隔膜对双电层电容器和混合型电池-超级电容器的电化学性能的影响
孙现众 张 熊 黄 博 马衍伟
(中国科学院电工研究所应用超导重点实验室,北京100190)
1 Introduction
Electrical double-layer capacitor(EDLC),also known as supercapacitor,is a type of important electrochemical energy storage device.1It has attracted increasing attentions due to its advantages over traditional electrochemical energy storage devices,e.g.,the high rate capability in both charge and discharge processes and long cycle life as high as 105cycles.2Moreover,the hybrid battery-supercapacitor devices,3-6also called hybrid capacitor-batteries7,8or Li-ion capacitors,9-12have been recently developed.In these devices the internal hybridization of electrochemical capacitor and lithium-ion battery can be achieved by employing the battery electrode materials and the electrochemical capacitor materials within one device.13-16The hybrid devices can have the merits of high rate capability and long cycle life of EDLC and high energy density of lithium-ion battery.17In both the electrochemical energy storage devices,separator serves as an inactive fundamental and critical component,and it plays an important role in determining the device performance.18A separator is usually a porous membrane/mat sandwiched between anode and cathode.Its main function is to physically separate the anode and cathode to avoid electrical short circuits,and at the same time provide a pathway for ionic charge carriers transport in liquid electrolyte throughout the interconnected porous structure.19Up to now,yet few studies on the application of separators for EDLC and hybrid batterysupercapacitor have been performed.
In general,the separators can be porous polymeric membrane,nonwoven mat,and composite separator.19-22In this work,the physical properties of four different types of separator and the electrochemical behaviors of EDLCs and hybrid battery-supercapacitors with these separators have been comparatively studied.The separators are nonwoven polypropylene(PP)mat,porous PP membrane,Al2O3-coated PP membrane,and nonwoven cellulose paper.
2 Experimental
2.1 Materials
Nonwoven PP mat(Nonwoven,MPF30AC100)and nonwoven cellulose paper(Cellulose,NKK4030)were purchased from Nippon Kodoshi Corporation,Japan.Porous PP membrane(PP,Celgard 2400)was purchased from Celgard LLC,USA.Al2O3-coated PP membrane(Modified PP,LS-4025-C)was provided by Henan Best New Energy Technology Corporation,China.The thickness,areal density,and porosity of separators are listed in Table 1.Capacitor-grade activated carbon(AC,YP50F)with a specific surface area of 1600 m2·g-1was purchased from Kuraray Chemicals,Japan.LiNi0.5Co0.2Mn0.3O2powder(NCM)was provided by Hebei Strong-Power Li-ion Battery Technology Corporation,China.The flake-like artificial graphite(CAG)was purchased from Shanghai Shanshan Tech.,China.The EDLC electrolyte is 1 mol·L-1tetraethyl ammonium tetrafluoroborate in propylene carbonate(Et4NBF4/PC),purchased from Shenzhen Capchem,China.The Li-ion electrolyte is 1 mol·L-1lithium hexafluorophate(LiPF6)in the mixture solvent of ethylene carbonate(EC),dimethyl carbonate(DMC),and diethyl carbonate(DEC)in a volume ratio of 1:1:1,purchased from Beijing Institute of Chemical Research,China.All the chemicals are battery grade,and were used as received.
2.2 EDLC and hybrid battery-supercapacitor
All the electrodes were fabricated by doctor-blade casting the slurry of a mixture onto a current collector.The EDLC electrode is composed of AC,carbon black(Super C,Timcal,Switzerland),conductive graphite(KS-6,Timcal,Switzerland),sodium carboxymethyl cellulose(CMC,Nippon Paper Chemicals,Japan),styrene-butadiene rubber(SBR,JSR company,Japan)in a mass ratio of 80:8:2:2:8.The cathode and anode in the(NCM+AC)/graphite hybrid battery-supercapacitor are described as A13 sample in our previous publication.17The cathode electrode of hybrid battery-supercapacitor consists of 60%(w)NCM,20%(w)AC,10%(w)Super C,2%(w)KS-6,and 8%(w)poly(vinylidene fluoride)(PVdF,N9001,Triquo Chemical,Shanghai).The anode electrode of hybrid device contains 90%(w)CAG,5%(w)Super C,2%(w)CMC,and 3%(w)SBR.The electrodes were dried thoroughly to remove the solvent(water orN-methyl-2-pyrrolidone),and punched into circular discs of 13 mm diameter.The cell was assembled in a two-electrode CR2032 coin cell using an anode and a cathode separated by a separator.For EDLC cells,the masses of AC loaded on anode and cathode in one cell are equal(4.6 mg).For hybrid batterysupercapacitor cells,the mass loading of activated materials on cathode and anode are 5.7 and 4.5 mg,respectively.
