醋酸铅作为铅源合成CH3NH3PbBr3−xClx纳米晶体颗粒
王亚楠 马 品 彭路梅 张 迪 方艳艳 周晓文 林 原,*
(1中国科学院化学研究所,光化学重点实验室,北京分子科学国家实验室,中国科学院分子科学科教融合卓越中心,北京 100190;2中国科学院大学,北京 100049)
醋酸铅作为铅源合成CH3NH3PbBr3−xClx纳米晶体颗粒
王亚楠1,2马 品1,2彭路梅1张 迪1,2方艳艳1周晓文1林 原1,2,*
(1中国科学院化学研究所,光化学重点实验室,北京分子科学国家实验室,中国科学院分子科学科教融合卓越中心,北京 100190;2中国科学院大学,北京 100049)
采用醋酸铅作为铅源,成功制备出CH3NH3PbBr3-xClx(MA = CH3NH3, 0 ≤ x ≤ 3)发光纳米晶体颗粒。醋酸铅比卤化物铅盐,尤其是氯化铅,能更好地溶解在NʹN-二甲基甲酰胺(DMF)溶剂中,解决了卤化物盐溶解度低的问题。在MAPbBr3-xClx中,不同比例的Br/Cl可以产生不同的光谱性质,荧光光谱(PL)可以从399 nm调控到527 nm。所有荧光光谱的半峰宽(FWHM)在20 nm左右,说明色谱比较纯。制备的MAPbBr3-xClx纳米晶体颗粒尺寸分布在~(11 ± 3) nm,可以很好地分散在甲苯中。其中,MAPbBr3纳米晶体颗粒的荧光量子产率(PLQY)高达73%,其平均荧光寿命为97.4 ns。
钙钛矿;CH3NH3PbBr3−xClx;纳米晶体;醋酸铅
1 Introduction
The field of solution processed organic-inorganic halide perovskite based solar cells has emerged in the last couple of years1−6. Intensive research has led to a rapid rise in powerconversion efficiency (PCE) since their original publication in 2009 to values reaching 22.1% in 20167, showing superiority over all other third generation photovoltaic devices. The most studied compound in photovoltaic is the methylammonium lead iodide CH3NH3PbI3(CH3NH3= MA) perovskite, due to its strong bandgap absorption of about 1.6 eV8,9. While the predominant research have since been oriented towards the use of these materials as active layer in light-harvesting devices,more recent studies have demonstrated photoelectronic applications of perovskite materials in light-emitting-devices(LEDs)10−14. In these studies, the compound of the perovskite is MAPbBr3with bandgap of about 2.3 eV. The above mentioned perovskite has a generic structure of APbX3(A = cationic organic molecules; X = halogens), which can be made from abundant and low-cost starting compounds.
Nano-structured halide perovskite hold great promise for various optoelectronic applications, especially for electroluminescent devices and lasers15−21. Researchers have been developing synthesis approaches to create a variety of nanostructures of organic-inorganic halide perovskite to expand the property space and to achieve new properties. Pé rez-Prieto and co-workers did pioneering works about non-template synthesis of perovskite nanopaticles (NPs)22. Nanoscaled MAPbBr3NPs were first synthesized at mild temperature.MAPbBr3NPs can be prepared by fine-tuning the molar ratios of all the componments, which either form part of the framework (MABr and PbBr2) or act as the organic capping(octylammonium bromide and 1-octadecene, ODE)23. Zhong and co-workers demonstrated a strategy to prepare MAPbBr3NPs by ligand-assissted reprecipitation (LARP) technique in order to solve the poor solubility of perovskite precursors in ODE24. All precursors were dissolved into the DMF and dropped into the poor solvent. Typical products had an average diameter of 3.3 nm with a size deviation of ±0.7 nm and the PLQY up to 70%. Ogale and co-workers have recently reported the preparation of MAPbBr3NPs by electrospay antisolventsolvent extraction and intercalation25.
