Time-dependent Appearances of Myofibroblasts during the Repair of Contused Skeletal Muscle in Rat and Its Application for Wound Age Determination
YU Tian-shui,GUAN Da-wei,CHANG Lin,WANG Xu,ZHAO Rui,ZHANG Hai-dong,BAI Ru-feng
(1.Department of Forensic Pathology,School of Forensic Medicine,China Medical University,Shenyang 110001,China;2.Collaborative Innovation Center of Judicial Civilization,Beijing 100088,China;3.Key Laboratory of Evidence Science,China University of Political Science and Law,Ministry of Education,Beijing 100088,China)
Abstract∶Objective To research the relation between the time-dependent appearances of myofibroblasts during the repair of contused skeletal muscle in rat and wound age determination.Methods A total of 35 SD male rats were divided into the control and six injured groups according to wound age as follows∶12h,1d,5d,7d,10d and 14d after injury.The appearances of myofibroblasts were detected by HE staining,immunohistochemistry and confocal laser scanning microscopy.Masson’s trichrome staining was utilized to examine collagen accumulation in the contused areas.Results Immunohistochemical staining showed that α-SMA+myofibroblasts were initially observed at 5 d post-injury.The average ratio of myofibroblasts was highest at 14d post-injury,with all samples,ratios more than 50%.In the other five groups,the average of α-SMA positive ratios were less than 50%.The collagen stained areas in the contused zones,concomitant with myofibroblast appearance,were increasingly augmented along with advances of posttraumatic interval.Conclusion The immunohistochemical detection of myofibroblasts can be applied to wound age determination.The myofibroblasts might be involved in collagen deposition during the repair of contused skeletal muscle in rat.
Key words∶forensic pathology;wounds and injuries;muscle,skeletal;myofibroblasts
Introduction
The repair of contused muscles consists of two overlapping phases∶regeneration of damaged myofibers and formation of fibrosis.The two processes are at the same time supportive but also competitive with each other[1].After muscle injury,satellite cells proliferate early and differentiate rapidly to myoblasts,and thereafter fuse with each other into multinucleated myotubes.Myotubes also rapidly regenerate the injured muscle by fusing with surviving parts of local injured myofibers[2].However,the functional recovery of the injured muscle is often hindered by the development of fibrosis,which typically appears during the second week after muscle injury[3].During the early step of fibrotic development,fibrin and fibronectin derived from blood cross-link to form primary matrix,which plays scaffold and anchorage roles for invading fibroblasts and gives the initial strength to withstand the forces applied on it[4].As fibrosis proceeds,fibroblasts start showing the myofibroblastic phenotype characterized by large bundles of actin-containing microfilaments disposed along the cytoplasmic face of the plasma membrane and dense bodies visible by electron microscopy[5].The typical myofibroblasts were reported to be alpha-smooth muscle actin (α-SMA) positive by immunostaining,α-SMA becoming the most reliable marker of myofibroblastic identification[6].To replace the damaged tissues,myofibroblasts participate actively in the synthesis of extracellular matrix components such as typesⅠ/Ⅲ collagen,tenascin and fibronectin,followed by the production of a connective tissue scar.In an experimental study[7],myofibroblasts showed the time-dependent appearance in the granulation tissue of skin wounds in rat.Betz et al.[8]further confirmed their application as a marker for determining the duration in human skin wounds.Thus,the tenable hypotheses were that the appearance of myofibroblasts can provide information for determining the age of skeletal muscle contusion in rat.
In continuation of the previous investigations on fibroblastic cells (FBC),we investigated the distribution and appearance of α-SMA+myofibroblasts in the contused zones by immunostaining in the current study,with a focus on the participation of myofibroblasts during the repair of contused skeletal muscle in rat,and an insight into their practical suitability as a marker for wound age determination.
