聚乙烯吡咯烷酮辅助的水热法合成形貌可控的银纳米结构
徐丽红 阚彩侠,2,* 王长顺 从 博 倪 媛 施大宁,*
(1南京航空航天大学理学院应用物理系,南京211106;2南京航空航天大学,纳智能材料器件教育部重点实验室,南京211106)
1 Introduction
Metal nanostructures have been of extensive interest in many different areas because of their unique or improved electronic,catalytic,optical properties.1-5The synthesis of shapecontrolled metal nanocrystals has achieved great progress.The top-down solid-state methods,such as sol-gel,6molten salt,7physical grinding,and high energy ball milling,8,9etc.have taken the lead in industrial production of nanomaterials.But preparation cost of these methods is relatively expensive and products are not uniform in size and morphology.Solution-phase chemical approaches provide economic routes to obtain highquality nanomaterials in specified morphology.These include template-assistant deposition approach,10chemical reduction method,11-15irradiation-photoreduction processes.16-18Among these methods,the common chemical reduction method especially the polyol process,which is the most convenient,versatile,and low cost,is usually adopted for the synthesis of noble metal nanoparticles.By changing the preparation parameters in the system,all kinds of nanostructures with different morphologies can be obtained,and the morphology could be further well controlled through introducing surfactant.For example,polyvinylpyrrolidone(PVP)plays a remarkable important role to control the shapes of different nanostructures such as Ag nanowires,19,20Ag nanocubes,21and newly shaped Au nanoplates22etc.It is believed that the selective adsorption of surfactant molecules onto particular crystal facets of nanoparticles results in various morphologies due to different growth rates along different directions.23Moreover,surrounding environment of the reaction system does affect the properties of metal nanocrystals.Many research groups synthesize metal nanostructures mostly in oil bath which can afford uniform heat distribution under a controllable temperature.The reaction solution was placed in a vial or flask,capped as appropriate and heated with stirring.In such an open circumstance,oxygen plays a vital role in the formation of nanostructures.24,25
Currently,Ag nanostructures are of great interest because of their unique microstructures,optical properties,and potential applications.Shape control provides one of the most powerful means to tailor the optical properties of metal nanostructures.The number,position,and intensity of surface plasma resonance(SPR)have a strong correlation with their exact morphology.The uniformity of size and monodispersity of nanoparticles are important physical parameters for both technological usage and foundational research.
In this paper,we report a simple way for the synthesis of Ag nanostructures(nanowires and nanodecahedrons)with welldefined shapes by hydrothermal method in a 60 mL stainless steel autoclave.This hydrothermal synthesis process assisted by PVP provides a sealed,high-pressure surrounding.PVP polymers of various average molecular weight(MW)marked asK17,K30,K60,and,K90 are applied(Kis usually used to represent the characteristic value of relative viscosity of PVP solution.The larger MW of PVP,the higher relative viscosity of the PVP solution is).Based on the optical evolution and microscopy results,the effects of PVP and solvents(such as ethylene glycol(EG)and deionized water)on the morphology of Ag nanostructures are studied in detail.Plausible growth mechanisms can be proposed through analyzing the structural characteristics of Ag nanostructures as well as the role of PVP surfactants.
2 Experimental
2.1 Synthesis
2.1.1 Materials
Silver nitrate(AgNO3,99.8%,Sinopharm Chemical Reagent Co.,Ltd.),sodium chloride(NaCl,99.5%,Nanjing Chemical Reagent Co.,Ltd.),hydrochloric acid(HCl,37%,Sinopharm Chemical Reagent Co.,Ltd.),and ethylene glycol(EG,98%,Nanjing Chemical Reagent Co.,Ltd.)were used in this work.Polyvinylpyrrolidone(PVP,Sinopharm Chemical Reagent Co.,Ltd.)with various average MW values of 8000,40000,160000,and 360000 were chosen,which were marked asK17,K30,K60,andK90,respectively.All reagents were used without further purification.Water was purified using a MilliQ setup(QYFX,Chongqing Qianyan water treatment equipment Co.LTD.)for ultrapure water(18.25 MΩ·cm).