The photoluminescence (PL) spectrum of halide perovskite also can be modified via controlling the ratio of halide26,27.However, tuning the band-gap in the blue-green region using solution processed chloride-bromide mixed halide perovskite has been a challenging task, given the low solubility of the chloride containing precursor (PbCl2) in solvent DMF. With the lead acetate to synthesis the perovskite NCs, the growth of perovskite crystal is much faster28. Lead acetate also has a high solubility in DMF. So we here use an organic lead source of lead acetate Pb(Ac)2to synthesis the perovskite NCs.
In this work, we use an organic lead source of lead acetate as Pb precursor to synthesis the mixed halide perovskites MAPbBr3−xClx(0≤ x ≤3) (NCs), especially for the blue-green perovskite MAPbBr3−xClxNCs. The obtained colloidal MAPbBr3NCs with absolute quantum yield reaches 73% at room temperature, which is comparable to the reported MAPbBr3QDs24. We conducted surface characterization,optical properties and thermal stability to illustrate the MAPbBr3colloidal NCs obtained by the lead acetate. Finally,we tuned the color by varying the chloride to bromide ratios in the MAPbBr3−xClx(0 ≤ x ≤ 3) perovskite using lead acetate.The PL emission peak can be tuned from 399 to 527 nm. Their FWHM are about 20 nm, indicating better color purity.
2 Experimental
2.1 Synthesis of CH3NH3Br and CH3NH3Cl
The precursors CH3NH3Br and CH3NH3Cl were synthesized from HBr (48% in water, Aladdin) and HCl (37% in water,Beijing Chemical Works) respectively, by reaction with methylamine solution (27%−32% in ethanol, Sinopharm) as follows. First, acid solution (HBr or HCl) was added dropwise to CH3NH2solution at 0 °C with stirring for 2 h. The mixture was evaporated in a rotary evaporator under vacumme at 60 °C.The resulting solid was washed with diethyl ether (AR, Beijing Chemical Works) for three times and then recrystallized from ethanol (AR, Beijing Chemical Works). The obtained CH3NH3Br and CH3NH3Cl crystals were dried under vacuum and used without further purification.
2.2 Synthesis of CH3NH3PbBr3−xClxnanoparticles
For MAPbBr3, 36 mmol·L−1of Pb(Ac)2∙3H2O (Ac =CH3COO, AR, Sinopharm) and 108 mmol·L−1of MABr were dissolved in 5 mL DMF (AR, Beijing Chemical Works) with 20 μL of n-octylamine (99%, Aladdin) and 0.5 mL of oleic acid(85%, Aladdin) to form a precursor solution. 0.2 mL of precursor solution was dropped into 5 mL of toluene (AR,Beijing Chemical Works) with vigorous stirring. Along with the mixing, strong green PL emission was observed. The MAPbCl3-based NCs were fabricated using the same strategy with 36 mmol·L−1of Pb(Ac)2∙3H2O and 108 mmol·L−1of MACl as the precursor. For the synthesis of mixed-halid-based perovskite NCs, separate precursor solutions of MAPbBr3and MAPbCl3were mixed with different volume ratios.
2.3 Characterization
Transmission electron microscopy (TEM) images were captured using HITACHI HT7700 (Japan). X-ray diffraction(XRD) patterns were recorded on a Rigaku D/Max 2500 X-ray diffractometer (Cu Kαradiation, λ = 0.15402 nm, Japan). X-ray photoelectron spectroscopy (XPS) analysis was carried out using a VG Scientific ESCALab 220i-XL (England)spectrometer with standard Al Kαradiation. The UV-Visible spectra (UV-Vis) were recorded using HITACHI U3010 UV-Vis spectrophotometer (Japan). The steady-state PL spectra were performed at room temperature on a HITACHI F-7000 fluorescence spectrophotometer (Japan). The PL quantum yields of colloidal perovskites were measured by using a Edinburgh FLS980 (England) absolute PL quantum yield measurement system with monochromatic light source (Xe lamp, 150 W) and integrating sphere. The lifetime was measured using a Compact fluorescence lifetime spectrometer C11367 (Japan), Quantaurus-Tau, with LED excitation wavelength of 365 nm.

