稀溶液中Rod-Coil-Rod三嵌段共聚物组装结构的耗散粒子动力学模拟
范中相 黄建花
(浙江理工大学化学系,杭州310018)
1 Introduction
The self-assembly of rod-coil block copolymer in a dilute solution has attracted much scientific interest due to the formation of a variety of aggregates,which have a wide range of potential applications in different fields,such as biological sciences and materials sciences.In the past ten years,many novel structures assembled by rod-coil block copolymers have been reported,such as vesicles,1,2wormlike micelles,3honeycombs,4bundles,5spheres,3,6-8cylinders,5-8and lamellae.6,9-11Chen and co-workers1used a transmission electron microscopy techniqueto investigate the aggregate formation of poly[2,7-(9,9-dihexylfluorene)]-block-poly(acrylic acid)dilock copolymers in various coil fractions and solvent quality.They observed spherical micelles with a rod corona,cylindrical micelles with a rod corona,cylindrical micelles with a coil corona,and lamellar sheet aggregates,as the solvent quality for rod block was decreased.In contrast to the rod-coil bock copolymer that contains one rigid or rodlike block,the arrangement of rod in rod-coil-rod triblock copolymer is strongly influenced by the behavior of the other rod in the polymer chain due to the connectivity of each block.12In recent years,the self-assembly of rod-coil-rod triblock copolymers has attracted special interest.13-15Xie and his partners13reported that poly(anilene)98-poly(ethylene glycol)136-poly(anilene)98(PANI98-PEG136-PANI98)triblock copolymers could assemble into particles,rods,fibers,networks,and fiber bands inN-methyl-2-pyrrolidone,dimethyl formamide,ethanol and water,respectively.With an increase of the polymer concentration in ethanol,the mono-layer fibrous network transformed into a multi-layer,tri-dimensional fibrous network,and petaline structures.13Depending on the initial polymer concentration in toluene,oligo(p-phenyleneethynylene)-polystyrene-oligo(p-phenyleneethynylene)(OPE-PS-OPE)triblock copolymers self-organized into a series of structures including vesicles,onion-like structures,spheres and rod-like fibers.14The self-assembly of rod-coil-rod copolymers also depends on the solution acidity.For example,(ANI)4-b-PEG600-b-(ANI)4formed vesicles with a mean diameter of 258 nm in aqueous solution.And the diameter of the vesicles increased to 471 nm and 1.19 μm when the concentration of HCl in solution was 10-3and 10-1mol·L-1,respectively.Once the concentration of HCl rose to 1 mol·L-1,hollow spheres and bowl-like aggregates were obtained,indicating a characteristic morphology dependence on the acidity of the solution.15
Computer simulation has played an important role in investigating the phase behavior of copolymers.Yuan and his coworkers16observed different morphologies formed by copolymers at various concentrations in solution.Shenget al.17investigated the solvent-induced self-assembly behaviors of rod-coil copolymersviadissipative particle dynamics(DPD).It shows that the solvent quality exerts a significant impact on the rod alignment when the rod block reaches a certain length.Thus the rod length and solvent quality strongly determine the morphological preference on the rod domain scale.In a very recent simulation,Chen and Ruckenstein18reported the formation of complex structure.It was found that the assembled nanostructures strongly depended on the chain structure of the polymer,the interactions between the solvent and polymer segments,as well as the interactions between the segments of the polymer chain.We19previously performed DPD simulations to study the self-assembly behavior of rod-coil-rod copolymers in a rodselective solvent.Bowl-like aggregates were observed in a quite large region in the phase diagram.The aggregate structure was also found to be dependent on the solvophobicity of the coil block.The bowl-like aggregate could change to a disklike aggregate as well as a vesicle by varying the interaction strength between the coil block and solvent.19
In the present simulation,we perform DPD simulations to further investigate the aggregate structure of rod-coil-rod triblock copolymers in a rod-repulsive solvent.Our simulation results reveal a rich variety of complex aggregates depending on the copolymer concentration,coil length,and coil-solvent interaction,as well as the coil-rod interaction.
