RhoA和Snail在涎腺腺样囊性癌中的表达及意义
胡瑞利,安峰,林媛媛,马赛,郭博伟
RhoA和Snail在涎腺腺样囊性癌中的表达及意义
胡瑞利1,安峰2△,林媛媛2,马赛2,郭博伟2
目的探讨RhoA和Snail在涎腺腺样囊性癌(SACC)中的表达及其与癌症侵袭转移的关系。方法采用免疫组织化学方法检测RhoA和Snail在55例SACC(SACC组)与20例癌旁正常组织(对照组)中的表达情况,分析RhoA和Snail的表达与SACC临床病理特征的关系及其在SACC组织中表达的相关性。结果SACC组的RhoA(69.1%vs 5.0%)和Snail(72.7%vs 10.0%)蛋白阳性表达率高于对照组(均P<0.05);有淋巴结转移者的RhoA和Snail阳性表达率高于无转移者,Ⅲ+Ⅳ期的RhoA和Snail阳性表达率高于Ⅰ+Ⅱ期者;实体型的RhoA阳性表达率高于筛孔型,实体型和管状型的Snail阳性表达率高于筛孔型(均P<0.05),而不同性别、年龄及肿瘤部位的RhoA和Snail阳性表达率差异无统计学意义;RhoA和Snail在SACC中的表达呈正相关(rs=0.414,P<0.001)。结论RhoA和Snail蛋白可能通过RhoA/ROCK/PKD1/NF-κB/Snail信号传导通路联合作用促进了SACC的浸润和转移。
涎腺肿瘤;癌;Rho相关激酶类;肿瘤浸润;肿瘤转移;RhoA;Snail;涎腺腺样囊性癌
涎腺腺样囊性癌(salivary adenoid cystic carcino⁃ma,SACC)是较常见的口腔颌面部恶性肿瘤之一,侵袭性强,易侵入神经和血管,易沿神经及血循环发生远处转移,肺转移率较高[1]。SACC的侵袭性生长、复发和转移是导致患者死亡的主要原因之一。锌指转录因子Snail为锌指蛋白超家族的第1个成员,由1个高度保守的羧基末端及1个高度可变的氨基末端组成[2]。其超家族的功能为转录抑制,这与它复杂保守的蛋白结构域有密切关联。因为Snail可诱导上皮-间充质的转化,间接增强了细胞转移侵袭的功能,所以Snail被看作是肿瘤转移的促进因素[3-4]。而蛋白RhoA是小G蛋白超家族的成员之一,它不仅参与了细胞凋亡的过程,而且在细胞凋亡的起始中起着开关的作用[5-6]。RhoA基因与肿瘤浸润转移关系密切,但目前关于RhoA蛋白在SACC中的作用尚不明确,有关RhoA和Snail两者在SACC中表达的报道较少。本研究运用免疫组化方法检测RhoA和Snail在SACC中的表达,探讨两者与SACC侵袭转移的关系,为有效控制SACC的发生发展提供依据。
1 资料与方法
1.1一般资料选取1997年9月—2013年9月于河北北方学院附属第一医院病理科存档的手术切除SACC石蜡标本55例(SACC组),其中男25例,女30例,年龄17~80岁,平均(54.24±12.52)岁,依照2005年WHO对涎腺肿瘤的病理分类进行分型[7]:筛状型20例,管状型19例,实体型16例;TNM分期法(UICC)进行临床分期:Ⅰ+Ⅱ期37例、Ⅲ+Ⅳ期18例。同时选取癌旁正常的组织20例作为对照组,所选取患者术前均未接受放疗和化疗。HE染色切片由2位资深的病理医师按照WHO诊断标准进行诊断。
1.2主要试剂兔抗人RhoA多克隆抗体、兔抗人Snail多克隆抗体均购自上海广锐生物试剂有限公司。
