Bi3.25La0.75Ti3O12纳米线的可见光催化性能
林 雪 吕 鹏 关庆丰,* 李海波 李洪吉 蔡 杰 邹 阳
(1江苏大学材料科学与工程学院,江苏镇江212013;2吉林师范大学化学学院,环境友好材料制备与应用教育部重点实验室,吉林四平136000;3吉林师范大学物理学院,吉林四平136000)
Bi3.25La0.75Ti3O12纳米线的可见光催化性能
林 雪1,2吕 鹏1关庆丰1,*李海波3李洪吉2蔡 杰1邹 阳1
(1江苏大学材料科学与工程学院,江苏镇江212013;2吉林师范大学化学学院,环境友好材料制备与应用教育部重点实验室,吉林四平136000;3吉林师范大学物理学院,吉林四平136000)
研究了用一步水热法制备的掺镧钛酸铋(Bi3.25La0.75Ti3O12,BLT)纳米线的光学和可见光催化性能,并对其晶体结构和微观结构用X射线衍射(XRD)、透射电子显微镜(TEM)和高分辨透射电子显微镜(HRTEM)等手段进行了表征.结果表明,制备的纳米线为纯相的Bi3.25La0.75Ti3O12,平均直径约为25 nm.室温光致发(PL)光谱显示BLT纳米线在433和565 nm附近有较强的发射峰,分别对应激子发射和表面缺陷发光.紫外-可见漫反射光谱(UV-Vis DRS)表明BLT样品的带隙能约为2.07 eV.利用可见光(λ>420 nm)照射下的甲基橙(MO)降解实验评价了BLT样品的光催化性能.结果表明,BLT的光催化活性比商用TiO2催化剂P25、掺氮TiO2和纯相钛酸铋(Bi4Ti3O12,BIT)高得多.BLT光催化剂具有更高催化活性的原因是La3+离子的掺杂拓展了BIT对可见光的吸收范围,同时抑制了BIT的光生电子-空穴的复合.
钛酸铋;掺镧;纳米线;水热合成;光催化降解;可见光照射
1 Introduction
The worldwide quest for clean and renewable energy sources has encouraged a great deal of research activities and development in the field of solar energy in the last twenty years.Solar energy as a clean energy is inexhaustible in supply and always available for use.Therefore,high efficient catalyst,photochemical cell and solar cell have become the hotspot of scientific research.Since the discovery of the photocatalytic splitting of water on the TiO2electrodes by Fujishima and Honda1in 1972,the application of semiconductor photocatalysts on degradation ofpollutantshasreceived greatattention.2-7Among all photocatalysts,TiO2attracts the most attention due to its chemical stability,low cost,nontoxicity,and high photocatalytic activity.8-12However,the band gap energy of the TiO2is 3.2 eV.It absorbs only the ultraviolet light(λ≤386.5 nm) which only accounts for about 4.0%of the sunlight.In order to improve the efficiency of the sunlight utilization,the development of photocatalysts with high activity under a wide range of visible light is highly desirable.13-15
In recent years,bismuth titanate photocatalysts,such as Bi12TiO20,16Bi2Ti2O7,17and Bi4Ti3O12(BIT),18have been widely studied as a class of promising photocatalysts which can respond under visible light.Among bismuth titanate photocatalysts,Bi4Ti3O12(BIT)has received more attention for its high photocatalytic ability in degrading the organic pollutants.18-20Photocatalytic properties of BIT have been examined before. Kudo et al.18had reported the preparation of BIT by the solidsolid method and examined the photocatalytic activity of BIT for water splitting.Recently,Yao et al.19,20reported the preparation of BIT by using the chemical solution decomposition (CSD)method and examined the photocatalytic activity for oxidizing methyl orange(MO).In our previous work,21we presented the hydrothermal synthesis of BIT microspheres and tested the photocatalytic properties of the as-prepared BIT spheres. Furthermore,Metal element doping is one of the typical approaches to extend the spectral response of BIT photocatalysts by providing defect states in the band gap.22-24
However,few papers have revealed the photocatalytic activity of Lanthanum doped bismuth titanate(Bi3.25La0.75Ti3O12,BLT) crystals for oxidizing organic contaminants in water.It is known that the activity of photocatalysts is influenced by a wide variety of factors,such as the catalyst preparation conditions,crystal morphology,the adsorption affinity and capacity for organic contaminants,pH,intrinsic solid state defects and so on.The aim of the present paper is to study the influence of La-doping on the microstructure,optical properties,and photocatalytic properties of BIT photocatalysts.