2 Simulation details
The DPD method was developed by Hoogerbrugge and Koelman20and cast in the present form by Español.21In DPD,fluid particles are coarse-grained into DPD particles.These DPD particles interact with each otherviapairwise forces that locally conserve momentum leading to a correct hydrodynamic description.22The pairwise forces consist of the conservative forcerandom forceθijis an uncorrelated symmetric random noise with zero mean and unit variance,σis the amplitude of the thermal noise,andγis a friction coefficient.The combined effect of the dissipative and random forces is a thermostat to ensureσ2=2γkBT.The softness of the interaction is determined by the weight functionw(rij)with a commonly used choice:w(rij)=1-rij/rcforrij≤rcandw(rij)=0 forrij>rc,wherercis the cutoff radius.
The rod-coil-rod triblock copolymer is modeled as a coarsegrained linear chain AxByAxwithxDPD particles in each rod(A)block andyDPD particles in the coil(B)block,which are connectedviaa finitely extensible non-linear elastic(FENE)potential:23
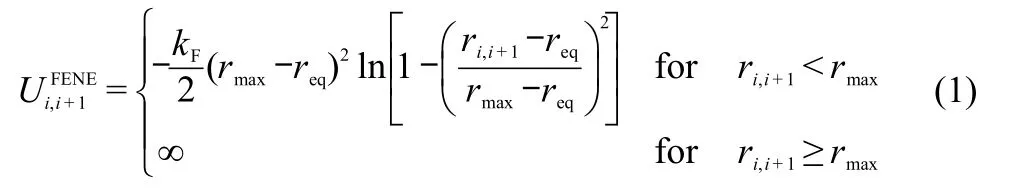
where the equilibrium bond lengthreq=0.7,the maximum bond lengthrmax=1.3,and the elastic coefficientkF=40.For the rod block A,an additional bond-bending energy between consecutive bonds is introduced aswithkθ=100 andθ0=π,kθandθ0are the bending constant and the equilibrium angle between consecutive bonds,respectively.24
The repulsive interaction parameteraijbetween different types of DPD particles is specified by
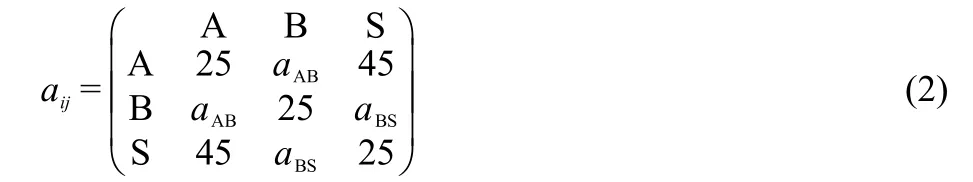
Here the solvent is represented by S.The repulsion parameter between the same types of particles is set to be 25,and the interactions between different types of particles are specified by the off-diagonal values.The valueaAS=45 means a repulsive interaction between rodAblock and solvent.