2.3 Characterization
The surface morphologies of the separators were observed with a Zeiss Sigma field emission-scanning electron microscopy(FE-SEM).The differential scanning calorimetry(DSC)measurements were conducted using a DSC-Q2000 TA Instrument.The samples were heated to 300 °C with a heating rate of 10 °C·min-1under nitrogen atmosphere.The measurements of apparent contact angle at the boundary between the surface of separators and EDLC/Li-ion electrolyte droplets were taken on a KRÜSS EasyDrop system.The liquid electrolyte uptake of the separators was measured according to the procedure:firstly,the dry separators were weighed,and then immersed in liquid electrolyte for 12 h;finally,the separator surface was wiped off with filter paper,and the wet separators were weighed again.The electrolyte uptake(%)was calculated according to the formula:23
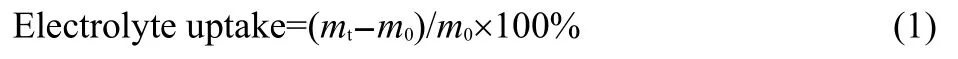
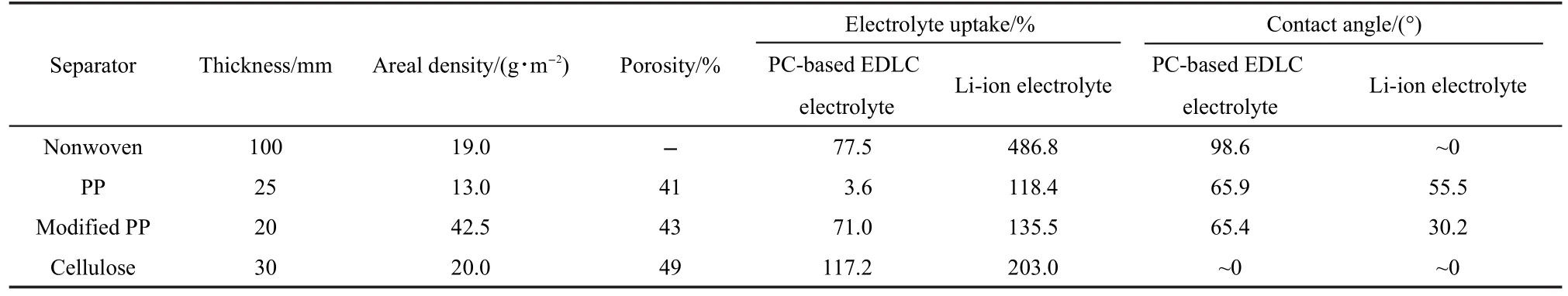
Table 1 Physical characterization of separators:thickness,areal density,porosity,electrolyte uptake,and contact angle
where,mtandm0are the masses of the wet and dry separators,respectively.
2.4 Electrochemical tests
Electrochemical impedance spectroscopy(EIS)measurements were carried out using an Autolab PGSTAT302N electrochemistry workstation(Eco Chemie,the Netherlands).The EIS spectra were obtained in the frequency range from 100 kHz to 10 mHz under a sinusoidal excitation potential of 10 mV,at the open circuit voltage of 0 and 4.0 V for EDLC and hybrid battery-supercapacitor cells,respectively.The galvanostatic chargedischarge measurements of EDLC cells were performed using anArbin MSTAT 4 system with the current density of 0.5-10A·g-1(based on the active materials in both electrodes).The hybrid cells were charged at the current density of 25 mA·g-1,and discharged at the current density of 25-7580 mA·g-1(based on the active materials in cathode)using LAND CT2001A battery testers.The self-discharge measurements were conducted according to the following steps:firstly,the cells were charged to the rated voltage followed by charging at constant voltage mode for 1 h,then the open circuit voltage decay of the devices was measured in the following 24 h.All the measurements were conducted at room temperature(20°C).