Fig.1 (a) Schematic of MAPbBr3 NCs formation process by the reprecipitation technique.(b) Photographs taken under UV irradiation at indicated volume period with the precursor dropped into the toluene.
3 Results and discussion
Colloidal perovskite NCs were prepared by the modified ligand-assisted reprecipitation method as reported previously24.The scheme of the synthesis process is shown in Fig.1. In this work, Pb(Ac)2and MABr for the synthesis of MAPbBr3, were dissolved together with oleic acid (OA) and octylamine (OLA)in the good solvent dimethylformamide (DMF), resulting in a transparent solution (Fig.1a), which has no light emitting under 365 nm UV-lamp. The precursor solution was subsequently dropwise to the poor solvent toluene under vigorous stirring at room temperature (Fig.1a). Poor solvent means that in which both perovskite precursors are completely insoluble. Initially,the sample fluoresces blue, but with each addition of further precursor solution, the color shifts to green (Fig.1b). Finally,the semitransparent colloidal with green emitting under UV-lamp was formed. Smaller MAPbBr3NCs were formed when tiny amounts of the precursors (7 μL) were added into the toluene. The smaller MAPbBr3NCs exhibited blue-shifted emission due to quantum confinement analogous to conventional semiconductors. The change in photoluminescence with increasing amount of precursor indicates that the formation takes place via seed-mediated growth15,17.
Fig.2a shows a typical TEM image of MAPbBr3NCs, it is observed that typical MAPbBr3NCs have an average diameter of 11 nm with a size deviation of ±3 nm (Fig.2b). The particle size distribution of MAPbBr3NCs is uniform without large crystals. In order to analyze the phase structure, XRD (Fig.2b)patterns were applied to characterize the obtained samples. The diffraction peaks of MAPbBr3NC at 15.07°, 21.26°, 30.28°,33.90°, 43.31°, 46.00° can be index to the (100), (110), (200),(210), (220) and (300) planes, respectively, corresponding to a cubic phase group29,30. In order to understand the chemical composition and the surface properties of the MAPbBr3NCs,the samples were subjected to XPS analyses and the results are shown in Fig.2(d−f). The XPS data in Fig.2d show two symmetric peaks of Pb 4f7/2and Pb 4f5/2at binding energy value 138.4 and 143.2 eV, respectively. No sign of metallic Pb was observed in the NCs. The Br 3d peak can be fitted into two peaks with binding energies of 68.5 and 69.5 eV, respectively.The N 1s XPS data are plotted in the Fig.2e. In the MAPbBr3NCs, N 1s peak can be fitted to two peaks at 399.3 and 402.1 eV, indicating existence two chemical states. The peak at 399.3 eV can be assigned to the presence of ―NH2from OLA, while the peak at binding 402.1 eV originates from methylamine.The ―NH2group as a ligand intercalate with the MAPbBr3NCs and control the size of the MAPbBr3NCs15−17.
The optical properties of MAPbBr3NCs were investigated by steady-state absorption, photoluminescence (PL), and recombination lifetime. Fig.3a shows the PL behavior and absorption spectra MAPbBr3NCs. The abrupt absorption onsets and emission peaks at 527 nm correspond well with the band-to-band transition of bromide perovskite. Its FWHM is~20 nm. This is comparable to previous colloidal MAPbBr3quantum dots (QDs) solution results23,24, indicating better color purity. The absolute PLQY of colloidal solutions was 73%. The high PLQY indicated the reduction of nonradiative decay in high-quality MAPbBr3NCs. The recombination lifetime of MAPbBr3NCs was determined by measuring PL decay at the emission peak wavelength (λpeak). The PL decay curve of colloidal MAPbBr3NCs was shown in the Fig.3b. The curve is fitted with a triexponential function of time F(t), where τiis the decay time and aiis a prefactor.

Fig.2 (a) Transmission electron micrograph of colloidal MAPbBr3 NCs, inset shows photograph of the NCs dispersion in toluene under ambient light. (b) Size distribution histogram of MAPbBr3 NCs. (c) X-ray diffraction patterns of MAPbBr3 NCs.(d−f) XPS spectra corresponding to Pb 4f (d), Br 3d (e), and N 1s (f) of MAPbBr3 NCs.