1.3免疫组织化学PV两步法检测RhoA、Snail蛋白表达水平石蜡切片经过常规脱蜡、水化、抗原修复、一抗(RhoA和Snail一抗的工作浓度分别为1∶50和1∶75)和二抗分别孵育、DAB显色、苏木精复染,中性树脂封片后观察两者的表达情况。用PBS代替一抗作为阴性对照。阳性标准为细胞出现棕黄色颗粒,阴性为无显色。阳性细胞比例评分方法:阴性为0分,阳性细胞比例<25%为1分,25%~50%为2分,51%~75%为3分,>75%为4分。细胞染色程度评分法:肿瘤细胞不着色为0分,淡黄色为1分,棕褐色为3分。阳性细胞比例评分×细胞染色程度评分=肿瘤细胞的染色评分,总分0分为阴性(-),1~2分为(+),3~6分为(++),7~12分为(+++)。
1.4统计学方法采用SPSS 17.0统计软件分析。计量资料采用表示,组间比较用t检验,计数资料用χ2检验,相关分析采用Spearman等级相关,以P<0.05为差异有统计学意义。
2 结果
2.1RhoA和Snail在2组中的表达RhoA表达于上皮细胞膜,部分表达于细胞质,呈棕黄色,见图1;Snail表达于细胞核中,呈棕黄色或棕褐色颗粒,见图2。SACC组的RhoA和Snail阳性表达率明显高于对照组,见表1。
2.2RhoA和Snail的表达与SACC临床病理特征的关系RhoA和Snail在有淋巴结转移者中的阳性表达率高于无转移者,Ⅲ+Ⅳ期的RhoA和Snail阳性表达率高于Ⅰ+Ⅱ者;实体型的RhoA阳性表达率高于筛孔型,实体型和管状型的Snail阳性表达率高于筛孔型(均P<0.05),而不同性别、年龄及肿瘤部位的RhoA和Snail阳性表达率差异无统计学意义,见表2。
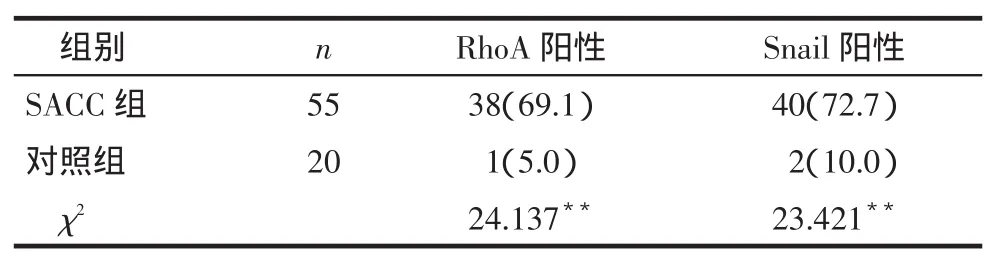
Tab.1The positive expression of RhoA and Snail in two groups表1 RhoA和Snail在2组中阳性表达情况例(%)
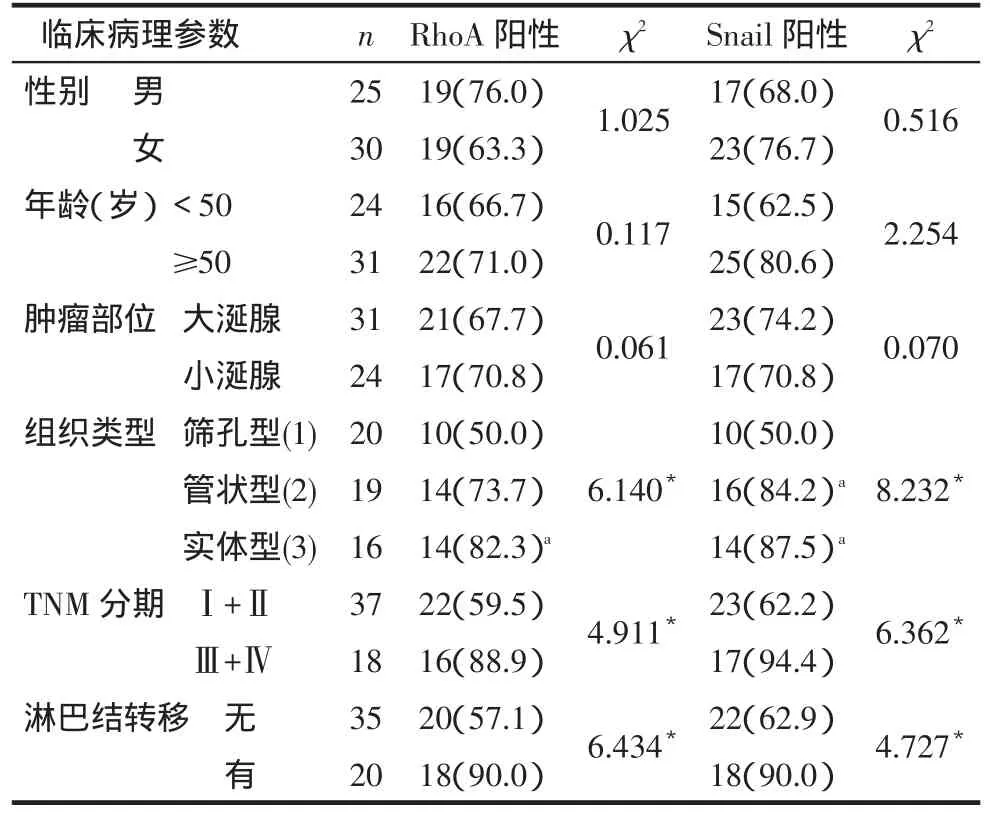
Tab.2The relationship between positive expression of RhoA,Snail and clinicopathological feature in SACC表2 RhoA、Snail阳性表达与SACC临床病理参数的关系例(%)
2.3SACC组织中RhoA和Snail的相关性RhoA和Snail共同阳性30例,共同阴性7例,两者呈正相关(rs=0.414,P<0.001),见表3
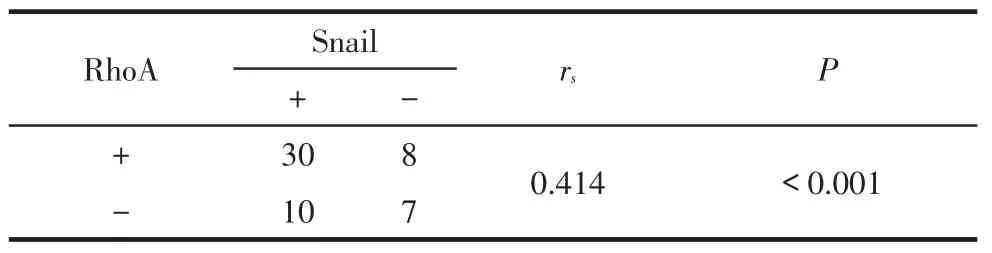
Tab.3Correlation between expression of RhoA and Snail proteins in SACC表3 在SACC中RhoA与Snail蛋白表达的相关性
3 讨论