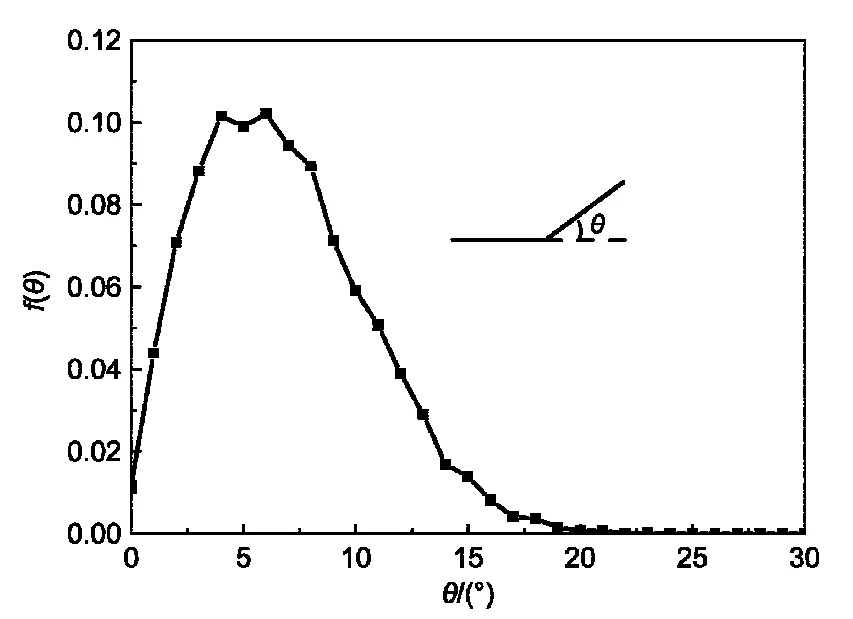
Fig.1 Probability distribution function f(θ)for the bond angle of rod block in A5B10A5copolymer chain with kθ=100
DPD particlesmoveaccording to Newton'sequationwith fiis the force experienced by particlei.The positions and velocities of the particles are solved using a modified velocity-Verlet algorithm proposed by Groot and Warren.25
Our simulations are performed in a cubic simulation box with periodic boundary conditions in all three directions.The overall particle density is set as 3 throughout this study.The concentrations of AxByAxchains is defined asCp=(2x+y)Np/N,hereN=Ns+(2x+y)Npis the total number of DPD particles,whereNsis the number of solvent particles andNpis the number of AxByAxchains.In the present work,we set the cutoff distancerc=1,energy scalekBT=1,and mass of DPD particlem=1.In the simulations,we useσ=3 and a time step δt=0.01 for integration.The total simulation time for each independent run is 5×104DPD time units.Our simulations have been carried out in different systems with size from 30×30×30 to 45×45×45 and similar aggregates are observed at the same condition.So we mainly discuss the results obtained in the system of size 40×40×40.
3 Results and discussion
For A5B10A5rod-coil-rod triblock copolymers with the concentrationCp=0.075,we have first calculated the bond angle between two adjacent bond vectors in the rod block withkθ=100.HereaAS,aBS,andaABare kept to be 45,30,and 35,respectively.The probability distribution functionf(θ)is presented in Fig.1.We find that more than 80%of the bond angles have a valueθ<10°and the peak distribution locates about 5°,indicating that the rigidity of the rod block is well modeled withkθ=100.
3.1 Effect of mutual compatibility between rod and coil blocks
The mutual compatibility between rod-coil blocks(aAB)is known to affect the aggregate morphologies of diblock copolymer systems.26In the present work,we first study the effect ofaABon the aggregate morphology of A5B10A5rod-coil-rod triblock copolymers with the concentrationCp=0.075.HereaASandaBSare kept to be 45 and 30,respectively.When A and B blocks are compatible,such asaAB=27,a spherical aggregate is observed,as shown in Fig.2(a).The density distributionρmeasured from the mass center of aggregate is calculated,which is plotted in Fig.2(b).It is clearly observed that segments A and B roughly distribute uniformly in the aggregate,except in its center and surface,where the density of distribution of B segments is bigger than that of segments A.This is because that A rods are more hydrophobic than B coils.WhenaABreaches 30,onion-like aggregate is generated,see Fig.2(c)and 2(d).Segments A and B are found to distribute alternately in the aggre-gate.Similar onion-like structure was also observed in experiment for OPE-PS-OPE triblock copolymer when the polymer concentration is 2.5 mg·mL-1.14With a continuous increase ofaABto 40,the aggregate transforms into coil-block cage(Fig.2(e)).Similar cage-like structure was also observed by Chen27and Li28et al.Chen reported that the coil-block cages can be changed into rod-block cages by varying the selectivity of the solvent for the coil blocks and/or by modifying the interaction between the coil blocks and rod blocks.27The coil-block cages are formedviadisks possessing holes,which decrease the contact between the solvophobic moiety of the polymer and solvent.The small domains B prefer staying at the surface because they would like to contact with solvent rather than with rod blocks.The corresponding density distribution shows that the aggregate size is a little bigger than the above two ones(Fig.2(f)).With further increase ofaABup to 70,a cylindrical structure occurs(Fig.2(g)).We find that these structures shown in Fig.2 are stable and can be reproduced with a probability larger than 90%from 20 independent samples.