3 Results and discussion
3.1 SEM morphology
The surface morphologies of separators are shown in Fig.1(a-d).Both the Nonwoven and Cellulose separators are made of micro/submicron fibers.The fiber diameters of the Nonwoven and Cellulose separators are about 2-6 μm and 0.1-1 μm,respectively.In the PP separator,the nanosized fibrils connect adjacent crystalline regions and form a three-dimensional(3D)network.The Modified PP separator is a composite membrane which binds micron-sized Al2O3particles on porous PP membrane.The gap between adjacent Al2O3particles forms the path for ion transport in liquid electrolyte.
3.2 DSC measurements
Fig.2 shows the DSC curves of the four kinds of separator from room temperature to 300°C.The Cellulose separator reveals only one wide heat flow peak around 100°C,which should be ascribed to the evaporation of absorbed water by the cellulose fibers.From the other three curves the melting temperatures are determined to be 162.92,167.63,and 168.38°C for Nonwoven,PP,and Modified PP separators,respectively.The PP membrane has higher melting temperature than the nonwoven PP mat,and the thermal stability of Al2O3-particle coated PP membrane is also slightly improved.
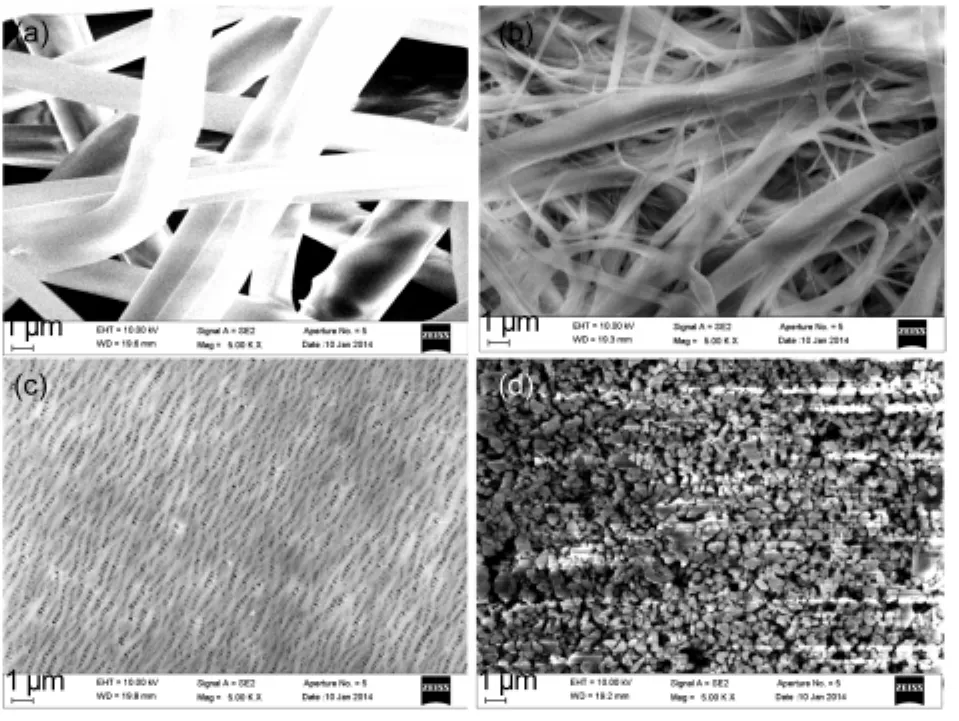
Fig.1 SEM images of(a)nonwoven PPmat,(b)cellulose paper,(c)PPmembrane,and(d)Al2O3-coated PPmembrane
3.3 Wettability
The wettability of separator can affect the internal ionic resistance and the electrolyte filling time during the cell assembly.19The apparent contact angles at the boundary between the surfaces of separator and the droplets of PC-based EDLC electrolyte or Li-ion electrolyte are listed in Table 1.The Cellulose separator is completely wettable by both EDLC and Li-ion electrolyte,and the Nonwoven separator is completely wettable by Li-ion electrolyte.The electrolyte droplet is absorbed as soon as it touches the separator.Meanwhile,the two types of separators exhibit high electrolyte uptakes.The contact angle increases in the order of Cellulose<Modified PP<PP<Nonwoven for PC-based EDLC electrolyte,and in the order of Cellulose,Nonwoven<Modified PP<PP for Li-ion electrolyte.The Li-ion electrolyte uptake is 486.8%and 203.0%for the Nonwoven and the Cellulose separator,respectively.The PC-based electrolyte uptake is 117.2%for the Cellulose separator,much higher than those of other separators.The photographs of electrolyte droplet shapes on separators are shown in Fig.3.TheModified PP separator possesses enhanced wettability than the PP separator,accompanying with higher electrolyte uptake.It should be noticed that the PC-based electrolyte uptake for PP separator is only 3.6%,much lower than other separators.