Materials and methods
Animal model of contused skeletal muscle
As previously reported[9],we established a standardized animal model of contused skeletal muscle of rat.A total of 35 healthy,adult SD male rats,weighing 230-250 g,were anesthetized with an intraperitoneal injection with 2%sodium pentobarbital(30 mg/kg).Subsequently,the rats were placed on the experimental table in a prone position,and a single impact was delivered to the site of the right posterior limb 2.5 cm away from calcaneus.After contusion,the rats were individually housed in the cage and fed with commercial rat chow and tap water ad libitum.Sacrificed by an intraperitoneal injection of over dose of sodium pentobarbital(350 mg/kg)at 12 h,1 d,5 d,7 d,10 d and 14 d after impact(five rats at each interval),the muscle samples were derived from the contused areas.No bone fracture was detected at dissection.The remaining 5 rats were used as control,dissected from the same site after anesthetization with over dose of pentobarbital.
The experiments were conformed to the Principles of Laboratory Animal Care published by National Institutes of Health and performed according to the Guidelines for the Care and Use of Laboratory Animals of China Medical University.
Immunohistochemistry and morphometric analysis
The muscle specimens were immediately fixed in 4%paraformaldehyde in phosphate-buffered saline(pH=7.4) and embedded in paraffin.And 5 μm-thick sections wereprepared.Immunostaining wasperformed using the streptavidin-peroxidase method as follows.The sections were deparaffinized in xylene,rehydrated with a series of graded alcohol,and then underwent microwave antigen retrieval in 0.01mol/L sodium citrate buffer (pH=6.0).The endogenous peroxidase activity was blocked by hydrogen peroxide pretreatment,and then further blockaded by incubation with 10%non-immune goat serum.Afterwards,the sections were incubated with mouse anti-α-SMA mAb (dilution 1∶200,MS-113,Lab Vision Corporation,Fremont,USA)overnight at 4℃,followed by incubation with Histostain-Plus Kit(Zymed Laboratories,South San Francisco,USA)according to the manufacturer’s instructions.The sections were routinely counterstained with hematoxylin.As the immunohistochemical controls for immunostaining procedures,some sections were incubated with normal mouse IgG or PBS in place of the primary antibody.HE staining was conventionally conducted.
As previously reported[10-11],a morphometrical analysis was performed for a semi-quantitative evaluation of immunohistochemical findings.Five highpower microscopic fields were randomly selected in the contused zones of each section(original magnification, ×400),and the ratio of the number of α-SMA+myofibroblasts to the total number of polymorphonuclear leukocytes (PMN),round-shaped mononuclear cells (MNC) and spindle-shaped FBC was counted in each microscopic field.The average ratio of five randomly chosen visual fields was evaluated as α-SMA expression score of each specimen.All measurements were performed blind by two different investigators who had no prior knowledge.
Confocal laser scanning microscopy (CLSM)
The deparaffinized sections were blocked with 5%BSA before incubated with mouse anti-α-SMA mAb overnight at 4℃.Afterwards,they were further incubated with Alexa Fluor®488 donkey anti-mouse IgG (dilution 1∶200,A21202,Invitrogen,USA)at room temperature for 2 h,protected from light.Normal mouse IgG or PBS was used instead of primary antibodies as the negative controls.The sections were observed by CLSM with parameters as follows.
A confocal laser scanning microscope system(FV1000S,Olympus,Japan),mounted on an inverted optical microscope (IX81,Olympus,Japan),was used,and the observation and image acquisition,using 20× and 60× oil immersion objective lens,were performed by 488nm excitation/519nm emission for Alexa Fluor®488.In order to test for localization,a single section at the same focus plane was scanned at 0.62 μm-thickness in z-axle,and the two channels were merged into a 12-bit RGB tif-file using FV1000 Viewer(Ver 1.6b) software.After recording was done by CLSM,no alternations of image files were performed by additional image processing.
Masson’s trichrome staining
The 5 μm-thick tissue sections were stained with Masson’s trichrome for the detection of collagen accumulation in the contused areas.The collagen fibers showed blue color in the sections.
The areas of collagen deposition,expressed as a percentage of the contused areas,were assessed by analyzing five fields ofMasson’s trichrome stained sections per animal.Acquired at 400×magnification,each field was analyzed using Image-Pro Plus 6.0 (Media Cybernetics,USA).Morphometrical analysis was performed by two investigators without prior knowledge on the experimental procedures.