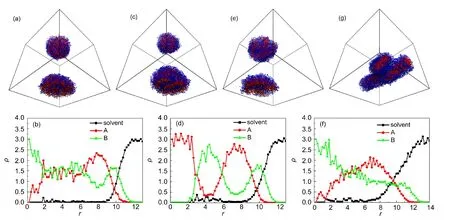
Fig.2 Equilibrium structures assembled byA5B10A5rod-coil-rod copolymers with Cp=0.075 at various aAB(a,c,e,g)and radial density profiles at various aAB(b,d,f)
3.2 Effect of the solvent selectivity for the coil blocks
Chen and Ruckenstein27found that the selectivity of the solvent for the coil blocks affected the aggregate morphology of coil-rod-coil(ABA)copolymer systems.They observed vesicles ataBS=30 and a rod block cage partially covered by coils ataBS=40,respectively.Whereas a cage completely covered by rod blocks was formed ataBS=90.In their work,the repulsion between rod block and solventaASwas kept to be 50.In the A5B10A5rod-coil-rod copolymer systems,we obtain different results.Fig.3 shows a series of structural changes and the corresponding radial density profiles whenaBSchanges from 27.5 to 70 withaASandaABfixed at 45 and 35,respectively.When the repulsion between the coil segment and solvent is small,such asaBS=27.5,cage-like aggregate is generated(Fig.3(a)).AsaBSincreases to the region of 30-35,onion-like structures with segments B lying at the outmost surface are observed.Fig.3(b)shows the onion-like structure formed ataBS=35.It is clearly observed that its innermost core is occupied by rods A and the outmost shell is covered by coils B.The corresponding radial density profile plotted in Fig.4(a)further provides the structure information.With a continuous increase ofaBSto 45-50,the repulsions between solvent and the two blocks are comparable,and a patchy aggregate occurs mainly induced by the miscibility between segments A and B.Fig.3(c)shows the patchy shape formed ataBS=50.WhenaBSincreases to 70,onion-like structure is observed again,see Fig.3(d).However since the hydrophobicity of segments B is stronger than that of segments A,we notice that the innermost core of onion is occupied by coils B and the outmost shell is covered by rods A,contrary to Fig.3(b).It is further confirmed by the radial density profile shown in Fig.4(b).
3.3 Dependence of morphology on the coil length and concentration of copolymer chains
Fig.5 shows the effects of the coil length and polymer concentration on the aggregate morphology of A5BxA5rod-coil-rod triblock copolymers withaAB=35,aAS=45,andaBS=30.For copolymers withx≤10,cage structure changes into onion structure with the increase of the copolymer concentration.However,when the coil length reaches 12,only cage structure is observed.And complicated aggregate is formed instead atCp=0.10.Our results clearly indicate that the copolymer concentration and the coil length strongly affect the aggregate morphology.Copolymers with longer coil block favor to form cage structure,whereas those with shorter coil block tend to aggregate into onion structure at relatively high concentrations.