2 Experimental
2.1 Preparation of BLT photocatalysts
All the chemicals were analytically graded(purchased from Shanghai Chemical Industrial Company)and used without further purification.BLT photocatalysts were prepared with a one-step hydrothermal synthesis.24Bismuth nitrate(Bi(NO3)3· 5H2O),lanthanum nitrate(La(NO3)3·6H2O),and titanium tetrachloride(TiCl4)were chosen as starting materials with the bismuth:lanthanum:titanium ions molar ratios of 3.25:0.75:3.00. TiCl4(10 mL)was dissolved in cold water(50 mL)under vigorous stirring,then mixed with Bi(NO3)3·5H2O and La(NO3)3· 6H2O.The concentration of the alkali solution was adjusted using KOH.Before being transferred to a 20 mL stainless steel autoclave,the solution mixture was prepared under an ultrasonic water bath for 30 min and kept at a filling ratio of 70%(volume fraction).The autoclave was kept at 180°C for 24 h,and cooled to room temperature after the reaction.The precipitates were washed with deionized water and ethanol three times,separately.The final products were dried at 100°C for 2 h in a vacuum box.The samples prepared for comparison are(i)BIT and (ii)N doped TiO2(N-TiO2).For more details about the preparation of BIT and N-TiO2,the readers can refer to our previous work.24-26
2.2 Characterization of photocatalysts
The crystal structures of the samples were characterized by X-ray diffraction(XRD,America PE,D/max 2500)with Cu Kαradiation.Transmission electron microscopy (TEM)and high-resolution transmission electron microscopy(HRTEM) were conducted using a JEM-2100F(Japan JEOL)instrument. The surface areas of samples were measured by TriStar 3000-BET/BJH Surface Area.The optical property was obtained by thephotoluminescence(PL)measurementusing HR800 LabRam Infinity Spectro photometer excited by a continuous He-Cd laser with a wavelength of 325 nm at a power of 50 mW.The UV-Vis diffuse reflectance spectra(DRS)were recorded for the dry-pressed disk sample using a scan UV-Vis spectrophotometer(UV-Vis,Japan SHIMADZU,UV-2550) equipped with an integrating sphere assembly.
2.3 Photocatalytic activity test
The photocatalytic degradation of MO was employed to evaluate the photocatalytic activities of the samples.A 300 W Xe lamp(λ>420 nm)was used to provide visible light irradiation.Photocatalyst(0.10 g)was added to 100 mL of MO solution(0.01 mmol·L-1).Before irradiation,the suspensions were magnetically stirred in the dark for 30 min to ensure the adsorption-desorption equilibrium between the photocatalysts and MO.Then the solution was exposed to visible light irradiation under magnetic stirring.At given time intervals,4 mL of suspension was sampled and centrifuged to remove the photocatalyst particles.Then,the catalyst-free dye solution was analyzed by a UV-2550 spectrometer to record intensity of the maximum band at 462 nm in the UV-Vis absorption spectra.
3 Results and discussion
3.1 XRD analysis
XRD patterns of the as-prepared BLT products synthesized at OH-concentrations of 3 and 8 mol·L-1,respectively,are shown in Fig.1.All the reflection peaks can be indexed according to the JCPDS card No.36-1486,suggesting that the as-prepared products are of layered-perovskite structure(Bi4Ti3O12). No peaks of impurities were detected from the patterns.The strong and sharp peaks indicate high crystallinities of BLT samples.