Statistical analysis
Results
Expression of histology,immunohistochemistry and immunofluorescent method
The histological observation showed that,in the sections with HE staining,the normal myofibers,stained pink,arranged closely and the nuclei,stained blue,were located in the sarcolemma (Fig.1A).A large number of PMN and MNC accumulated in the contused areas,and necrotized skeletal muscle fibers were phagocytosed increasingly at 12 h and 1 d post-injury.And the skeletal muscle contusion was recognized as interstitial hemorrhage with PMN and MNC infiltration at 1 d post-injury (Fig.1B).A great quantity of regenerated myofibers,concomitant with FBC and multinucleated myotubes,were initially present in the contused zones at 5 d postinjury (Fig.1C),and damaged myofibers were mostly phagocytosed at 7 d post-injury.A large number of spindle-shaped FBC segregated by large masses of collagen fibers remained abundant at 10 d and 14d post-injury (Fig.1D).
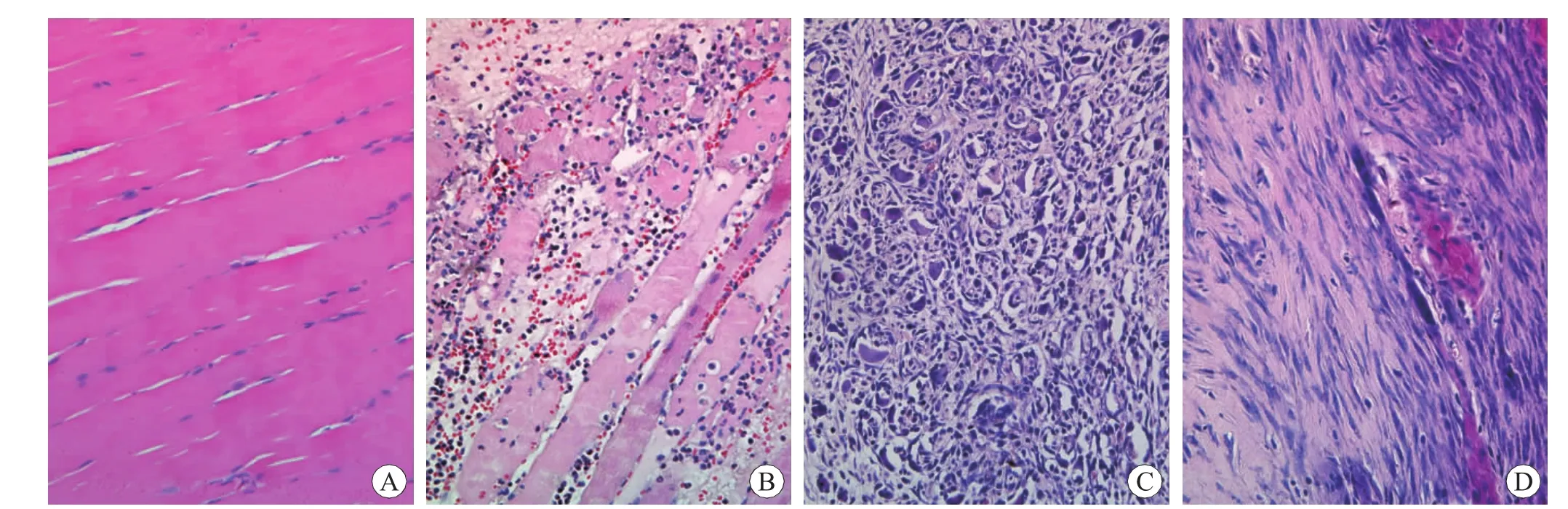
Fig.1 Histological observation of myofibroblasts after skeletal muscle contusion HE×400
For the identification of myofibroblasts in FBC,immunostaining with α-SMA was conducted by immunohistochemical staining.No positive staining was detected in FBC in the control,12 h and 1 d postinjury groups.Afterwards,α-SMA positive staining was observed in FBC in the contused zones from 5 d to 14 d.The α-SMA positive myofibroblasts and vascular smooth muscle cells of new vessels were detected in the contused areas at 7 d post-injury(Fig.2A),and a large number of α-SMA positive myofibroblasts separated by extracellular matrix were present in the contused areas at 14 d post-injury(Fig.2B).