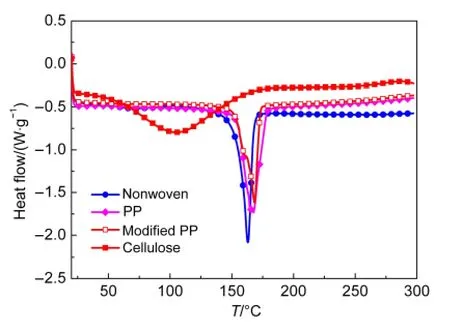
Fig.2 DSC curves of the separators from room temperature to 300°C
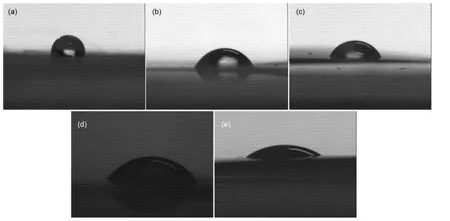
Fig.3 Photographs of electrolyte droplet shapes on the separators
3.4 Electrochemical properties
EIS is a powerful tool to study the electrochemical behaviors of cells.Fig.4a shows the EIS spectra of EDLC cells using various separators.Each Nyquist plot consists of one semicircle in the high frequency range,a straight line with a slope of close to 45°in the middle frequency region,and a nearly vertical line in the low frequency range.The intercept with the real impedance axis at high frequency(Z″(ω)=0)gives the equivalent series resistance(Rs),which includes the resistance of electrolyte and separator,the intrinsic resistance of activated carbons,the contact resistance between activated carbons and carbon black particles,and the contact resistance between the electrode film and the current collector.24-26The semicircle observed in the high frequency range can be correlated to the interface electrode/current collector,the particle-particle contact,the electrode porosity,and/or the charge transfer resistance of possible pseudocapacitance arising from the redox reactions of impurities and surface functionalities of carbons.26,27The inclined 45°line is attributing to the transmission-line-like behavior correlating to the distributed resistance/capacitance in the porous activated carbon electrodes.28,29The straight line nearly vertical to the realistic impedance axis is the characteristic of capacitive behavior.It can be found that the value ofRsincreases in the order of Cellulose<Modified PP<PP<Nonwoven.This trend indicates thatRsvalue increases with the contact angle between the PC-based electrolyte droplet and the separator.
The galvanostatic charge-discharge curves at the current density of 0.5 A·g-1are shown in Fig.4b.The specific capacitance of single electrode(Cs)for EDLC was calculated from the following formula:30

where,Iis the discharge current density,tis the discharge time,ΔUis the discharge potential after theIRdrop,andMis the total mass of activated carbon in both anode and cathode electrodes.TheCsvalue of the capacitor with a Cellulose separator is 101.4 F·g-1at a current density of 0.5 A·g-1,higher than those of other capacitors.The specific capacitance as a function of current density is shown in Fig.4c,which decreases as the current density increases.Among the four cells,the capacitor with the Cellulose separator has superior rate capability,with specific capacitances of still up to 97.2 and 89.6 F·g-1at the current densities of 5 and 10 A·g-1,respectively.The rate capabilities of the capacitors with various separators increase with the decrease of contact angles andRsvalues.Fig.4d shows the self-discharge properties of the capacitors.It reveals that the open circuit voltage of the cell with the Cellulose separator decays slightly faster than that of other three cells.