Rho蛋白属于Ras超家族的亚家族成员,由相对分子质量为20 000~30 000的GTP结合蛋白组成[8]。目前为止,最少已经发现了20余个Rho家族成员,根据其序列同源性和功能的相似性,可分为RhoA类、Rac1类、Cdc42类和缺乏内源性GTP酶活性类。RhoA是细胞内重要的中间信号分子,在肌动蛋白的细胞骨架重组、细胞间黏附移动和基因转录等过程中均有RhoA参与[9]。已有研究证实,RhoA在胃癌、肝癌、大肠癌等组织中均呈高表达,参与了肿瘤的转移、侵袭、增殖、凋亡和周期调控等多个重要环节[10],并且扮演着许多信号通路分子开关的角色。在对乳腺癌的研究中,Cho等[11]发现如果抑制了RhoA的活性,可对Snail的活化起到间接阻断的作用,进一步对Snail介导的靶基因转录起到了抑制作用,从而使乳腺癌细胞失去了浸润和转移的功能。Cowell等[12]研究表明,如果细胞的连接被破坏,可以使RhoA/ROCK/PKD1/NF-κB/Snail信号传导通路激活,而RhoA也是Snail的一个上游因子。本课题组前期研究已证明,SACC的浸润和转移与血管内皮生长因子(VEGF)有关[13],且有研究显示,RhoA可通过ROCK信号通路参与VEGF诱导的内皮细胞运动和血管生成,也可促进肿瘤血管的生成,同时协助肿瘤细胞穿越脉管内皮向远处转移[14]。
Snail为锌指蛋白超家族的第一个成员,人的Snail基因定位于第20号染色体20q12.3,全长为5 882 bp,包含了3个外显子。锌指转录因子Snail属于转录抑制子中的Snail超家族,它可通过诱导上皮-间充质转化的发生,提高细胞侵袭转移的能力,因此Snail被视为肿瘤转移的促进因素[3-4]。研究表明,Snail在口腔癌、乳腺癌、宫颈癌的阳性表达率明显高于癌旁组织[15-16],与本研究结果相一致,本研究还发现RhoA与Snail蛋白表达与不同类型SACC、TNM分期和有无淋巴结转移有关,但与患者的年龄大小、性别及肿瘤所生长的部位无关,且两者表达呈正相关,提示RhoA和Snail的高表达可能随着SACC的恶性程度的增高而增高,有可能提示预后不良。这一结果与Cowell等[12]发现若破坏细胞的连接可使信号传导通路RhoA/ROCK/PKD1/NF-κB/ Snail激活相一致,并且RhoA还可通过ROCK信号通路参与VEGF的诱导,提示在SACC中如果抑制RhoA的活性,很有可能在一定程度上为SACC的治疗提供新的理论依据和治疗靶点。
(图1、2见插页)
[1]Lyv CT,Zhou ZH.The biological property and biotherapy of salivary adenoid cystic carcinoma[J].Journal of Oral and Maxillofacial Surgery, 2009,19(3):153-158.[吕春堂,周中华.涎腺腺样囊性癌生物学特性与生物治疗[J].口腔颌面外科杂志,2009,19(3):153-158].
[2]LKenouchi J,Matuda M,Furuse M.Regulation of tight junctions dur⁃ing the epithelium-mesenchyme transition:direct repression of the gene expression of claudins/occluding by Snail[J].J Cell Sci,2003,116(Pt10):1959-1967.