3.2 TEM analysis
TEM was employed to observe the morphologies and structure details of BLT products.Fig.2a shows the TEM images of a typical example of nanoparticles.It can be observed that BLT product prepared at OH-concentration of 3 mol·L-1was composed of nano-sized particles with average size about 20 nm, and each particle is nearly spherical in shape(as shown in Fig.2b).Fig.2c gives BLT sample obtained at OH-concentration of 8 mol·L-1.It can be seen that there are BLT nanowires with width of approximate 25 nm and lengths up to several micrometres.Further structure details of BLT nanowires were obtained by TEM,as illustrated in Fig.2d.It reveals that BLT nanowires are made up of nanoparticles with average size of about 4 nm.These results show that OH-concentration seems to play a key role in controlling the morphologies of BLT crystals.
Although the crystal growth habit is mainly determined by the intrinsic structure,it is also affected by the external conditions such as pH of the solution,saturation,temperature and so on.23As we all know,OH-concentration in the precursor solution has been found to be very important for the microstructure.It has been reported that the morphologies of Bi2Ti2O7crystals can be controlled by adjusting the OH-concentrations suggesting that OH-ionscan behave as a surfactant,17obtaining a better understandingof the role of OH-ions in the hydrothermal process.On the basis of our previous report about Bi3.25Nd0.75Ti3O12(BNdT)nanostructure and BNdT nanosheets and nanowires,24at lower concentration of OH-,only nanoparticles were obtained.When the OH-concentration was increased,nanowires were obtained.Our experimental results are in accordance with the above mechanism.Thus,the pH value plays an important role in controlling the formation of seeds and the growth rates to shape BLT particles.

Fig.1 XRD patterns of BLT and BIT samplesT=180°C,t=24 h
In this work,the condition of the alkaline medium as a factor is considered to play a key part in the formation of BLT nanowires.At lower OH-concentration(3 mol·L-1),BLT nuclei produced in solution can aggregate to form small particles. These particles may serve as crystal seeds to grow the nanowire structure.With the increase of alkalinity(OH-concentration:8 mol·L-1),a large amount of BLT nuclei produced in the solution,leads to form the very high supersaturation solution, which favors the formation of nanowires structure.Hence,the formation of BLT nanowires in the present route is resulted from the highly alkaline medium.27
In order to investigate the detailed crystal structure of the as-prepared BLT sample,HRTEM images for BLT nanowires were measured,as shown in Fig.3.The lattice distance for BLT sample is calculated to be 0.38 nm,which is in agreement with the(111)lattice plane of the layered-perovskite BIT.Thus,we can conclude that the low concentration doping of La3+ions does not induce the formation of separate purity phases(lanthanum metal).
Fig.4 shows EDX analytical results of BLT composite,the molar ratios of Bi/Ti and Bi/La are calculated to be 1.09 and 4.74,respectively,which are in accordance with the nominal molar ratio of Bi3.25La0.75Ti3O12.
3.3 PL spectral analysis

Fig.2 TEM images of BLT samples obtained at different concentrations of OH-c(OH-)/(mol·L-1):(a,b)3,(c,d)8
Fig.5 shows PL spectra of BLT and BIT samples measured at room temperature at an excitation wavelength of 325 nm. The as-prepared BLT sample showed the presence of two PL bands.The first band was detected at 400 nm(violet emission), while the second was observed at 596 nm(yellow emission).It could be deduced that there exited surface oxygen vacancy in BLT nanowires and the two emission bands might arise respectively from the excitonic emission and surface-defect.28The fluorescence intensity of BLT was significantly weaker than that of BIT product,which showed the recombination restraint of the e-/h+pairs resulting from doping of La3+ions.The reduction of fluorescence indicates that the surface-defect of BLT is much less than that of BIT.And the decrease of surface-defect enhances the activity of photocatalysis.