2.1.2 Synthesis of Ag nanowires
An improved hydrothermal PVP-directed polyol process was used to synthesize Ag nanowires.Firstly,10 mL EG,2 mL HCl(3 mmol·L-1solution in EG),6 mL PVP(0.15 mol·L-1in terms of the repeating unit,K30,K60,K90 solution respectively in EG)and 6 mLAgNO3(0.1 mol·L-1solution in EG)were respectively added into a 60 mL stainless steel autoclave and heated at 140°C for 15 h.The molar ratio(R)of PVPrepeatunitto AgNO3is 1.5.After cooling to room temperature naturally,the obtained suspensions were washed with acetone and deionized water by centrifugating for several times.
2.1.3 Synthesis of Ag nanodecahedrons
In a typical experiment,the reaction solution was prepared by dissolving 0.710 g of PVP(K17)and 0.068 g AgNO3in 40 mL deionized water(R=16).Then,the solution was transferred into the 60 mL stainless steel autoclave and heated at 195°C for 10 h,followed by cooling to room temperature naturally.After the hydrothermal reaction,the obtained suspensions were washed with deionized water and centrifugated for several times.
2.2 Characterization
The optical absorption spectra of the prepared samples were collected using a UV-Vis spectrophotometer(UV-6300)in thewavelength range of 200-1100 nm.For transmission electron microscopy(TEM,JEM-1010)analysis,the products were diluted with deionized water,and a droplet of the dispersions placed onto the carbon-coated copper grids.For field emissionscanning electron microscopic(FE-SEM,Nova NanoSEM 230-FEI)measurement,the condensed products were dispersed on copper sheets.All the samples were dried at room temperature.
3 Results and discussion
3.1 Ag nanowires
Fig.1 shows the TEM images of synthesized silver nanowires.It is found that the aspect ratios of the obtained Ag nanowires increased with increasing the MW of PVP.Ag nanowires synthesized with PVP-K30 are not uniform in size and diameter,and a lot of nanocubes and other polyhedral nanostructures appear in the products,as presented in Fig.1(a).With the MW of PVP increasing,the yields of Ag nanowires increase greatly and only few particles could be observed in the products,as shown in Fig.1(b,c).The diameter of these Ag nanowires is quite uniform which is around 85 and 120 nm produced with the existence of PVP-K60 and PVP-K90,respectively.Fig.1(d)shows the typical products synthesized in the same condition except for the