Fig.4e shows the EIS spectra of hybrid(NCM+AC)/graphite hybrid batter-supercapacitor cells at charged state at 4.0 V.It can be observed that there is little difference inRsresistance for the four cells with different separators.The two overlapping semicircles in the high and middle frequency ranges(higher than 4.0-6.3 Hz)in each Nyquist plot can be mainly assigned to the lithium-ion diffusion through the solid electrolyte interphase(SEI)film(Rx)and the charge transfer resistance(Rct),respectively.31The curve in the low frequency region deviates from the 45°line of the Warburg line for Li-ion battery or the transmission line for porous activated carbon electrode,and the nearly vertical line for capacitor.It can be ascribed to the combined action of the redox reactions occurred in battery materials and the capacitive behavior existed in activated carbon.The values ofRsandRxof the four cells are proximately the same.TheRctvalues of the cells with the Cellulose and the Modified PP separators are slightly smaller than those of the
cells with the Nonwoven and the PP separators.Moreover,in the low frequency region,the impedances of cells with the Cellulose and the Modified PP separators are larger than those of the ones with the Nonwoven and the PP separators.
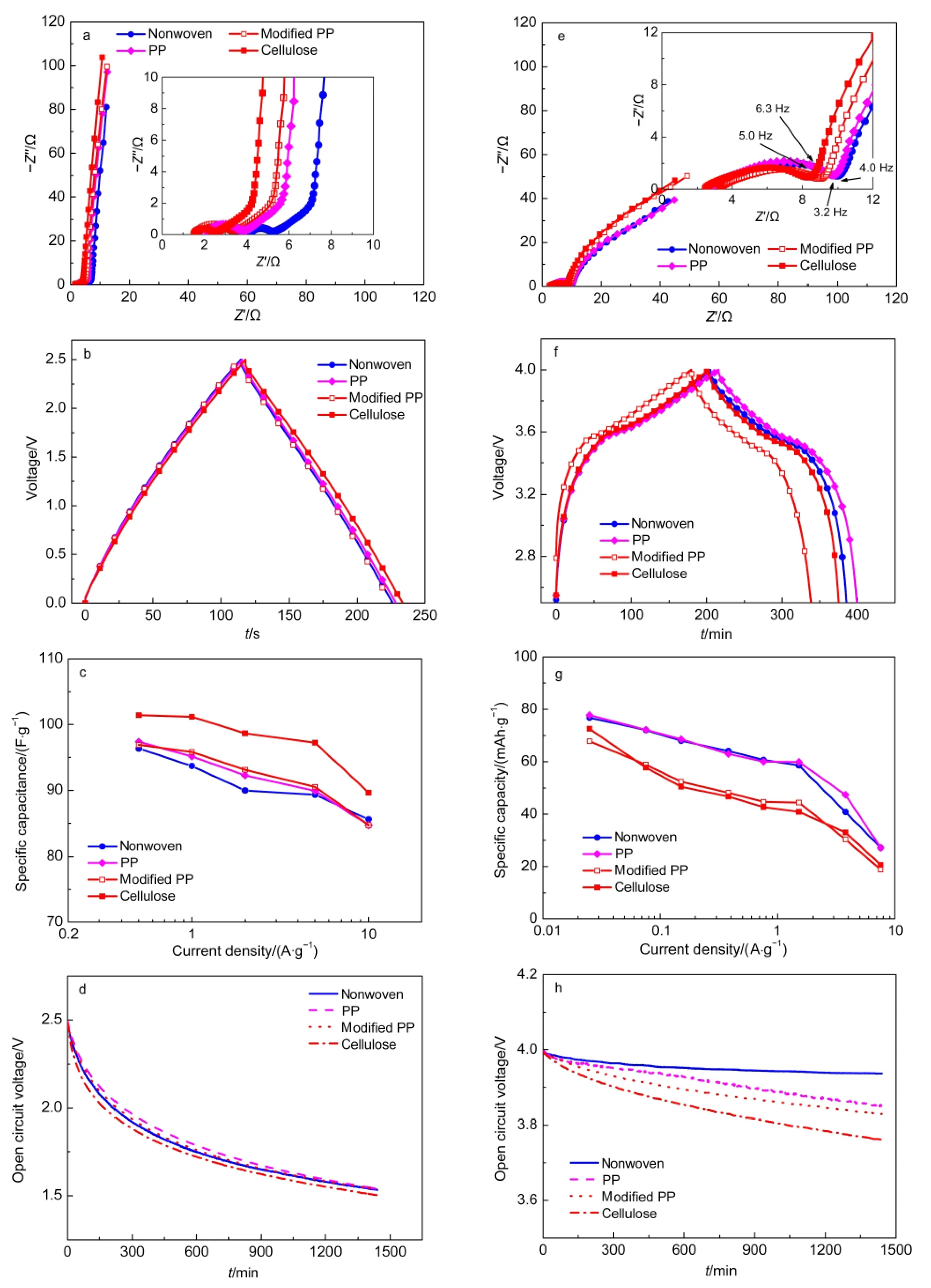
Fig.4 (a,e)EIS,(b,f)galvanostatic charge-discharge,(c,g)rate capability,and(d,h)self-discharge performances for(a-d)the EDLCs and(e-h)the(NCM+AC)/graphite hybrid battery-supercapacitors
The constant current charge-discharge curves at the current density of 25 mA·g-1are depicted in Fig.4f.The specific capacityversuscurrent density curves are depicted in Fig.4g.The specific capacity was calculated based on the total mass of NCM and activated carbon in cathode.It reveals that the cells with the PP and the Nonwoven separators display higher specific capacity and superior rate capability than the ones with the Cellulose and the Modified PP separators.It indicates that the thermal stability and the wettability of the Modified PP separator are