To further identify the expression of α-SMA in myofibroblasts,α-SMA immunolocalization was performed with CLSM.Myofibroblasts showed cytoplasmic immunoreactivity for α-SMA at 5 d and 7 d post-injury (Fig.2C).With the extension of a posttraumaticinterval,intensive positive signalswere observable in microfilament bundles of myofibroblastic cytoplasm.Myofibroblasts displayed a higher elongated morphology in longitudinal sections at 10 d and 14 d post-injury (Fig.2D) than myofibroblasts of 5d and 7d post-injury.
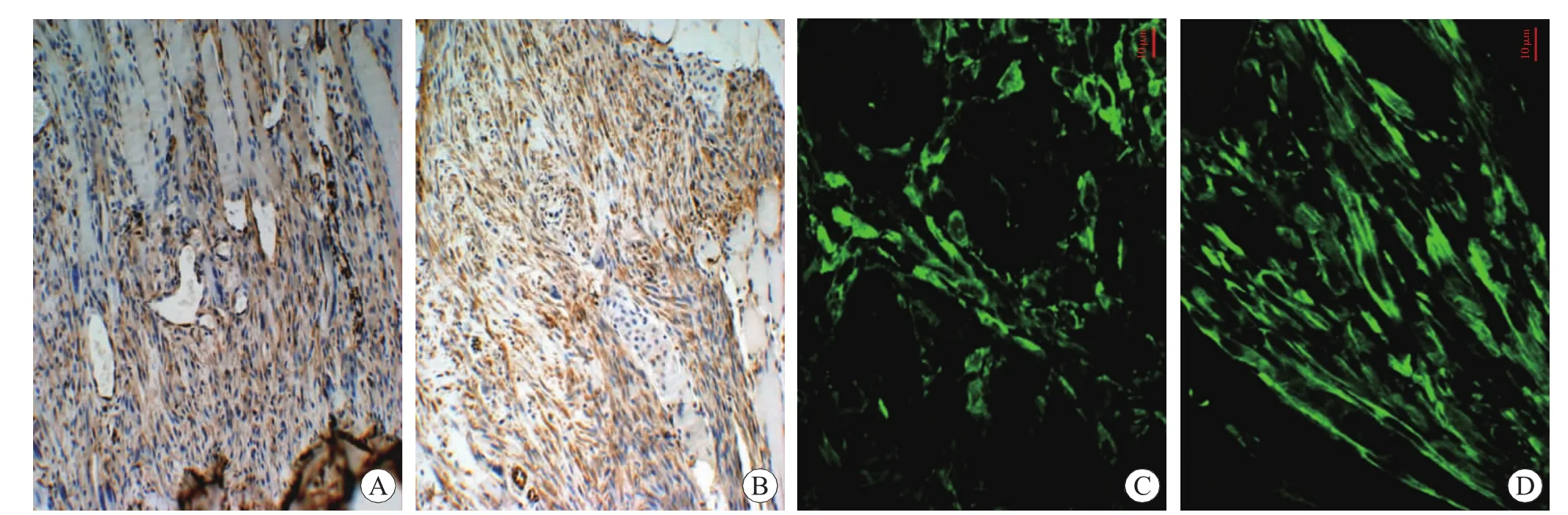
Fig.2 Immunohistochemical staining and immunolocalization of α-SMA in myofibroblasts after skeletal muscle contusion
Analysis of immunohistochemistry
The ratios of α-SMA+myofibroblasts in relation to wound age were observed,α-SMA positive ratios being zero in the control and the contused zones at 12 h and 1 d post-injury and low in the sections aged 5 d and 7 d post-injury,and all specimens presenting values of less than 30%.The α-SMA positive ratios increased almost two fold in 10 d injury group when compared with those in 5 d and 7 d injury groups,and remained elevated until 14 d post-injury.The ratios were over 50%in all samples at 14 d post-injury,and one specimen showed the highest value (66.9%).The α-SMA positive ratios were observed significant differences between 5 d,10 d,14 d injury groups and their preceding respective group (P<0.05) (Table 1).

Table 1 The average of α-SMA positive ratios after skeletal muscle contusion(n=5,%)
Analysis of Masson’s trichrome staining
The collagen deposition was not detected at 12h and 1 d post-injury and the control.From 5 d postinjury onward,the collagen fibers stained blue were deposited in the contused zones,and then began to progressively increase at 7 d post-injury (Fig.3A).A large quantity of collagen fibers were observed around the myofibroblasts at 10 d post-injury,and a peak value in the collagen content was observed at 14 d post-injury (Fig.3B).In the areas of collagen deposition were observed significant differences between 5 d,7 d,10 d,14 d injury groups and their preceding respective group (P<0.05)(Table 2).