Fig.3 HRTEM image of BLT nanowiresT=180°C,t=24 h
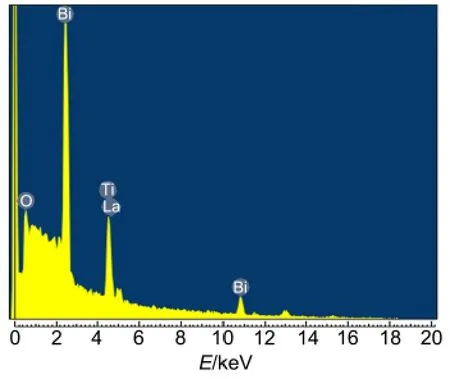
Fig.4 EDX spectrum of the as-prepared BLT sampleT=180°C,t=24 h
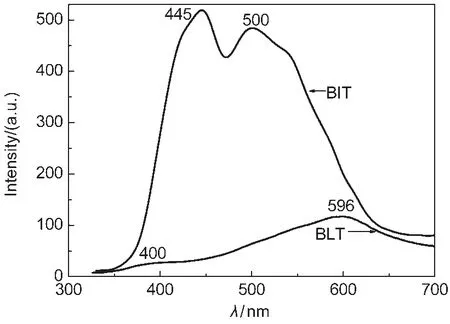
Fig.5 PLspectra of BLT and BIT samples T=230°C,t=24 h
3.4 UV-Vis DRS spectral analysis

Fig.6 UV-Vis DRS of different samples(a)N-TiO2,(b)P25 TiO2,(c)BIT,(d)BLT;A:absorbance.The inset shows the plot of(Ahv)2as a function of photon energy.
Fig.6 shows the UV-Vis DRS of P25 TiO2,N-TiO2,BIT,and BLT photocatalysts.It shows that the absorption onset wavelength(λg)of BLT sample is around 600 nm,which is shifted 175,150,and 50 nm to visible region compared to P25 TiO2, N-TiO2and BIT,respectively.The absorption coefficient(α)as a function of photon energy can be expressed by the Tauc relation:29

where hv,C,and Egare the photon energy,a constant,and the energy gap,respectively.And n is an index determined by the nature of the electron transition during the absorption process. It is well known that there are two types of fundamental optical transitions,namely direct(n=1/2)and indirect(n=2).For BLT, it is a direct band gap semiconductor,so here n=1/2.Since absorbance(A)is proportional to absorption coefficient,we use absorbance to substitute absorption coefficient.22The plot of (Ahv)2versus hv is presented in the inset of Fig.6.Thus,the band gap energy of BLT is calculated to be 2.07 eV,which shows a marked red shift in the absorbance compared to P25 TiO2,N-TiO2,due to the contribution of 6s electrons from Bi3+.22It indicates that BLT photocatalyst has a suitable band gap for photocatalytic decomposition of organic contaminants under visible light irradiation.The absorption spectrum of BLT photocatalyst has steep shape which shows that the absorption relevant to the band gap is due to the intrinsic transition of the nanomaterials.30
3.5 Degradation of MO using BLT photocatalysts
Photodegradation experiments of MO were employed under visible light irradiation to test the photocatalytic performance of N-TiO2,P25 TiO2,BIT,and BLT.UV-Vis spectral changes of MO solution by BLT are displayed in Fig.7(A)while the temporal courses of the photodegradation of MO in different catalyst aqueous dispersions are shown in Fig.7(B).It can be observed that the peaks at 462 and 271 nm are reduced with the increase of irradiation time(Fig.7(A)).It shows that MO solution is stable under visible light irradiation in the absence of any catalyst(Fig.7(B)).The degradation rates of P25 TiO2, N-TiO2,BIT,and BLT are 95.0%,80.0%,52.0%,and 18.0%, respectively.Thus,the addition of BLT photocatalyst leads to the obvious degradation of MO.