absence of HCl.The products are dominated by Ag nanoparticles and other polyhedral nanostructures with size larger than 100 nm,and no Ag nanowires can be seen no matter which PVP was used.We can see the application of HCl is important for the growth of Ag nanowires under the hydrothermal condition,which is quite different from that produced in an open atmosphere,as reported previously.26,27In an open atmosphere,polyhedral Ag nanostructures are the dominant products without the addition of HCl in the reaction system.
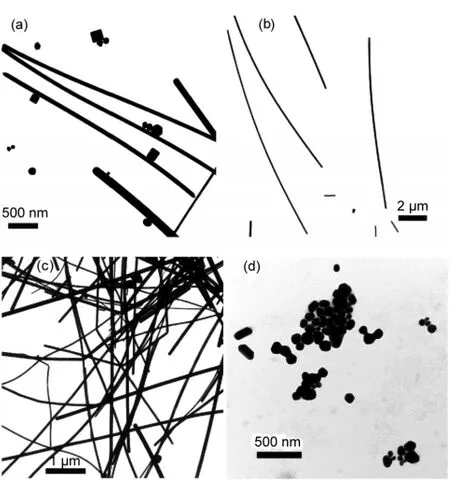
Fig.1 TEM images of the synthesizedAg nanostructures using PVP of different molecular weights(MWs)
Since Ag nanostructures with different shapes and sizes exhibit different SPR bands at different frequencies,we also carried out UV-Vis spectrum measurements for the samples.Fig.2 shows the optical absorption spectra of Ag colloid solutions synthesized using PVP of different MW values.All of the obtained products show the evident plasma peaks at~350 and~385 nm,which should be attributed to the quadrupole resonance and dipole resonance of the Ag nanowires,respectively.28-30For curve a,there is another obvious peak at~450 nm attributed to the SPR resonance of the polyhedral Ag nanostructures which indicates that the final products should be a mixture of Ag nanowires and polyhedral nanostructures.This is consistent with the results of TEM observations which contain nanocubes and other polyhedral nanostructures.Inset of Fig.2 shows the absorption spectra of Ag nanostructures obtained in the absence of HCl,all of which show one main SPR peak at~425 nm together with a weak absorption shoulder at~350 nm,belonging to Ag nanoparticles and other polyhedral nanostructures.31
Meanwhile,we also explored the influence of the reaction temperature on the Ag nanostructures.Figs.3(a)and 3(b)show the optical absorption spectra of Ag colloid solution sampled at 160 and 180°C,respectively.With the reaction temperature rising,the absorption peak of the products synthesized by PVPK30 becomes wider and shifts to~420 nm.In the case of PVPK60,the absorption peak drops slowly at a higher temperature and even shows a weak shoulder peak at~410 nm when the temperature rises up to 180°C.As for PVP-K90,we can see red shift of the absorption peak atT=160°C and a new shoulder peak at~410 nm at 180 °C.We can conclude