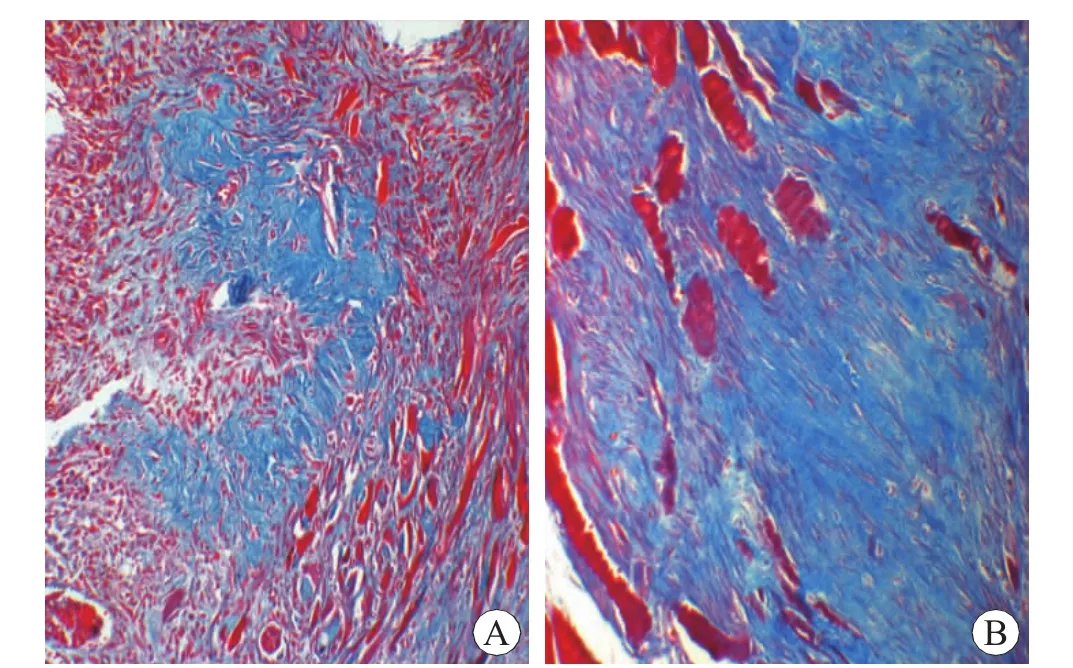
Fig.3 Collagen fibers after skeletal muscle contusion Masson×200
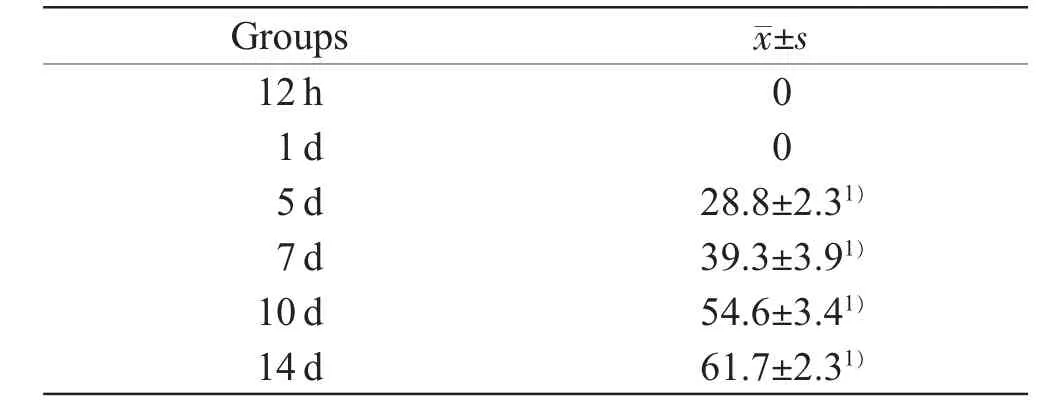
Table 2 The percentage of collagen deposition after skeletal muscle contusion(n=5,%)
Discussion
In forensic pathology,the molecular and cellular pathophysiology of wound healing process is applied to wound age determination.Forensically,most studies to date on wound age determination have been focused on analyses of extracellular matrix,adhesion molecules,growth factors and cytokines[12-14].A few studies have demonstrated that the numbers of various types of cells in autoptical human skin specimens were applicable to early and late wound age estimation[15-18].However,there has been little literature on the timedependent appearances of myofibroblasts for wound age estimation in mammalian skeletal muscles.