Fig.7 (a)UV-Vis spectral changes of MO by BLT sample; (b)Temporal courses of the photodegradation of MO in different catalyst aqueous dispersions;(c)First-order plots for the photocatalytic degradation of MO using different catalysts(a)without catalyst,(b)P25 TiO2,(c)N-TiO2,(d)BIT,(e)BLT
To quantitatively study the photocatalytic reaction kinetics of the MO degradation in the experiments,the degradation data was analyzed with the pseudo-first-order model,as expressed by Eq.(2)as follows:31

where C0and C represent MO concentrations at time zero and t,respectively,and k is the pseudo first-order rate constant.The first-order linear relationship was revealed by the plots of the ln(C/C0)vs irradiation time(t),as shown in Fig.7(C).The reaction rate constant k for P25 TiO2,N-TiO2,BIT,and BLT are determined to be 5.81×10-4,1.94×10-3,4.13×10-3,and 7.85×10-3min-1,respectively(Table 1),indicating the highest photocata-lytic performance of BLT nanowires.The BET surface areas of samples were also measured(as shown in Table 1).Based on the above analysis,it can be concluded that La doping is one of the typical approaches to improve the performance of BIT photocatalysts.

Table 1 BET specific surface area(SBET)and reaction rate constants(k)of samples
3.6 Stability of BLT as the photocatalyst
Fig.8 shows the XRD patterns of the BLT sample before and after 360 min of visible light irradiation.It can be seen that both the position and the intensity of the peaks in the XRD pattern are almost the same to those of BLT before irradiation. Thus,BLT photocatalyst is considered to be relatively stable to visible light irradiation under the present experimental conditions.This result indicates a possibility for application of BLT photocatalyst in the waste water treatment.
The stability tests were also conducted by using recycling reactions four times for the photodegradation of MO over BLT photocatalyst under visible light irradiation,and the results are displayed in Fig.9.No significant decrease in catalytic activity was observed in the recycling reactions.Combined with the XRD patterns,all evidences demonstrate that the BLT photocatalyst is a stable photocatalyst for degradation of MO under visible light irradiation.
3.7 Photocatalytic activity mechanism
On the basis of our previous work,24a schematic diagram of the band levels of doped BIT and the possible reaction mechanism of the photocatalytic procedure are proposed and illustrated.Thus,La doping would result in the improvement of the corresponding photocatalytic properties,as clarified by following equations:

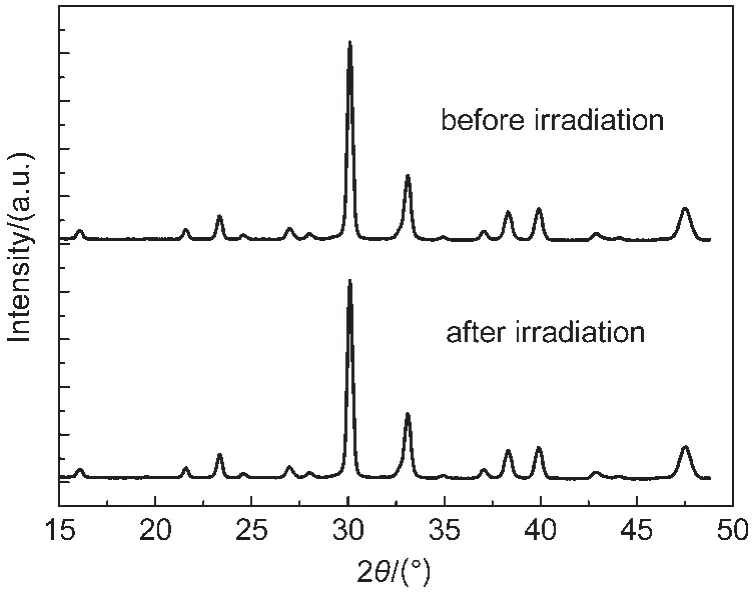
Fig.8 XRD patterns of BLT before and after visible light irradiation

Fig.9 Stability evaluation for BLTfour reaction cycles for photodegradation of MO under visible light irradiation

Furthermore,the photocatalytic activity of the semiconductor is very closely related to its corresponding band structure. The band gap of oxides is generally defined by the O 2p level and transition metal d level.31,32As calculated by Goto et al.33the conduction band(CB)and valence band(VB)of Nd doped bismuth titanate(BNdT)consist mostly of empty Ti 3d and occupied O 2p orbitals,respectively,and the latter is hybridized with Bi 6s and Nd 5d.These bands meet the potential requirements of organic oxidation.Therefore,in the present hybridized VB composed of O 2p and Bi 6s,the photogenerated carriers may own a high mobility.Then it will reduce the recombination opportunities of the photogenerated electron-hole pairs that could effectively move to the crystal surface to degrade the absorbed MO molecules.Based on the above consideration,we presume that the CB and VB of BLT consist mostly of empty Ti 3d and occupied O 2p orbitals,respectively,and the latter is hybridized with Bi 6s and La 5d.The higher photocatalytic activity of BLT over TiO2and BIT is attributed to be suitable band gap and stable e-/h+pair formation in the VB formed by the hybrid orbitals of Bi 6s,La 5d,and O 2p,and the CB of Ti 3d.