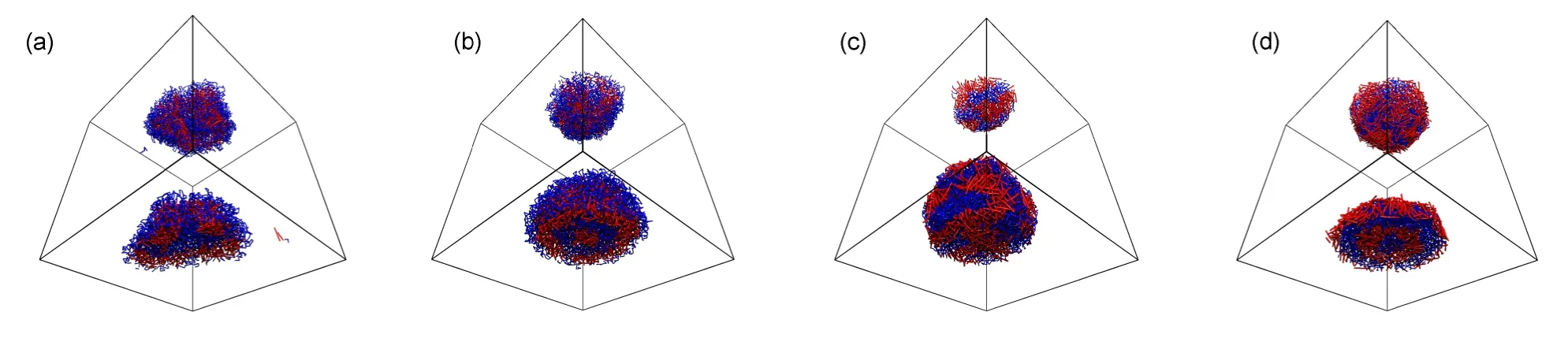
Fig.3 Equilibrium structures aggregated byA5B10A5rod-coil-rod triblcok copolymers with Cp=0.075 at various aBS
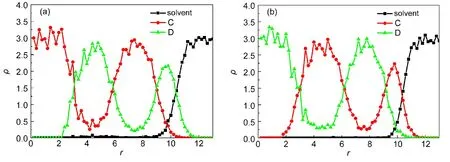
Fig.4 Radial density profiles ofA5B10A5rod-coil-rod copolymers in onion-like aggregate
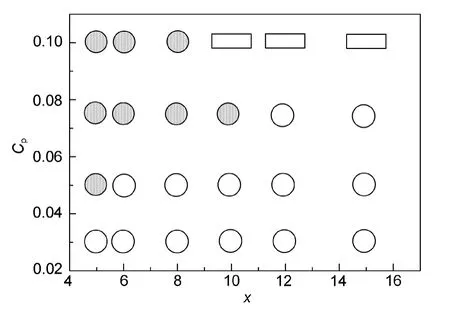
Fig.5 Morphology variation ofA5BxA5rod-coil-rod copolymers as a function of concentration Cpand coil length x
4 Conclusions
In summary,the aggregate morphology of A5BxA5rod-coilrod triblock copolymers in a rod-repulsive solvent is studied by using dissipative particles dynamics method.It is found that the aggregate morphology strongly depends on the mutual compatibility between rod and coil blocksaAB,the hydrophobicity of coil blockaBS,the coil length,as well as the copolymer concentration.For A5B10A5rod-coil-rod triblock copolymers,the aggregate changes from sphere,onion,cage,and ultimately to cylinder with an increase inaAB.And cage-like aggregate changes into onion-like,patch-like and then inverted onion-like with the increase ofaBS.We also find that copolymers with longer coil block favor to form cage structure,whereas those with shorter coil block tend to aggregate into onion structure at relatively high concentrations.
(1)Tung,Y.C.;Wu,W.C.;Chen,W.C.Macromol.Rapid Commun.2006,27(21),1838.