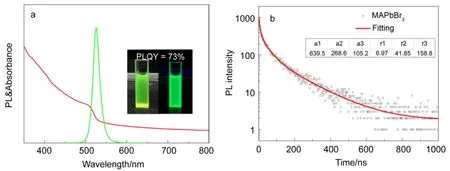
Fig.3 (a) PL and UV-Vis spectra of green MAPbBr3 NCs, insets show photographs of a toluene dispersion of MAPbBr3 NCs under white light and UV-light. (b) PL decay (black circle) and fitting curves (red line) for excitation at 365 nm and emission at 527 nm of MAPbBr3 NCs, the inset table is the fitting result. color online.

The average recombination lifetime (τave) was obtained from the triexponential decays according to the equation (2):

The PL decay fitting result is shown in the table of Fig.3b. The τaveof MAPbBr3NCs is 97.4 ns, indicating a low recombination rate compared to the MAPbBr3QDs. The triexponential decay suggests that there are three components in the colloidal solution. The fast decay is related to trap-assisted recombination between the NCs, whereas the slow decay is related to exciton recombination inside the NCs13.
We used circulating water controller system to test the thermal stability (Fig.4). The experimental temperature ranged from 20 to 80 °C. The PL intensity decrease when the temperature increased (Fig.4a). Along with the quenching of MAPbBr3NCs emission, the peak wavelength has a little blue shift and there is no obvious change about the FWHM. The intensity of the NCs emission increased with the cooling of the temperature (Fig.4b). After the temperature cooling to the original temperature, the peak wavelength and the FWHM can fully recover, but the PL intensity cannot recover. The relative intensity of MAPbBr3NCs is shown in Fig.4c. From Fig.4c, we can see that the PL intensity was decreased to about 75% after heat treatment. It is possibly due to some surface breakage that may promote the recombination which is similar to the conventional inorganic QDs33.
We fabricated a series of colloidal MAPbBr3−xClxNCs with Pb(Ac)2. Fig.5a shows the photo of NCs with compositions varying from pure Br to pure Cl colloidal NCs. The colloidal samples show different emission colors from green to blue when illuminated with UV-lamp. We use XRD pattern to study the structural properties of the MAPbBr3−xClxNCs, and the result is shown in the Fig.5b. Multiple reflections demonstrate that all the MAPbBr3−xClxNCs associated with the cubic Pm3m space group. Most remarkably, we find that all Bragg peaks slightly shift towards a high angle along with the increase in the smaller Cl substitution ratio. The inset of Fig.5b shows a cubic lattice constant a as a function of x. The cubic lattice constant a is extracted from the angular position of the (100) Bragg reflection 2θ using λ/2a = sinθ. The monotonic trend in the lattice constants from MAPbBr3to MAPbCl3indicates an expansion of the perovskite cage. The normalized PL peak wavelength of the MAPbBr3−xClxNCs is shown in Fig.5c. The change in emission indicates the increase in the bandgap as Br is replaced with Cl. The PLQYs of the MAPbBr3−xClxNCs were measured using a fluorescence spectrometer equipped with an integrating sphere and excitation at a wavelength of 365 nm and the data were summarized in Table 1. It is surprising that such a slight variation in the MAPbBr3−xClxcan lead to such a remarkable difference in their PL emission intensity. The exact reason is still not clear, it might be associated with the orientation and vibration restraint of the MA cation in the MAPbBr3−xClxNCs30. In addition, Pb(Ac)2also can be used to synthesis the MAPbBr3−xIxNCs and the PL emission spectra are shown in the inset of Fig.5c. The PL decay curves of MAPbBr3−xClxNCs are shown in the Fig.5d. The fitting results are shown in Table 1. We can see that the corresponding τaveof MAPbBr3−xClxNCs decreases with the increase of Cl substitution ratio. This trend is the same as that of PLQY, indicating the reduction of nonradiative decay in high-quality MAPbBr3NCs.