that more nanoparticles appear in the products with the reaction temperature rising that might attribute to a higher reaction rate.As we know,in such a polyol synthesis process,EG serves as solvent as well as reducing agent.To figure out the effect of EG,we replaced EG with deionized water as the solvent at 140°C with-out any other changes.The products were almost Ag nanoparticles in this case,although there is a weak absorption shoulder at~350 nm,as shown in Fig.3(c).When PVP-K60 and PVPK90 were used,only one resonance peak at~405 nm of Ag nanoparticles was observed in the absorption spectra.

Fig.2 UV-Vis absorption spectra ofAg nanowires synthesized using PVPof different MWs
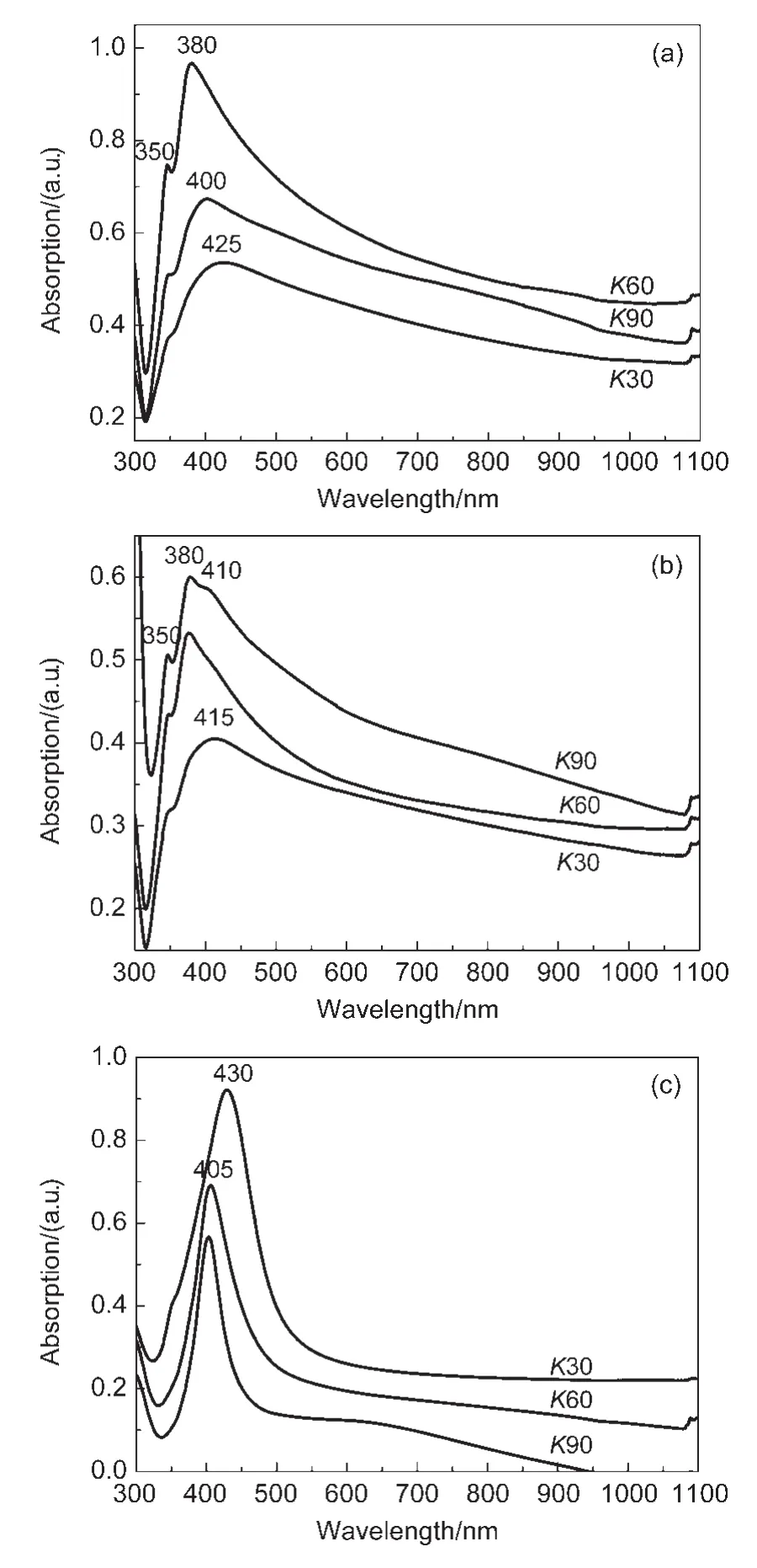
Fig.3 UV-Vis absorption spectra ofAg nanostructures synthesized using PVPof different MWs under different conditions(in the presence of HCl)
Furthermore,we carried our experiment by replacing HCl with NaCl in the same concentration(2 mL of 3 mmol·L-1solution in EG).The SEM images(Fig.4)of the products demonstrate that Ag nanowires are obtained with the application of NaCl.Meanwhile,the aspect ratios of Ag nanowires increase with the MW of PVP as well,as presented in Fig.4(a-c)that were respectively synthesized by PVP-K30,K60,andK90.One can also observe in Fig.4(d)that the Ag nanowires should be five-fold symmetry from the cross section.The UV-Vis absorption spectra(Fig.5)show that the absorption peak ofK60 is narrow and drops more quickly than the other two curves.Compared with Fig.2a,it is worth noting that Fig.5a does not show a peak at 450 nm.That might because of the absence of nanocubes in the products,as we can see in Fig.4(a),all of which are nanowires with some irregular nanoparticles.
According to the above results,we can conclude that Clplays a critical role in fabricating Ag nanowires.With no Clwas applied,the chemical reactions of the growth and reduction processes are as follows:
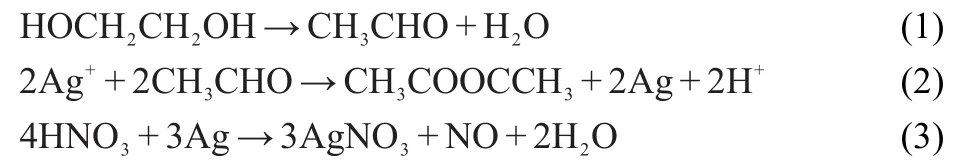
One plausible growth mechanism of Ag nanowires is that,at a high temperature,ethylene glycol can be oxidized to aldehyde(reaction(1))which reduces Ag+to Ag atoms(reaction(2))that will start to nucleate and grow into nanoparticles.At the same time,nitric acid was generatedin situdue to the generation of protons and could dissolve Ag solids into Ag+at high temperature again(reaction(3)).