The resident fibroblasts by recruitment,proliferation and differentiation were an important source of myofibroblasts through two stages described as fibrotic development of skin,lung and kidney∶One was that with a change in the connective tissue microenvironment caused by several agents or stimuli,the resident fibroblast differentiates into the proto-myofibroblast,which could be seen in early granulation tissue 2-4 d after an open wound;the other,with extension of posttraumatic interval,the proto-myofibroblast further evolved toward the differentiated myofibroblast characterized by the expression of α-SMA under the stimulation of growth factors and newly synthesized extracellular matrix components[19].In addition,muscle-derived stem cells(MC13 cells)[20],bone marrow-derived fibrocytes[21-22],and epithelial cells[23]could also differentiate into myofibroblasts in the fibrotic pathogenesis of skeletal muscle,liver,kidney and lung.However,the number of myofibroblasts decreased gradually,starting from the 15th day after wounding and on the 30th day,myofibroblast was no longer present in scar tissue by apoptotic mechanism[7].
In view of which,it is conceivable that myofibroblasts are of time-dependent presence in wounds.Betz et al.[8]further investigated time-dependent appearance of α-SMA+myofibroblasts in human skin wounds,demonstrating that myofibroblasts were initially detected in 5-day wounds and subsequently increased at the wound site.Our experimental model mimicked the contusion-repair process in the human skeletal muscles with a high degree of accuracy and objectivity,with all data obtained from the well-controlled animalexperiments.In agreement with the previous study[8],our results also showed that myofibroblasts initially appeared on 5 d post-injury,and subsequently α-SMA positive ratios increased gradually in the contused sites,and peaked on 14 d post-injury.From the viewpoint of forensic pathological applications,all samples at 14 d injury group showed α-SMA positive ratios of >50%.In the other five groups,no samples showed ratios of>50%.Thus,the α-SMA positive ratio of >50%can strongly indicate the wound age on 14 d,which demonstrated that the time-dependent appearance of myofibroblast can be applicable to determining skeletal muscle wound age.
Biosynthesis of collagen in the granulation tissue is known to be a prerequisite for wound healing.The synthesis of extracellular collagen in injured skeletal muscle was reported to occur as early as 2 d after contusion,with a gradual enhancement between 5 d and 21 d,and a significant decrease in the following 3 weeks[24].Moreover,typeⅢcollagen was prominent in the injured area during the first week post-injury;thereafter,the role of type Ⅰcollagen rapidly elevated,with a simultaneous decrease in the amount of typeⅢcollagen[25].Almost consistent with the previous observations,Masson’s trichrome staining in this study revealed that collagen deposition in the contused sites was detected as early as 5 d and remained elevated until 14 d after contusion.The change tendency of α-SMA positive ratios was the same as one of collagen content,which gradually increased with an extension of posttraumatic interval.Moreover,tissue fibrosis evolving from the impaired tissue remodeling after injury is characterized by myofibroblast accumulation.Myofibroblasts from rat granulation tissue at the early passage produced threefold more procollagens than did fibroblasts at the same stage[26].Therefore,we speculate thatmyofibroblastscan be involved in collagen deposition and scar formation during the repair of the contused skeletal muscle in rat.
Conclusion
We investigated the dynamic appearance of myofibroblasts and collagen fibers,indicating that the cells can provide significant information for the wound age determination of skeletal muscle,as well as promote the development of fibrosis in injured skeletal muscle after contusion.We presented a new perspective on wound age estimation,although further studies are needed using human samples from autopsy.
Acknowledgements
This study was financially supported in part by the research funds from the National Natural Science Foundation of China (81202383),the Doctoral Program of Higher Education of China (20122104110025)and Program for Young Innovative Research Team in China University of Political Science and Law.