Fig.4 (a) PL spectra of MAPbBr3 NCs with the increase of temperature. (b) PL spectra of MAPbBr3 NCs with the decrease of temperature.(c) Temperature-dependent PL intensity of MAPbBr3 NCs. color online.

Fig.5 (a) Photographs of MAPbBr3−xClx NCs under 365 nm UV-lamp. (b) X-ray diffraction patterns of MAPbBr3−xClx NCs.Inset: evolution of the lattice parameter as a function of x. (c) PL emission spectra of MAPbBr3−xClx NCs.Inset: PL emission spectra of MAPbBrxI3−x. (c) PL decay curves of MAPbBr3−xClx NCs for excitation at 365 nm.

Table 1 Detailed information of halide substituted samples
4 Conclusions
In summary, mixed halide perovskites MAPbBr3-xClxNCs are successfully synthesized by using lead acetate. We also tune the color by varying the chloride to bromide ratios in the MAPbBr3−xClx(0 ≤ x ≤ 3) perovskite using lead acetate. The PL emission peak can be tuned from 399 to 527 nm. Their FWHM are about 20 nm, indicating better color purity. The NCs are uniformly dispersed in toluene with an average size of(11 ± 3) nm. The photoluminescence quantum yield of MAPbBr3NCs reaches ~73%. The τaveof MAPbBr3NCs is 97.4 ns, indicating a low recombination rate. Above all,Pb(Ac)2can be used to prepared MAPbBr3−xClxNCs, so it may be a promising lead source to fabricate the perovskite optoelectronic devices.
(1) Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. J. Am. Chem. Soc.2009, 131, 6050. doi: 10.1021/ja809598r
(2) Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei,H.; Li, B.; Wan, J.; Yang, G.; Yan, Y. J. Am. Chem. Soc. 2015, 137,6730. doi: 10.1021/jacs.5b01994
(3) Bi, D.; Tress, W.; Dar, M. I.; Gao, P.; Luo, J.; Renevier, C.; Schenk,K.; Abate, A.; Giordano, F.; Correa Baena, J. P.; Decoppet, J. D.;Zakeeruddin, S. M.; Nazeeruddin, M. K.; Grä tzel, M.; Hagfeldt, A.Sci. Adv. 2016, 2, 1. doi: 10.1126/sciadv.1501170
(4) Wang, Y. Q.; Li, L.; Nie, L. H.; Li, N. N.; Shi, C. W. Acta Phys. -Chim. Sin. 2016, 32, 2724. [王艳青, 李 龙, 聂林辉,李楠楠, 史成武. 物理化学学报, 2016, 32 (11), 2724.]doi: 10.3866/pku.whxb201607272.
(5) Mejí a Escobar, M. A.; Pathak, S.; Liu, J.; Snaith, H. J.; Jaramillo, F.ACS App. Mater. Inter. 2017, 9 , 2342. doi: 10.1021/acsami.6b12509.
(6) Zhou, L.; Zhu, J.; Xu, Y. F.; Shao, Z. P.; Zhang, X. H.; Ye, J. J.;Huang, Y.; Zhang, C. N.; Dai, S. Y. Acta Phys.-Chim. Sin. 2016, 32 ,1207. [周 立, 朱 俊, 徐亚峰, 邵志鹏, 张旭辉, 叶加久, 黄阳, 张昌能, 戴松元. 物理化学学报, 2016, 32 (5), 1207.]doi: 10.3866/pku.whxb201602241.
(7) NREL, B. R. C. E. http://www.nrel.gov, accessed: November 2016.
(8) Yin, X.; Xu, Z.; Guo, Y.; Xu, P.; He, M. ACS Appl. Mater. Inter.2016, 8, 29580. doi: 10.1021/acsami.6b09326.
(9) Yin, X.; Guo, Y.; Xue, Z.; Xu, P.; He, M.; Liu, B. Nano Res. 2015, 8,1997. doi: 10.1007/s12274-015-0711-4.