The introduction of Cl-could slow down reaction(2)through the formation of colloid AgCl.Therefore,in our synthesis,HCl or NaCl was used to introduce Cl-so as to decrease the concentration of free Ag+ions that will reduce the generation rate of Ag crystal which results in the generation of more small Ag nanoparticles as Ag sources for the formation of nanowires.Furthermore,Ag+ions can be released to the solution and reduced again when the solution is heated to a high temperature(140°C here).32,33It is known that the oxygen may etch multiply twinned seeds since defects inherently present insuch a structure and would provide active sites for oxidative dissolution.25Therefore,the sealed circumstance of the autoclave with little oxygen is benefit for the formation of multiply twinned structure which is required for wire growth.34,35
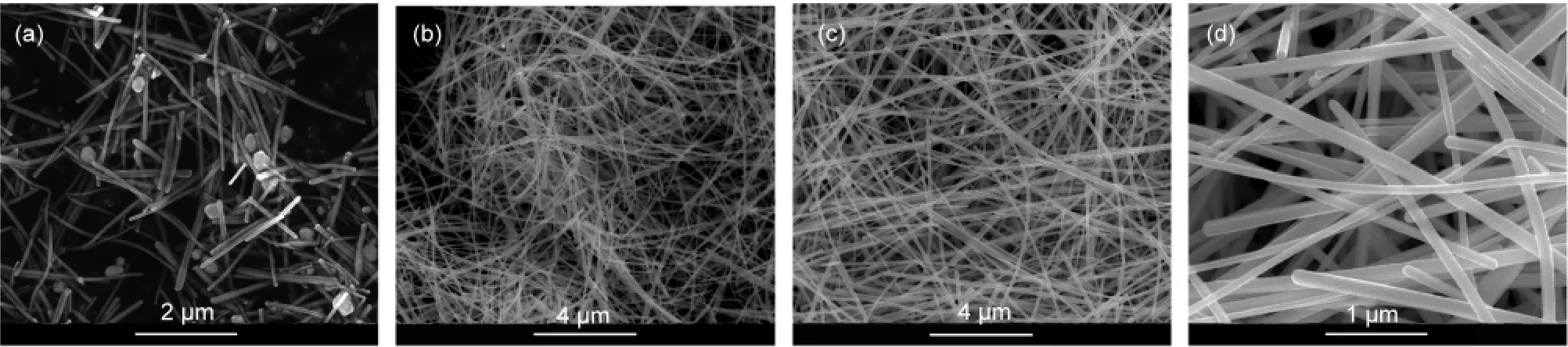
Fig.4 FE-SEM images ofAg nanowires synthesized with PVPof different MWs by replacing HCl with NaCl

Fig.5 UV-Vis absorption spectra ofAg nanowires synthesized with PVPof different MWs by replacing HCl with NaCl
Bounded by five{111}facets at each end and five{100}facets at the side,the five-fold twinned Ag nanoparticles in the initial stage are of minimized surface-energy.36As we know that the growth morphology is often determined by the surface free energies under thermal equilibrium.However,crystal growth is usually far from the thermal equilibrium;thus,the shape is not characterized by minimizing the surface energy,but rather the growth rate of each face as determined by the kinetics.37It is commonly believed that PVP interacts more strongly with sliver atoms on the{100}facets than those on the{111}facets.38Once the five-fold twinned particles formed,PVP would selectively adsorb onto the{100}facets as a result that{100}facets are completely passivated.Hence,the reduced Ag atoms preferentially deposited onto the{111}facets leading to the anisotropic growth ofAg nanowires.36
Moreover,as we know that PVP has the structure of polyvinyl skeleton with strong polar group(pyrrolidone ring),it has an affinity toward many chemicals to form coordinative compounds.The polar groups,such as the>C=O groups of PVP chain,can interact with metal ions and form coordinating complex.39The long-chain PVP has higher degree of polymerization compared with the short one,the average number of repeating units in one PVP macromolecule chainnis very high(7207,1802,and 522 respectively of PVP-K90,K60,andK30).Thus,there are more carbonyl groups in one PVP macromolecule and more Ag+coordinated along the long chain of PVP.Combining with our experiment,the heating process followed after all the reaction reagents were added into the stainless steel autoclave and maintained a period of time to heat up to the set temperature.That means Ag+might be reduced after combining with the long-chain PVP macromolecule.This induced the formation of long one-dimensional Ag nanostructures directly.So the previous interaction between Ag+and the PVP chains in the initial stage and the chain length of PVP both are critical factors to the synthesis of Ag nanowires.This interpretation seems more appropriate to explain our experimental results.
