface and bimetallic cluster.This result is faithfully in accordance with their big adsorption energies as noted above.In addition, the strong interaction can also be verified by the peaks of TiO2state shifting to the lower energy region.As for the Au-Au/ TiO2adsorption system,the trend of the changes in DOS shows a clear resemblance to that of TiO2(110)surface functionalized by Pt-Au bimetallic clusters.However,it is still interesting to realize that there is some essential disparity between Pt-Au and Au-Au adsorption,which we will discussed in the forthcoming section.

Fig.5 Total density of states(TDOS)and projected DOS for the adsorbate and substrate on the TiO2(110)surface functionalized by Pt-Au bimetallic clusters as a function of coverageThe corresponding DOS of clean surface is also shown for comparison. The Fermi level(EF)has been set to be zero.

Fig.6 TDOS and projected DOS for the adsorbate and substrate of the TiO2(110)surface functionalized byAu-Au clusters as a function of coverageThe corresponding DOS of bare surface is also shown for comparison. The Fermi level(EF)has been set to be zero.
3.4 Coverage effect on the adsorptive mechanisms of X clusters on TiO2(110)surface
A key aspect in the study of the metal-surface adsorption interaction may rely on the two competitive paths during the deposition process,i.e.,the inter-metal clustering and the metal binding with the surface.That is,the formation of supported metal cluster is mainly driven by two factors.One arises from the interaction between metal adatoms and the supported surface,and the other is the metal-metal interaction within the cluster.To better understand the adsorptive mechanism for X bimetallic clusters(X=Pt-Au,Au-Au)onto the rutile TiO2(110) surface,herein,the above related two interaction is introduced as follows:
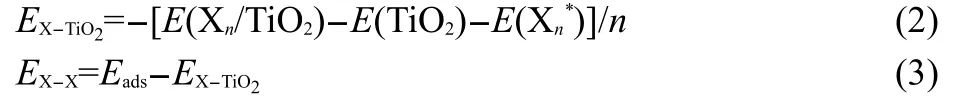
where EX-TiO2and EX-Xstand for the contribution of X-TiO2binding interaction and the X-X clustering interaction,respectively.Importantly,E(Xn*)is the total energy of adsorbed Xncluster,not in the gas phase,with n representing the number of adsorbed bimetallic clusters.

Fig.7 Plots of the binding energies per adsorbate(EX-TiO2)and the clustering energies(EX-X)with the different number(N)of X clusters(X=Pt-Au,Au-Au)on the perfect TiO2(110)surface
The variation of EX-TiO2and EX-Xwith the number of clusters is illustrated in Fig.7.As the the number of X gets larger,overall,the EX-TiO2decreases whereas the EX-Xincreases.In other words,the interaction between each metallic cluster and TiO2surface drastically decreases while the X-X interaction within the cluster becomes gradually stronger as the coverage increases.However,up to a saturated coverage of 1 ML,the EX-TiO2does not decrease but increase with respect to that of 5/6 ML which is in consistent with the change of their adsorption energies as mentioned above.It is clear that the interaction within the Pt-Au metallic clusters is much stronger than that of Au-Au clusters,which can be attributed to the smaller atomic volume of Pt vis-à-vis Au atom.Hence,it is more feasible to form a mono-layer film of Pt-Au bimetallic clusters onto the TiO2(110)surface than that of Au-Au clusters at the saturated coverage,albeit the adsorption energy of the Pt-Au/TiO2adsorption system is smaller in comparison with the Au-Au/TiO2system. Of particular interest,according to the prior discussion,is that the X-TiO2interaction is the main driving force at the initial stage of the adsorption process,whereas the X-X interaction turns out to control the process as the coverage increases.
4 Conclusions
In this work,first-principles calculations based on spinpolarized density functional theory and generalized gradient approximation method have been used to study the coverage-dependent adsorption of X bimetallic clusters(X=Pt-Au,Au-Au) onto the(3×2)TiO2(110)surface,in the absence of oxygen vacancy sites.The calculations employ slab geometry and periodic boundary conditions with full relaxation of all atomic positions.Notice that,herein,only the ground-state configurations corresponding to the given coverage patterns(θ=1/6-1 ML) for Pt-Au and Au-Au clusters have been discussed.Unambiguously,as for the adsorption energy,it increases as the coverage is less than 1/2 ML,while the adsorption energy for the half coverage is higher than that of the saturated coverage.Moreover,according to the interaction within X clusters,it is more feasible to yield a monolayer film of Pt-Au bimetallic clusters onto the surface than that of Au-Au clusters at all the coverage levels,albeit the adsorption energy of the Pt-Au/TiO2adsorption system is smaller in comparison with the Au-Au/TiO2system.Importantly,especially for the half and saturated coverages,there is a broadening of X peaks overlap with the TiO2state ranging from-3.0 eV to Fermi level,showing the reasonably strong interaction between the surface and bimetallic cluster. Of particular interest,as for the adsorptive mechanisms,is that the X-TiO2interaction is the main driving force at the initial stage of the adsorption process,whereas the X-X interaction turns out to control the process as the coverage increases.