(10) Tan, Z. K.; Moghaddam, R. S.; Lai, M. L.; Docampo, P.;Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; Hanusch, F.; Bein, T.; Snaith, H. J.;Friend, R. H. Nat. Nanotech. 2014, 9, 687.doi: 10.1038/nnano.2014.149
(11) Zhang, X.; Liu, H.; Wang, W.; Zhang, J.; Xu, B.; Karen, K. L.;Zheng, Y.; Liu, S.; Chen, S.; Wang, K.; Sun, X. W. Adv. Mater.2017, 1606405-1/7. doi: 10.1002/adma.201606405
(12) Yao, Q.; Fang, H.; Deng, K.; Kan, E.; Jena, P. Nanoscale 2016, 8, 17836. doi: 10.1039/c6nr05573g
(13) Cho, H.; Jeong, S. H.; Park, M. H.; Kim, Y. H.; Wolf, C.; Lee,C. L.; Heo, J. H.; Sadhanala, A.; Myoung, N.; Yoo, S. Science 2015, 350, 1222. doi: 10.1126/science.aad1818
(14) Ling Y.; Yuan, Z.; Tian. Y.; Wang, X.; Wang, J. C.; Xin, Y.;Hanson, K.; Ma, B.; Gao, H. Adv. Mater. 2015, 17, 1.doi: 10.1002/adma.201503954
(15) Sichert, J. A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.;Milowska, K. Z.; Garcí a Cortadella, R.; Nickel, B.;Cardenas-Daw, C.; Stolarczyk, J. K.; Urban, A. S.; Feldmann,J. Nano Lett. 2015, 15, 6521.doi: 10.1021/acs.nanolett.5b02985
(16) Tyagi, P.; Arveson, S. M.; Tisdale, W. A. J. Phys. Chem. Lett.2015, 6, 1911. doi: 10.1021/acs.jpclett.5b00664
(17) Tong, Y.; Ehrat, F.; Vanderlinden, W.; Cardenas-Daw, C.;Stolarczyk, J. K.; Polavarapu, L.; Urban, A. S. ACS Nano 2016, 10, 10936. doi: 10.1021/acsnano.6b05649.
(18) Hassan, Y.; Song, Y.; Pensack, R. D.; Abdelrahman, A. I.;Kobayashi, Y.; Winnik, M. A.; Scholes, G. D. Adv. Mater.2016, 28, 566. doi: 10.1002/adma.201503461.
(19) Di, D.; Musselman, K. P.; Li, G.; Sadhanala, A.; Ievskaya, Y.;Song, Q.; Tan, Z. K.; Lai, M. L.; MacManus-Driscoll, J. L.;Greenham, N. C. J. Phys. Chem. Lett. 2015, 6 (3), 446.doi: 10.1021/jz502615e
(20) Huang, H.; Susha, A. S.; Kershaw, S. V.; Hung, T. F.; Rogach,A. L. Adv. Sci. 2015, 2 (9), 1500194-1/5.doi: 10.1002/advs.201500194.
(21) Bhaumik, S.; Veldhuis, S. A.; Ng, Y. F.; Li, M.; Muduli, S. K.;Sum, T. C.; Damodaran, B.; Mhaisalkar, S.; Mathews, N.Chem. Commun. 2016, 52, 7118. doi: 10.1039/C6CC01056C.
(22) Gonzalez-Carrero, S.; Galian, R. E.; Pé rez-Prieto, J. J. Mater.Chem. A 2015, 3, 9187. doi: 10.1039/c4ta05878j
(23) Schmidt, L. C.; Pertegs, A.; Gonzlez-Carrero, S.;Malinkiewicz, O.; Agouram, S.; Mnguez Espallargas, G.;Bolink, H. J.; Galian, R. E.; Prez-Prieto, J. J. Am. Chem. Soc.2014, 136, 850. doi: 10.1021/ja4109209
(24) Zhang, F.; Zhong, H.; Chen, C.; Wu, X. G.; Hu, X.; Huang,H.; Han, J.; Zou, B.; Dong, Y. ACS Nano 2015, 9 (4), 4533.doi: 10.1021/acsnano.5b01154
(25) Naphade, R.; Nagane, S.; Shanker, G. S.; Fernandes, R.;Kothari, D.; Zhou, Y.; Padture, N. P.; Ogale, S. ACS Appl.Mater. Inter. 2016, 8, 854. doi: 10.1021/acsami.5b10208.