improved at the expense of specific capacity and rate capability.The Cellulose separator with better affinity to Li-ion electrolyte shows unexpectedly low specific capacity,and high self-discharge rate(Fig.4h).On the contrary,the cell with the Nonwoven PP separator(100-μm-thick)displays superior selfdischarge performances.
4 Conclusions
The nonwoven PP mat,porous PP membrane,Al2O3-coated PP membrane,and nonwoven cellulose paper have been studied for the application as separators for EDLCs and hybrid battery-supercapacitors.The specific capacitance and the rate capability of EDLC decrease in the order of cellulose paper>modified PP membrane,PP membrane>Nonwoven PP mat.On the other hand,the(NCM+AC)/graphite hybrid battery-supercapacitor cells with PP membrane and Nonwoven PP separators display superior specific capacity and rate capability.The devices with Cellulose separators show relatively high self-discharge rates.These results are expected to provide valuable information for further exploring high-performance EDLCs,lithiumion capacitors,and hybrid battery-capacitors.
(1) Miller,J.R.;Simon,P.Science2008,321,651.doi:10.1126/science.1158736
(2)Yang,X.;Cheng,C.;Wang,Y.;Qiu,L.;Li,D.Science2013,341,534.doi:10.1126/science.1239089
(3) Hu,X.B.;Deng,Z.H.;Suo,J.S.;Pan,Z.L.J.Power Sources2009,187,635.doi:10.1016/j.jpowsour.2008.11.033
(4) Hu,X.B.;Huai,Y.J.;Lin,Z.J.;Suo,J.S.;Deng,Z.H.J.Electrochem.Soc.2007,154,A1026.
(5) Lei,Y.;Huang,Z.H.;Shen,W.C.;Kang,F.Y.;Zheng,Y.P.Electrochim.Acta2013,107,413.doi:10.1016/j.electacta.2013.06.002
(6) Lei,Y.;Huang,Z.H.;Yang,Y.;Shen,W.C.;Zheng,Y.P.;Sun,H.Y.;Kang,F.Y.Sci.Rep.2013,3,2477.
(7)Davies,A.;Yu,A.P.Can.J.Chem.Eng.2011,89,1342.doi:10.1002/cjce.v89.6
(8) Liu,S.Q.;Liu,S.Q.;Huang,K.L.;Liu,J.S.;Li,Y.K.;Fang,D.;Wang,H.M.;Xia,Y.F.J.Solid State Electrochem.2012,16,1631.doi:10.1007/s10008-011-1573-7
(9) Böckenfeld,N.;Kuhnel,R.S.;Passerini,S.;Winter,M.;Balducci,A.J.Power Sources2011,196,4136.doi:10.1016/j.jpowsour.2010.11.042
(10) Ping,L.N.;Zheng,J.M.;Shi,Z.Q.;Qi,J.;Wang,C.Y.Chin.Sci.Bull.2013,58,689.