3.2 Ag nanodecahedrons
Fig.6(a,b)shows the TEM and SEM images for the typical samples of Ag nanodecahedrons,the mean edge length of which is~100 nm.There are also a small amount of nanorods and nanoplates in the products.Fig.6(c)shows a magnified TEM image of one Ag decahedron.It gives obvious evidence of the five-fold twinned structure of the Ag decahedron.We can clearly see the five truncated corners of the decahedrons and the twin boundaries between two neighboring{111}facets.Fig.6(d)is the UV-Vis absorption spectrum of the final products.The weak absorption shoulder located at~350,~415 nm and the broad peak of~510 nm are assigned to be the out-ofplane quadrupole,in-plane quadrupole,and dipole resonances of theAg nanostructures,respectively.40,41
Since there are only two reagents(AgNO3and PVP-K17)in the synthesis of Ag decahedron,PVP acts as reducing agent as well as surface capping agent.With the presence of high PVP concentration(R=16),the reduction rate of Ag+increases in the closed system,and the rates of nuclei formation and crystal growth increase simultaneously.Under thermodynamic growth,stable five-twinned seeds which are of the lowest surface energy are more easily formed.It is well known that twin boundary is the locus of high energy where defects accumulate due to configurational misfit.42The defect zones in twinned seeds are most susceptible to an oxidative environment,with their atoms being attacked by the etchant,oxidizer,and dissolved into the solution.43While in our experiment,the defect zones are not easily etched because of little oxygen and protected by PVP in the sealed hydrothermal reaction system.The subsequent growth process of Ag decahedrons has been studied by many groups and substantially divided into two ways:fivefold twinned Ag seeds grow into Ag decahedrons uniformly and five tetrahedrons assemble into the Ag decahedrons.42,44Reactions in such a high-pressure and sealed autoclave,it is difficult to track the morphological evolution involved in the growth process.A detailed growth mechanism for Ag decahedrons needs further study.However,it is reasonable to believe that the Ag decahedrons formed in the first way in our experiment since nanorods existed in the final products(seen in Fig.6(b))which were also grown from five-fold twinned nanostructures.Ag atoms reduced by PVP preferentially fall onto the stabilized twin boundaries of five-fold twin seeds which leads to the emergence of five{100}facets,consequently the formation of the truncated decahedrons.
As for Ag nanorods,PVP-K17 selectively adsorbs onto the{100}facets of few five-fold twinned seeds leading to the existence of rod-like products.Moreover,PVP-K17 acts with the{111}facets of plate-seeds with stacking faults,which directs the growth of a small amount of Ag nanoplates.45However,in the presence of PVP-K30,K60,andK90 in EG solution,no nanorods or nanoplates exist in the final products,all of which are only nanowires and little nanoparticles.In this case,as wehave mentioned previously,PVP also selectively adsorbs onto the{100}facets of five-fold twinned seeds,which directs the formation of nanowires.It is interesting that under the similar growth mechanism,we obtain different nanostructures.The shape evolution is related with the PVP as well as the solvent(EG and deionized water).It is hard to explain why this happens since the growth of crystals depends deeply on the reaction conditions.37And it is difficult to sample in our experiment process to track the morphological evolution under a high-pressure and sealed autoclave condition.More efforts should be taken to enrich our research.

Fig.6 (a)TEM and(b)SEM images ofAg nanostructures including decahedron and a small amount of truncated nanorods and nanoplates;(c)magnified TEM image of a typical five-fold twinned decahedron;(d)UV-Vis absorption spectrum of theAg nanostructures
4 Conclusions
In summary,we developed a convenient,versatile,and low cost hydrothermal process for the synthesis of Ag nanostructures in different solution systems with the presence of PVP.PVP of higher MW,such asK30,K60,andK90,plays a critical role in directing the growth of Ag nanowires while PVPK17 is benefit for the synthesis of Ag nanodecahedrons.The results show that PVP plays a critical role in the process of ion reduction,nuclei formation,and further crystal growth.Plausible growth mechanisms of Ag nanowires and nanodecahedrons have been put forward.More efforts should be taken to track the morphological evolution which is required both in technological usage and foundational research.
(1) Lu,W.;Lieber,C.M.Nat.Mater.2007,6,841.doi:10.1038/nmat2028
(2) Lal,S.;Link,S.;Halas,N.J.Nat.Photonics2007,1,641.doi:10.1038/nphoton.2007.223
(3)Xiong,Y.;Wiley,B.J.;Xia,Y.Angew.Chem.Int.Edit.2007,46,7157.