4 Conclusions
Bi3.25La0.75Ti3O12nanowires were synthesized by a one-step hydrothermal process without the use of any surfactant or template.The optical band gap of BLT nanowires was estimated to be about 2.07 eV,which proved that BLT photocatalysts could respond to the visible light.Most importantly,BLT photocatalysts with good stability exhibited higher photocatalytic performance in the degradation of methyl orange under visible light irradiation(λ>420 nm)than traditional N doped TiO2,commercial P25 TiO2,and pure BIT.Over this catalyst,the 95.0%degradation of MO solution(0.01 mmol·L-1)was obtained after visible light irradiation for 360 min.In addition,after 4 recycles,there was no significant decrease in its photocatalytic activity,indicating that BLT is a stable photocatalyst for degradation of MO under visible light irradiation.
(1) Fujishima,A.;Honda,K.Nature 1972,238,37.doi:10.1038/ 238037a0
(2)Lu,S.Y.;Wu,D.;Wang,Q.L.;Yan,J.H.;Buekens,A.G.;Cen, K.F.Chemosphere 2011,82,1215.doi:10.1016/j. chemosphere.2010.12.034
(3) Xie,J.;Wang,H.;Duan,M.Acta Phys.-Chim.Sin.2011,27(1), 193. [谢 娟,王 虎,段 明.物理化学学报,2011,27(1), 193.]doi:10.3866/PKU.WHXB20110124
(4) Yang,X.H.;Liu,C.;Liu,J.K.;Zhu,Z.C.Acta Phys.-Chim. Sin.2011,27(12),2939.[杨小红,刘 畅,刘金库,朱子春.物理化学学报,2011,27(12),2939.]doi:10.3866/PKU. WHXB20112939
(5) Hu,Y.F.;Li,Y.X.;Peng,S.Q.;Lü,G.X.;Li,S.B.Acta Phys.-Chim.Sin.2008,24(11),2071.[胡元方,李越湘,彭绍琴,吕功煊,李树本.物理化学学报,2008,24(11),2071.] doi:10.3866/PKU.WHXB20081123
(6) Zhang,L.S.;Wang,H.L.;Chen,Z.G.;Wong,P.K.;Liu,J.S. Appl.Catal.B:Environ.2011,106,1.
(7) Zhang,L.;Cao,X.F.;Chen,X.T.;Xue,Z.L.J.Colloid Interface Sci.2011,354,630.doi:10.1016/j.jcis.2010.11.042
(8) Selishchev,D.S.;Kolinko,P.A.;Kozlov,D.V.J.Photochem. Photobiol.A 2012,229,11.doi:10.1016/j.jphotochem. 2011.12.006
(9) Zhang,J.W.;Jin,Z.S.;Feng,C.X.;Yu,L.G.;Zhang,J.W.; Zhang,Z.J.J.Solid State Chem.2011,184,3066.doi:10.1016/ j.jssc.2011.09.016
(10) Su,Y.R.;Yu,J.G.;Lin,J.J.Solid State Chem.2007,180,2080. doi:10.1016/j.jssc.2007.04.028
(11)Xu,J.;Wang,W.Z.;Shang,M.;Gao,E.P.;Zhang,Z.J.;Ren,J. J.Hazard.Mater.2011,196,426.doi:10.1016/j.jhazmat. 2011.09.010
(12)Wang,H.Q.;Wu,Z.B.;Liu,Y.;Wang,Y.J.Chemosphere 2008, 74,773.