(1) Harada,M.;Asakura,K.;Toshima,N.J.Phys.Chem.1993,97, 5103.doi:10.1021/j100121a042
(2)Luo,J.;Maye,M.M.;Petkov,V.;Kariuki,N.N.;Wang,L.Y.; Njoki,P.;Mott,D.;Lin,Y.;Zhong,C.J.Chem.Mater.2005,17, 3086.doi:10.1021/cm050052t
(3) Scott,R.W.J.;Sivadinarayana,C.;Wilson,O.M.;Yan,Z.; Goodman,D.W.;Crooks,R.M.J.Am.Chem.Soc.2005,127, 1380.doi:10.1021/ja044446h
(4)Wang,Y.;Toshima,N.J.Phys.Chem.B 1997,101,5301.doi: 10.1021/jp9704224
(5) Belloni,J.;Mostafavi,M.;Remita,H.;Marignier,J.L.; Delcourt,M.O.New J.Chem.1998,22,1239.doi:10.1039/ a801445k
(6) Link,S.;Wang,Z.L.;El-Sayed,M.A.J.Phys.Chem.B 1999, 103,3529.doi:10.1021/jp990387w
(7) Fang,Y.H.;Liu,Z.P.J.Am.Chem.Soc.2010,132,18214.doi: 10.1021/ja1069272
(8) Notoya,F.;Su,C.;Sasaoka,E.Ind.Eng.Chem.Res.2001,40, 3732.doi:10.1021/ie000972f
(9) Diebold,U.Surf.Sci.Rep.2003,48,53.doi:10.1016/S0167-5729(02)00100-0
(10) Pang,C.L.;Lindsay,R.;Thornton,G.Chem.Soc.Rev.2008, 37,2328.doi:10.1039/b719085a
(11)Wang,C.M.;Fan,K.N.;Liu,Z.P.J.Am.Chem.Soc.2007, 129,2642.doi:10.1021/ja067510z
(12) Gong,X.Q.;Liu,Z.P.;Raval,R.;Hu,P.J.Am.Chem.Soc. 2004,126,8.doi:10.1021/ja030392k
(13) Yudanov,I.;Pacchioni,G.;Neyman,K.;Ro1sch,N.J.Phys. Chem.B 1997,101,2786.doi:10.1021/jp962487x
(14) Rodriguez,J.A.;Liu,G.;Jirsak,T.;Hrbek,J.;Chang,Z.; Dvorak,J.;Maiti,A.J.Am.Chem.Soc.2002,124,5242.doi: 10.1021/ja020115y
(15) Pabisiak,T.;Kiejna,A.Surf.Sci.2011,605,668.doi:10.1016/ j.susc.2010.12.033
(16) Henderson,M.A.Surf.Sci.Rep.2002,46,1.doi:10.1016/ S0167-5729(01)00020-6
(17)Chen,S.H.;Xu,Y.;Lü,B.L.;Wu,D.Acta Phys.-Chim.Sin. 2011,27,2933.[陈淑海,徐 耀,吕宝亮,吴 东.物理化学学报,2011,27,2933.]doi:10.3866/PKU.WHXB20112933
(18)Dong,H,Q.;Pan,X.;Xie,Q.;Meng,Q.Q.;Gao,J.R.;Wang,J. G.Acta Phys.-Chim.Sin.2012,28,44.[董华青,潘 西,谢 琴,孟强强,高建荣,王建国.物理化学学报,2012,28, 44.]doi:10.3866/PKU.WHXB20122844
(19) Thompson,T.L.;Yates,J.T.Chem.Rev.2006,106,4428.