[3]Klymkowsky MW,Savagner P.Epithelial-mesenchymal transition:a cancer researcher’s conceptual friend and foe[J].Am J Pathol,2009,174(5):1588-1593.
[4]Evdokimova V,Tongon C,Ng T,et al.Translational activation of snail and other developmentally regulated transcription factors by YB-1 Promotes an Epithelial-Mesenchymal Transition[J].Cancer Cell,2009,15(5):402-415.
[5]Park GB,Kim YS,Song H,et al.Cross-linking of CD80 and CD86 diminishes expression of CD54 on EBV-transformed B cells through inactivation of RhoA and Ras[J].Immunc Netw,2011,11(6):390-398.
[6]David M,Petit D,Bertoglio J.Cell cycle regulation of Rho signaling pathways[J].Cell Cycle,2012,11(16):3003-3010.
[7]Lee J,Kang WK,Park JO,et al.Expression of activated signal trans⁃ducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma[J].APMIS,2009,117(8):598-606.
[8]Grise F,Bidaud A,Moreau V,et al.Rho GTPases in hepatocellular carcinoma[J].Biochim Biochim Biophys Acta,2009,1795(2):137-151.
[9]Loirand G,Sauzeau V,Pacaud P.Small G proteins in the cardiovas⁃cular system:physiological aspects[J].Physiol Rev,2013,93(4): 1659-1720.
[10]Wilson KF,Erickson JW,Antonyak MA,et al.Rho GTP ases and their roles in cancer metabolism[J].Trends Mol Med,2013,19(2):74-82.
[11]Cho SG,Li D,Stafford LJ,et al.KiSS I suppresses TNF-a-in⁃duced bresst cancer cell invasion via an inhibition of RhoA-mediat⁃ed NF-кb activation[J]J Cell Biochem,2009,107(6):1139-1149.