(13) Xu,J.J.;Chen,M.D.;Fu,D.G.Appl.Surf.Sci.2011,257, 7381.doi:10.1016/j.apsusc.2011.02.030
(14) Ghorai,T.K.;Biswas,S.K.;Pramanik,P.Appl.Surf.Sci.2008, 254,7498.doi:10.1016/j.apsusc.2008.06.042
(15) Zhang,Y.L.;Deng,L.J.;Zhang,G.K.;Gan,H.H.Colloids and Surfaces A:Physicochem.Eng.Aspects.2011,384,137. doi:10.1016/j.colsurfa.2011.03.043
(16) Zhang,H.P.;Lü,M.K.;Liu,S.W.;Xiu,Z.L.;Zhou,G.J.; Zhou,Y.Y.;Qiu,Z.F.;Zhang,A.Y.;Ma,Q.Surf.Coat.Tech. 2008,202,4930.doi:10.1016/j.surfcoat.2008.04.081
(17) Hou,J.G.;Jiao,S.Q.;Zhu,H.M.;Kumar,R.V.J.Solid State Chem.2011,184,154.doi:10.1016/j.jssc.2010.11.017
(18) Kudo,A.;Hijii,S.Chem.Lett.1999,28(10),1103.
(19)Yao,W.F.;Xu,X.H.;Wang,H.;Zhou,J.T.;Yang,X.N.; Zhang,Y.;Shang,S.X.;Huang,B.B.Appl.Catal.B:Environ. 2004,52,109.doi:10.1016/j.apcatb.2004.04.002
(20)Yao,W.F.;Wang,H.;Xu,X.H.;Shang,S.X.;Hou,Y.;Zhang, Y.;Wang,M.Mater.Lett.2003,57,1899.doi:10.1016/ S0167-577X(02)01097-2
(21) Lin,X.;Lv,P.;Guan,Q.F.;Li,H.B.;Zhai,H.J.;Liu,C.B. Appl.Surf.Sci.2012,258,7146.doi:10.1016/j.apsusc. 2012.04.019
(22)Wang,Z.Z.;Qi,Y.J.;Qi,H.Y.;Lu,C.J.;Wang,S.M.J.Mater. Sci.:Mater.Electron 2010,21,523.doi:10.1007/s10854-009-9950-z
(23)Yao,W.F.;Wang,H.;Shang,S.X.;Xu,X.H.;Yang,X.N.; Zhang,Y.;Wang,M.J.Mol.Catal.A:Chem.2003,198,343. doi:10.1016/S1381-1169(02)00699-4
(24) Lin,X.;Guan,Q.F.;Li,H.B.;Li,H.J.;Ba,C.H.;Deng,H.D. Acta Phys.-Chim.Sin.2012,28(6),1481. [林 雪,关庆丰,李海波,李洪吉,巴春华,邓海德.物理化学学报,2012,28(6), 1481.]doi:10.3866/PKU.WHXB201203313
(25) Lin,X.;Guan,Q.F.;Li,H.B.;Liu,Y.;Zou,G.T.Sci.China Ser.G 2012,55,33.[林 雪,关庆丰,李海波,刘 洋,邹广田.中国科学G,2012,55,33.]doi:10.1007/s11433-011-4574-8
(26) Xu,G.C.;Pan,L.;Guan,Q.F.;Zou,G.T.Acta Phys.Sin.2006, 55,3080.[徐国成,潘 玲,关庆丰,邹广田.物理学报, 2006,55,3080.]