(2) Vriezema,D.M.;Hoogboom,J.;Velonia,K.;Takazawa,K.;Christianen,P.C.M.;Maan,J.C.;Rowan,A.E.;Nolte,R.J.M.Angew.Chem.Int.Edit.2003,42(7),772.doi:10.1002/anie.200390204
(3) Lin,C.H.;Tung,Y.C.;Ruokolainen,J.;Mezzenga,R.;Chen,W.C.Macromolecules2008,41(22),8759.doi:10.1021/ma8016629
(4) Horsch,M.A.;Zhang,Z.;Glotzer,S.C.J.Chem.Phys.2006,125(18),184903.doi:10.1063/1.2363983
(5) He,L.L.;Zhang,L.X.;Ye,Y.S.;Liang,H.J.J.Phys.Chem.B2010,114(21),7189.doi:10.1021/jp101129p
(6) Jenekhe,S.A.;Chen,X.L.Science1999,283(5400),372.doi:10.1126/science.283.5400.372
(7) Cai,C.F.;Wang,L.Q.;Lin,J.P.;Zhang,X.Langmuir2012,28(9),4515.doi:10.1021/la204941w
(8) Ding,W.;Lin,S.;Lin,J.;Zhang,L.J.Phys.Chem.B2008,112(3),776.doi:10.1021/jp076939p
(9) Pinol,R.;Jia,L.;Gubellini,F.;Levy,D.;Albouy,P.A.;Keller,P.;Cao,A.;Li,M.H.Macromolecules2007,40(16),5625.doi:10.1021/ma071064y
(10) Olsen,B.D.;Segalman,R.A.Mater.Sci.Eng.R-Rep.2008,62(2),37.doi:10.1016/j.mser.2008.04.001
(11) Zhu,X.M.;Wang,L.Q.;Lin,J.P.J.Phys.Chem.B2013,117(18),5748.doi:10.1021/jp400882h
(12) Chen,J.Z.;Sun,Z.Y.;Zhang,C.X.;An,L.J.;Tong,Z.J.Chem.Phys.2008,128(7),074904.doi:10.1063/1.2831802
(13)Yang,Z.F.;Wu,J.G.;Yang,Y.K.;Zhou,X.P.;Xie,X.L.Front.Chem.Eng.China2008,2(1),85.doi:10.1007/s11705-008-0003-6
(14)Li,K.;Wang,Q.Chem.Commun.2005,4786.
(15)Yang,Z.F.;Wang,X.T.;Yang,Y.K.;Liao,Y.G.;Wei,Y.;Xie,X.L.Langmuir2010,26(12),9386.doi:10.1021/la100382s
(16)Yuan,S.L.;Wu,R.;Cai,Z.T.Acta Phys.-Chim.Sin.2004,20(8),811.[苑世领,吴 锐,蔡政亭.物理化学学报,2004,20(8),811.]doi:10.3866/PKU.WHXB20040806
(17) Chou,S.H.;David,T.W.;Tsao,H.K.;Sheng,Y.J.Soft Matter2011,7(19),9119.doi:10.1039/c1sm05808h
(18) Chen,H.Y.;Ruckenstein,E.Soft Matter2012,8(34),8911.doi:10.1039/c2sm26035b
(19) Huang,J.H.;Fan,Z.X.;Ma,Z.X.J.Chem.Phys.2013,139(6),064905.doi:10.1063/1.4818417
(20) Hoogerbrugge,P.J.;Koelman,J.M.V.A.Europhys.Lett.1992,19(3),155.doi:10.1209/0295-5075/19/3/001
(21) Español,P.Europhys.Lett.1997,40(6),631.doi:10.1209/epl/i1997-00515-8
(22) Ripoll,M.;Ernst,M.H.;Español,P.J.Chem.Phys.2001,115(15),7271.doi:10.1063/1.1402989
(23) Kremer,K.;Grest,G.S.J.Chem.Phys.1990,92(8),5057.doi:10.1063/1.458541
(24) AlSunaidi,A.;den Otter,W.K.;Clarke,J.H.R.Philos.Trans.R.Soc.London,Ser.A2004,362(1821),1773.15 doi:10.1098/rsta.2004.1414
(25) Groot,R.D.;Warren,P.B.J.Chem.Phys.1997,107(11),4423.doi:10.1063/1.474784
(26) Chou,S.H.;Tsao,H.K.;Sheng,Y.J.J.Chem.Phys.2011,134(3),034904.doi:10.1063/1.3537977
(27) Chen,H.Y.;Ruckenstein,E.Soft Matter2012,8(5),1327.doi:10.1039/c2sm06968g
(28) Kong,W.X.;Li,B.H.;Jin,Q.H.;Ding,D.T.;Shi,A.C.Langmuir2010,26(6),4226.doi:10.1021/la903292f