(11) Li,J.M.;Chang,K.H.;Wu,T.H.;Hu,C.C.J.Power Sources2013,224,59.doi:10.1016/j.jpowsour.2012.09.007
(12) Ping,L.N.;Zheng,J.M.;Shi,Z.Q.;Wang,C.Y.Acta Phys.-Chim.Sin.2012,28,1733.[平丽娜,郑嘉明,时志强,王成扬.物理化学学报,2012,28,1733.]doi:10.3866/PKU.WHXB201205092
(13)Amatucci,G.G.;Badway,F.;Du Pasquier,A.;Zheng,T.J.Electrochem.Soc.2001,148,A930.
(14) Cericola,D.;Kötz,R.Electrochim.Acta2012,72,1.doi:10.1016/j.electacta.2012.03.151
(15) Cericola,D.;Ruch,P.W.;Kötz,R.;Novak,P.;Wokaun,A.J.Power Sources2010,195,2731.doi:10.1016/j.jpowsour.2009.10.104
(16) Hantel,M.M.;Kaspar,T.;Nesper,R.;Wokaun,A.;Kötz,R.ECS Electrochem.Lett.2012,1,A1.
(17) Sun,X.Z.;Zhang,X.;Huang,B.;Zhang,H.T.;Zhang,D.C.;Ma,Y.W.J.Power Sources2013,243,361.doi:10.1016/j.jpowsour.2013.06.038
(18) Liang,Y.Z.;Cheng,S.C.;Zhao,J.M.;Zhang,C.H.;Sun,S.Y.;Zhou,N.T.;Qiu,Y.P.;Zhang,X.W.J.Power Sources2013,240,204.doi:10.1016/j.jpowsour.2013.04.019
(19) Huang,X.S.J.Solid State Electrochem.2011,15,649.doi:10.1007/s10008-010-1264-9
(20)Huang,B.;Sun,X.Z.;Zhang,X.;Zhang,D.C.;Ma,Y.W.Acta Phys.-Chim.Sin.2013,29,1998.[黄 博,孙现众,张 熊,张大成,马衍伟.物理化学学报,2013,29,1998.]doi:10.3866/PKU.WHXB201307031
(21)Sun,X.Z.;Zhang,X.;Zhang,D.C.;Ma,Y.W.Acta Phys.-Chim.Sin.2012,28,367.[孙现众,张 熊,张大成,马衍伟.物理化学学报,2012,28,367.]doi:10.3866/PKU.WHXB201112131
(22)Yi,T.F.;Xie,Y.;Zhu,Y.R.;Zhu,R.S.;Shen,H.Y.J.Power Sources2013,222,448.doi:10.1016/j.jpowsour.2012.09.020
(23) Idris,N.H.;Rahman,M.M.;Wang,J.Z.;Liu,H.K.J.Power Sources2012,201,294.doi:10.1016/j.jpowsour.2011.10.141
(24) Tonurist,K.;Thomberg,T.;Janes,A.;Romann,T.;Sammelselg,V.;Lust,E.J.Electroanal.Chem.2013,689,8.
(25)Sun,X.Z.;Zhang,X.;Zhang,H.T.;Zhang,D.C.;Ma,Y.W.J.Solid State Electrochem.2012,16,2597.doi:10.1007/s10008-012-1678-7
(26) Dsoke,S.;Tian,X.;Taubert,C.;Schluter,S.;Wohlfahrt-Mehrens,M.J.Power Sources2013,238,422.doi:10.1016/j.jpowsour.2013.04.031
(27) Lewandowski,A.;Olejniczak,A.;Galinski,M.;Stepniak,I.J.Power Sources2010,195,5814.doi:10.1016/j.jpowsour.2010.03.082
(28) de Levie,R.Electrochim.Acta1963,8,751.doi:10.1016/0013-4686(63)80042-0
(29) Kötz,R.;Carlen,M.Electrochim.Acta2000,45,2483.doi:10.1016/S0013-4686(00)00354-6
(30) Yang,X.;He,Y.S.;Jiang,G.;Liao,X.Z.;Ma,Z.F.Electrochem.Commun.2011,13,1166.doi:10.1016/j.elecom.2011.09.006
(31) Xu,J.;Chou,S.L.;Gu,Q.F.;Liu,H.K.;Dou,S.X.J.Power Sources2013,225,172.doi:10.1016/j.jpowsour.2012.10.033