(4) Feng,M.;Zhang,M.;Song,J.;Li,X.;Yu,S.ACS Nano2011,5,6726.doi:10.1021/nn202296h
(5) Pedireddy,S.;Li,A.;Bosman,M.;Phang,I.Y.;Li,S.;Ling,X.Y.J.Phys.Chem.C2013,117,16640.doi:10.1021/jp4063077
(6) Mackenzie,J.D.;Bescher,E.P.Accounts Chem.Res.2007,40,810.doi:10.1021/ar7000149
(7) Reddy,M.V.;Jose,R.;Teng,T.H.;Chowdari,B.V.R.;Ramakrishna,S.Electrochim.Acta2010,55,3109.doi:10.1016/j.electacta.2009.12.095
(8) Koch,C.C.Rev.Adv.Mater.Sci.2003,5,91.
(9) Zhang,D.L.Prog.Mater.Sci.2004,49,537.doi:10.1016/S0079-6425(03)00034-3
(10) Fang,J.Y.;Qin,S.Q.;Zhang,X.A.;Wang,G.;Wang,F.;Chang,S.L.Micro&Nano Lett.2011,6,971.doi:10.1049/mnl.2011.0480
(11) Duan,J.Y.;Zhang,Q.X.;Wang,Y.L.;Guan,J.G.Acta Phys.-Chim.Sin.2009,25,1405.[段君元,章桥新,王一龙,官建国.物理化学学报,2009,25,1405.]doi:10.3866/PKU.WHXB20090731
(12)Wu,H.;Kuo,C.;Huang,M.H.Langmuir2010,26,12307.doi:10.1021/la1015065
(13) Li,Z.C.;Shang,T.M.;Zhou,Q.F.;Feng,K.Micro&Nano Lett.2011,6,90.doi:10.1049/mnl.2010.0183
(14) Silva,J.N.;Saade,J.;Farias,P.M.A.;Falcão,E.H.L.Advances in Nanoparticles2013,2,217.doi:10.4236/anp.2013.23030
(15)Wang,Y.;Zheng,Y.;Huang,C.Z.;Xia,Y.J.Am.Chem.Soc.2013,135,1941.doi:10.1021/ja311503q
(16) Zhang,Q.;Ge,J.;Pham,T.;Goebl,J.;Hu,Y.;Lu,Z.;Yin,Y.Angew.Chem.Int.Edit.2009,48,3516.doi:10.1002/anie.v48:19
(17) Huang,X.;Qi,X.;Huang,Y.;Li,S.;Xue,C.;Gan,C.L.;Boey,F.;Zhang,H.ACS Nano2010,4,6196.doi:10.1021/nn101803m
(18) Bordenave,M.D.;Scarpettini,A.F.;Roldán,M.V.;Pellegri,N.;Bragas,A.V.Mater.Chem.Phys.2013,139,100.doi:10.1016/j.matchemphys.2012.12.061
(19) Korte,K.E.;Skrabalak,S.E.;Xia,Y.J.Mater.Chem.2008,18,437.doi:10.1039/b714072j
(20) Chen,D.;Qiao,X.;Qiu,X.;Chen,J.G.;Jiang,R.J.Colloid Interface Sci.2010,344,286.doi:10.1016/j.jcis.2009.12.055
(21) Im,S.H.;Lee,Y.T.;Wiley,B.;Xia,Y.Angew.Chem.Int.Edit.2005,117,2192.