(27) Yang,J.H.;Zheng,J.H.;Zhai,H.J.;Yang,L.L.;Lang,J.H.; Gao,M.J.Alloy.Compd.2009,481,628.doi:10.1016/j.jallcom. 2009.03.108
(28) Li,L.;Yang,H.Q.;Ma,J.H.;Jia,D.Z.Chin.J.Inorg.Chem. 2012,28(1),25.[李 丽,杨合情,马军虎,贾殿赠.无机化学学报,2012,28(1),25.]
(29)Xu,D.;Gao,A.M.;Deng,W.L.Acta Phys.-Chim.Sin.2008, 24(7),1219.[许 迪,高爱梅,邓文礼.物理化学学报,2008, 24(7),1219.]doi:10.3866/PKU.WHXB20080717
(30) Zhu,X.Q.;Zhang,J.L.;Chen,F.Chemosphere 2010,78,1350. doi:10.1016/j.chemosphere.2010.01.002
(31) Cheng,H.F.;Huang,B.B.;Dai,Y.;Qin,X.Y.;Zhang,X.Y.; Wang,Z.Y.;Jiang,M.H.J.Solid State Chem.2009,182,2274. doi:10.1016/j.jssc.2009.06.006
(32) Li,B.X.;Wang,Y.F.;Liu,T.X.Acta Phys.-Chim.Sin.2011, 27(12),2946.[李本侠,王艳芬,刘同宣.物理化学学报, 2011,27(12),2946.]doi:10.3866/PKU.WHXB20112946
(33)Goto,T.;Noguchi,Y.;Soga,M.;Miyayama,M.Mater.Res. Bull.2005,40,1044.doi:10.1016/j.materresbull.2005.02.025
April 9,2012;Revised:May 17,2012;Published on Web:May 17,2012.
Visible Light Photocatalytic Properties of Bi3.25La0.75Ti3O12Nanowires
LIN Xue1,2LU¨Peng1GUAN Qing-Feng1,*LI Hai-Bo3LI Hong-Ji2CAI Jie1ZOU Yang1(1School of Materials Science and Engineering,Jiangsu University,Zhenjiang 212013,Jiangsu Porvnce,P.R.China;2Key Laboratory of Preparation and Application Environmentally Friendly Materials of the Ministry of Education,College of Chemistry,Jilin Normal University,Siping 136000,Jilin Province,P.R.China;3College of Physics,Jilin Normal University, Siping 136000,Jilin Province,P.R.China)
Lanthanum-doped bismuth titanate(Bi3.25La0.75Ti3O12,BLT)nanowires were synthesized by a one-step hydrothermal process and their optical and photocatalytic properties were investigated.Their crystal structure and microstructures were characterized using X-ray diffraction(XRD),transmission electron microscopy(TEM),and high-resolution transmission electron microscopy(HRTEM).The BLT nanowires obtained are single-phase with an average diameter of 25 nm.The room temperature photoluminescence(PL)spectrum reveals two visible emission peaks at 400 and 596 nm,which are assigned to excitonic and surface-defect emissions,respectively.The UV-visible diffuse reflectance spectrum(UV-Vis DRS)reveals that the band gap of BLT nanowires is 2.07 eV.The prepared BLT nanowires are stable and exhibit higher photocatalytic activities in the degradation of methyl orange(MO) under visible light irradiation(λ>420 nm)compared with commercial P25 TiO2,traditional N-doped TiO2(N-TiO2),and pure bismuth titanate(Bi4Ti3O12,BIT).The high photocatalytic performance of BLT photocatalysts is attributed to the strong visible light absorption and the recombination restraint of the e-/h+pairs resulting from the presence of La3+ions.
Bismuth titanate;Lanthanum doping;Nanowire;Hydrothermal synthesis; Photocatalytic degradation; Visible light irradiation
10.3866/PKU.WHXB201205172
∗Corresponding author.Email:guanqf@ujs.edu.cn;Tel:+86-13852904936;Fax:+86-434-3290363.
The project was supported by the Key Laboratory of Preparation andApplication Environmentally Friendly Materials of the Ministry of Education of China.
环境友好材料制备与应用教育部重点实验室项目资助
O643