(20) Ganduglia-Pirovano,M.V.;Hofmanna,A.;Sauer,J.Surf.Sci. Rep.2007,62,219.doi:10.1016/j.surfrep.2007.03.002
(21) Tomohito,T.;Matsunaga,K.;Ikuhara,Y.;Yamamoto,T.Phys. Rev.B 2003,68,205213.doi:10.1103/PhysRevB.68.205213
(22) Perron,H.;Domain,C.;Roques,J.;Drot,R.;Simoni,E.; Catalette,H.Theor.Chem.Acc.2007,117,565.doi:10.1007/ s00214-006-0189-y
(23) Ramamoorthy,M.;Vanderbilt,D.;King-Smith,R.D.Phys.Rev. B 1994,49,16721.doi:10.1103/PhysRevB.49.16721
(24) Cui,H.F.;Ye,J.S.;Liu,X.;Zhang,W.D.;Sheu,F.S. Nanotechnology 2006,17,2334.doi:10.1088/0957-4484/17/9/ 043
(25) Qian,L.;Yang,X.R.J.Phys.Chem.B 2006,110,16672.doi: 10.1021/jp063302h
(26)Wu,M.L.;Chen,D.H.;Huang,T.C.Chem.Mater.2001,13, 599.doi:10.1021/cm0006502
(27) Lu,Y.;Yuan,J.Y.;Polzer,F.;Drechsler,M.;Preussner,J.Nano 2010,4,7078.
(28) Hernández-Fernández,P.;Rojas,S.;Ocón,P.;Gómez de la Fuente,J.L.;San Fabián,J.;Sanza,J.;Peña,M.A.;García-García,F.J.;Terreros,P.;Fierro,J.L.G.J.Phys.Chem.C 2007,111,2913.doi:10.1021/jp066812k
(29) Tenney,S.A.;Ratliff,J.S.;Roberts,C.C.;He,W.;Ammal,S. C.;Heyden,A.;Chen,D.A.J.Phys.Chem.C 2010,114,21652. doi:10.1021/jp108939h
(30) Park,J.B.;Conner,S.F.;Chen,D.A.J.Phys.Chem.C 2008, 112,5490.doi:10.1021/jp076027n
(31)Wen,D.;Guo,S.;Wang,Y.;Dong,S.Langmuir 2010,26, 11401.doi:10.1021/la100869r
(32) Boronat,M.;Corma,A.Langmuir 2010,26,16607.doi: 10.1021/la101752a
(33) Payne,M.;Teter,M.;Allan,D.;Arias,T.;Joannopoulos,J.Rev. Mod.Phys.1992,64,1045.doi:10.1103/RevModPhys.64.1045
(34) Hohenberg,P.;Kohn,W.Phys.Rev.B 1964,136,864.doi: 10.1103/PhysRev.136.B864
(35) Jones,R.;Gunnarsson,O.Rev.Mod.Phys.1989,61,689.doi: 10.1103/RevModPhys.61.689
(36) Kohn,W.;Sham,L.Phys.Rev.1965,140,1133.doi:10.1103/ PhysRev.140.A1133
(37) Perdew,J.P.;Wang,Y.Phys.Rev.B 1992,45,13244.doi: 10.1103/PhysRevB.45.13244
(38) Delley,B.J.Chem.Phys.1990,92,508.doi:10.1063/1.458452
(39) Delley,B.J.Chem.Phys.2000,113,7756.doi:10.1063/ 1.1316015
(40) Goniakowski,J.;Holender,J.M.;Kantorovich,L.N.;Gillan, M.J.Phys.Rev.B 1996,53,957.doi:10.1103/PhysRevB. 53.957
(41) Sanz,J.F.;Márquez,A.J.Phys.Chem.C 2007,111,3949.doi: 10.1021/jp0639952
(42) Goniakowski,J.;Gillan,M.J.Surf.Sci.1996,350,145.doi: 10.1016/0039-6028(95)01252-4
(43) White,J.A.;Bird,D.M.;Payne,M.C.;Stich,I.Phys.Rev.Lett. 1994,73,1404.doi:10.1103/PhysRevLett.73.1404
(44) Versluis,L.;Zeigler,T.J.Chem.Phys.1988,88,322.doi: 10.1063/1.454603
(45) von Barth,U.;Hedin,L.J.Phys.C 1972,5,1629.doi:10.1088/ 0022-3719/5/13/012
(46) Matsuzawa,N.;Seto,J.E.;Dixon,D.A.J.Phys.Chem.A 1997, 101,9391.doi:10.1021/jp952465v
(47) Monkhorst,H.J.;Pack,J.D.Phys.Rev.B 1976,13,5188.doi: 10.1103/PhysRevB.13.5188
(48) Fernandez,S.;Markovits,A.;Miont,C.J.Phys.Chem.C 2008, 112,14010.doi:10.1021/jp800708u
(49) Zou,X.J.;Ding,K.N.;Zhang,Y.F.;Li,J.Q.Int.J.Quantum Chem.2011,111,915.doi:10.1002/qua.22454
(50) Zhu,J.;Jin,H.;Chen,W.J.;Li,Y.;Zhang,Y.F.;Ning,L.X; Huang,X.;Ding,K.N;Chen,W.K.J.Phys.Chem.C 2009, 113,17509.doi:10.1021/jp906194t
(51) Mazheika,A.S.;Matulis,V.E.;Ivashkevich,O.A.J.Mol. Struct.2009,909,75.