[12]Cowell CF,Yan IK,Eiseler T,et al.Loss of cell-cell contacts in⁃duced NF-кB via RhoA-mediated activation of protein kinase DI[J].J Cell Biochem,2009,106(4):714-728.
[13]Zhang G,Xiang F,An F,et al.The expression of STAT3,VEGF pro⁃tein in salivary adenoid cystic carcinoma and the effect on angiogen⁃esis[J].Tianjin Med J,2012,40(6):563-565.[张果,项峰,安峰,等.STAT3、VEGF蛋白在涎腺腺样囊性癌中的表达及对血管生成的影响[J].天津医药,2012,40(6):563-565].doi:10.3969/i.issn.0253-9896.2012.06.011.
[14]Pille JY,Denoyelle C,Varet J,et al.AntiRhoA and antiRhoC siR⁃NAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro[J].Mol Ther,2005,11(2):267-274.
[15]Sun L,Diamond ME,Ottaviano AJ,et al.Transforming growth factorbetal promotes matrix metalloproteinase-9-mediated oral cancer in⁃vasion through snail expression[J].Mol Cancer Res,2008,6(1):10-20.
[16]Lee MY,Chou CY,Tang MJ,et al.Epithelial-mesenchymal transi⁃tion in cervical cancer:correlation with tumor progression,epider⁃mal growth factor receptor overexpression,and snail up-regulation[J].Clin Cancer Res,2008,14(15):4743-4750.
(2014-11-12收稿 2015-01-25修回)
(本文编辑 闫娟)
Significance of RhoA and Snail expression in salivary adenoid cystic carcinoma
HU Ruili1,AN Feng2△,LIN Yuanyuan2,MA Sai2,GUO Bowei2
1 Hebei North University,Zhangjiakou 075000,China;2 The First Affiliated Hospital of Hebei North University△
ObjectiveTo investigate the relationship of RhoA and Snail expressions,and the invasion and metastasis in salivary adenoid cystic carcinoma(SACC).MethodsThe expressions of RhoA protein and Snail protein in 55 samples of SACC(SACC group)and 20 samples of para-carcinoma normal tissues(control group)were detected using immunohisto⁃chemical method.The relationship between RhoA protein and Snail protein expressions and clinical and pathological charac⁃teristics were analyzed.ResultsThe positive expressions of RhoA protein(69.1%vs 5.0%)and Snail protein(72.7%vs 10.0%)were significantly higher in SACC group than those in control group(P<0.05).The positive expression rates of RhoA protein and Snail protein were significantly higher in patients with lymph node metastasis than those in patients with⁃out lymph node metastasis.The positive expression rates of RhoA protein and Snail protein were significantly higher in pa⁃tients atⅢ+Ⅳstage than those in patients atⅠ+Ⅱstage.The positive expression rates of RhoA protein and Snail protein were significantly higher in substantive carcinal tissues than those in screen roller type and tubular carcinal tissues.The posi⁃tive expression of Snail protein was significantly higher in substantive and tubular carcinal tissues than that in screen roller type carcinal tissues(P<0.05).There were no significant differences in positive expression rates of RhoA and Snail between different gender,age and different carcinal tissues.There was a positive correlation beween expression rates of RhoA and Snail protein in SACC(r=0.414,P<0.001).ConclusionRhoA and Snail may both facilitate the infiltration and metastasis of SACC through RhoA/ROCK/PKD1/NF-kappa B/Snail signaling pathways.
salivary gland neoplasms;carcinoma;Rho-associated kinases;neoplasm invasiveness;neoplasm metasta⁃sis;RhoA;Snail;salivary adenoid cystic carcinoma
R739.87
A
10.11958/j.issn.0253-9896.2015.07.016
张家口市科学技术研究与发展计划指令项目(11110012D);河北北方学院自然科学面上项目(2013010)
1河北北方学院(邮编075000);2河北北方学院附属第一医院
胡瑞利(1986),女,硕士在读,主要从事口腔颌面头颈部肿瘤研究
△通讯作者E-mail:kq126@126.com