(22) Kan,C.;Wang,C.;Li,H.;Qi,J.;Zhu,J.;Li,Z.;Shi,D.Small2010,6,1768.doi:10.1002/smll.201000600
(23) Sun,Y.;Xia,Y.Science2002,298,2176.doi:10.1126/science.1077229
(24) Wiley,B.;Herricks,T.;Sun,Y.;Xia,Y.Nano Lett.2004,4,1733.doi:10.1021/nl048912c
(25) Tang,X.;Tsuji,M.;Jiang,P.;Nishio,M.;Jang,S.;Yoon,S.Colloid Surface A2009,338,33.doi:10.1016/j.colsurfa.2008.12.029
(26) Zhu,J.;Kan,C.;Zhu,X.;Wan,J.;Han,M.;Zhao,Y.;Wang,B.;Wang,G.J.Mater.Res.2007,22,1479.doi:10.1557/JMR.2007.0222
(27) Zhao,T.;Sun,R.;Yu,S.;Zhang,Z.;Zhou,L.;Huang,H.;Du,R.Colloid Surface A2010,366,197.doi:10.1016/j.colsurfa.2010.06.005
(28) Kottmann,J.P.;Martin,O.J.F.;Smith,D.R.;Schultz,S.Phys.Rev.B2001,64,235402.doi:10.1103/PhysRevB.64.235402
(29) Kottmann,J.P.;Martin,O.J.F.;Smith,D.R.;Schultz,S.Opt.Express2000,6,213.doi:10.1364/OE.6.000213
(30) Rycenga,M.;Cobley,C.M.;Zeng,J.;Li,W.;Moran,C.H.;Zhang,Q.;Qin,D.;Xia,Y.Chem.Rev.2011,111,3669.doi:10.1021/cr100275d
(31) Kan,C.;Wang,C.;Zhu,J.;Li,H.J.Solid State Chem.2010,183,858.doi:10.1016/j.jssc.2010.01.021
(32) Gou,L.;Chipara,M.;Zaleski,J.M.Chem.Mater.2007,19,1755.doi:10.1021/cm070160a
(33) Hu,M.;Gao,J.;Dong,Y.;Yang,S.;Li,R.K.Y.RSC Adv.2012,2,2055.doi:10.1039/c2ra01162j
(34) Chen,D.;Qiao,X.;Qiu,X.;Chen,J.;Jiang,R.J.Mater.Sci-Mater.El.2011,22,6.doi:10.1007/s10854-010-0074-2
(35) Zhang,W.C.;Wu,X.L.;Chen,H.T.;Gao,Y.J.;Zhu,J.;Huang,G.S.;Chu,P.K.Acta Mater.2008,56,2508.doi:10.1016/j.actamat.2008.01.043
(36) Mao,H.;Feng,J.;Ma,X.;Wu,C.;Zhao,X.J.Nanopart.Res.2012,14,1.
(37) Wang,Z.L.J.Phys.Chem.B2000,104,1153.doi:10.1021/jp993593c
(38) Sun,Y.;Mayers,B.;Herricks,T.;Xia,Y.Nano Lett.2003,3,955.doi:10.1021/nl034312m
(39) Jiang,P.;Li,S.;Xie,S.;Gao,Y.;Song,L.Chem.-Eur.J.2004,10,4817.
(40) Sosa,I.O.;Noguez,C.;Barrera,R.G.J.Phys.Chem.B2003,107,6269.doi:10.1021/jp0274076
(41) Kan,C.;Zhu,J.;Zhu,X.J.Phys.D:Appl.Phys.2008,41,155304.doi:10.1088/0022-3727/41/15/155304
(42) Li,C.R.;Lu,N.P.;Xu,Q.;Mei,J.;Dong,W.J.;Fu,J.L.;Cao,Z.X.J.Cryst.Growth2011,319,88.doi:10.1016/j.jcrysgro.2011.01.068
(43) Xia,Y.;Xiong,Y.;Lim,B.;Skrabalak,S.E.Angew.Chem.Int.Edit.2009,48,60.doi:10.1002/anie.200802248
(44) Gao,Y.;Jiang,P.;Song,L.;Wang,J.X.;Liu,L.F.;Xiang,Y.J.;Zhang,Z.X.;Zhao,X.W.;Dou,X.Y.;Luo,S.D.;Zhou,W.Y.;Xie,S.S.J.Cryst.Growth2006,289,376.doi:10.1016/j.jcrysgro.2005.11.123
(45)Mo,B.;Kan,C.X.;Ke,S.L.;Cong,B.;Xu,L.H.Acta Phys.-Chim.Sin.2012,28,2511.[莫 博,阚彩侠,柯善林,从 博,徐丽红.物理化学学报,2012,28,2511.]doi:10.3866/PKU.WHXB201208132