(52)Zeng,Q.S.;Chen,W.K.;Dai,W.X.;Zhang,Y.F.;Li,Y.;Guo, X.Chin.J.Catal.2010,31,423. [曾庆松,陈文凯,戴文新,章永凡,李 奕,郭 欣.催化学报,2010,31,423.]
(53) Lopez,N.;Nørskov,J.K.Surf.Sci.2002,515,175.doi: 10.1016/S0039-6028(02)01873-3
(54) Markovits,A.;Paniagua,J.C.;López,N.;Minot,C.;Illas,F. Phys.Rev.B 2003,67,115417.doi:10.1103/PhyRevB. 67.115417
(55)Labat,F.;Baranek,P.;Domain,C.;Minot,C.;Adamo,C. J.Chem.Phys.2007,126,154703.doi:10.1063/1.2717168
(56) Burdett,J.K.;Hughbanks,T.;Miller,G.J.;Richardson,J.W., Jr.;Smith,J.V.J.Am.Chem.Soc.1987,109,3639.doi: 10.1021/ja00246a021
(57) Thiên-Nga,L.;Paxon,A.T.Phys.Rev.B 1998,58,13233.doi: 10.1103/PhysRevB.58.13233
(58)Yang,Z.;Wu,R.;Goodman,D.W.Phys.Rev.B 2000,61, 14066.doi:10.1103/PhysRevB.61.14066
(59) Lai,X.;Clair,T.P.S.;Valden,M.;Goodman,D.W.Prog.Surf. Sci.1998,59,25.doi:10.1016/S0079-6816(98)00034-3
(60) Bates,S.P.;Kresse,G.;Gillan,M.J.Surf.Sci.1997,385,386. doi:10.1016/S0039-6028(97)00265-3
(61) Mattsson,A.E.;Jennison,D.R.Surf.Sci.2002,520,L611.
March 20,2012;Revised:May 21,2012;Published on Web:May 21,2012.
Coverage-Dependent Adsorption of X Clusters(X=Pt-Au,Au-Au)on the Defect-Free(3×2)TiO2(110)Surface
ZHAO Wei-Na1LIN Hua-Xiang2LI Yi1ZHANG Yong-Fan1HUANG Xin1CHEN Wen-Kai1,2,*
(1College of Chemistry and Chemical Engineering,Fuzhou University,Fuzhou 350116,P.R.China;2Fujian Provincial Key Laboratory of Photocatalysis-State Key Laboratory Breeding Base,Fuzhou University,Fuzhou 350002,P.R.China)
Based on spin-polarized density functional theory and generalized gradient approximation (DFT-GGA)calculations,the coverage-dependent adsorption of X bimetallic clusters(X=Pt-Au,Au-Au)on the(3×2)TiO2(110)surface has been investigated utilizing periodic supercell models in the absence of oxygen vacancy sites.Only the ground-state structures corresponding to the given coverage patterns(θ= 1/6-1 ML)for X clusters are discussed in this work.The unambiguous results reveal that the adsorption energies increase with coverage up to 1/2 ML and then decrease except for when saturated coverage is reached.According to the interaction with X clusters,it is more feasible at all coverage levels to create a monolayer film of Pt-Au bimetallic clusters on the TiO2(110)surface than it is to create a monolayer of Au-Au clusters,even though the adsorption energy of the Pt-Au/TiO2adsorption system is smaller in comparison with that of the Au-Au/TiO2system.Importantly,especially for the half and saturated coverages,there is a broadening of X peaks overlapping with the TiO2state ranging from-3.0 eV to the Fermi level,suggesting a strong interaction between the surface and bimetallic cluster.Also of particular interest is the adsorptive mechanism where the X-TiO2interaction is the main driving force at the initial stage of the adsorption process,whereas the X-X interaction controls the process as the coverage increases.
Density functional theory;TiO2(110)surface;Bimetallic cluster
10.3866/PKU.WHXB201205214
∗Corresponding author.Email:qc2008@fzu.edu.cn;Tel:+86-591-22866162.
The project was supported by the National Natural Science Foundation of China(90922022),Natural Science Foundation of Fujian Province,China (2012101032,2012101041),and Foundation of State Key Laboratory of Coal Combustion of Huazhong University of Science and Technology, China(FSKLCC1110).
国家自然科学基金(90922022),福建省自然科学基金(2012J01032,2012J01041)和华中科技大学煤燃烧国家重点实验室开放基金(FSKLCC1110)资助项目
O641