(26) Sadhanala, A.; Ahmad, S.; Zhao, B.; Giesbrecht, N.; Pearce, P.M.; Deschler, F.; Hoye, R. L. Z.; Gö del, K. C.; Bein, T.;Docampo, P.; Dutton, S. E.; De Volder, M. F. L.; Friend, R. H.Nano Lett. 2015, 15, 6095. doi:10.1021/acs.nanolett.5b02369.
(27) Pathak, S.; Sakai, N.; Wisnivesky Rocca Rivarola, F.; Stranks,S. D.; Liu, J. W.; Eperon, G. E.; Ducati, C.; Wojciechowski,K.; Griffiths, J. T.; Haghighirad, A. A.; Pellaroque, A.; Friend,R. H.; Snaith H. J. Chem. Mater. 2015, 27, 8066.doi: 10.1021/acs.chemmater.5b03769
(28) Zhang, W.; Saliba, M.; Moore, D. T.; Pathak, S. K.; Hö rantner,M. T.; Stergiopoulos, T.; Stranks, S. D.; Eperon, G. E.;Alexander-Webber, J. A.; Abate, A.; Sadhanala, A.; Yao, S.;Chen, Y.; Friend, R. H.; Estroff, L. A.; Wiesner, U.; Snaith, H.J. Nat. Commun.2015, 6, 6142. doi: 10.1038/ncomms7142
(29) Zhuo, S.; Zhang, J.; Shi, Y.; Huang, Y.; Zhang, B. Angew.Chem. Inter. Edit. 2015, 54, 5693.doi: 10.1002/anie.201411956
(30) Comin, R.; Walters, G.; Thibau, E. S.; Voznyy, O.; Lu, Z. H.;Sargent, E. H. J. Mater. Chem. C 2015, 3, 8839.doi: 10.1039/C5TC01718A
(31) Zhao, Y.; Riemersma, C.; Pietra, F.; Koole, R.; de Mello Donegá, C.; Meijerink, A. ACS Nano 2012, 6, 9058.doi: 10.1126/science.1243167
Synthesis of Colloidal Perovskite CH3NH3PbBr3−xClxNanocrystals with Lead Acetate
WANG Ya-Nan1,2MA Pin1,2PENG Lu-Mei1ZHANG Di1,2FANG Yan-Yan1ZHOU Xiao-Wen1LIN Yuan1,2,*
(1Beijing National Laboratory for Molecular Sciences, Key Laboratory of Photochemistry, CAS Research/Education Center for Excellence in Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China;2University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
Lead acetate, which is highly soluble in dimethylformamide, was used to synthesize mixed halide perovskite CH3NH3PbBr3-xClx(MA = CH3NH3, 0 ≤ x ≤ 3) nanocrystals (NCs). This method provides an approach to address the low solubility of lead halides, especially lead chloride. Different Br/Cl ratios in MAPbBr3-xClxlead to various optical properties. The photoluminescence emission peak can be tuned from 399 to 527 nm. Their full-widths at half-maxima (FWHM) are about 20 nm. MAPbBr3-xClxNCs have an average diameter of ~(11 ± 3) nm and have uniform dispersion in toluene. The MAPbBr3NCs have a long average recombination lifetime (τave= 97.4 ns) and a photoluminescence quantum yield (PLQY) of up to 73%.
Perovskite; CH3NH3PbBr3-xClx; Nanocrystal; Lead acetate
April 3, 2017; Revised: May 2, 2017; Published online: May 11, 2017.
O646
10.3866/PKU.WHXB201705115 www.whxb.pku.edu.cn
*Corresponding author. Email: linyuan@iccas.ac.cn; Tel: +86-10-82615031; Fax: +86-10-82617315.
The project was supported by the National Natural Science Foundation of China (51303186, 51673204) and National Materials Genome Project, China(2016YFB0700600).
国家自然科学基金(51303186, 51673204)和国家重点研发计划材料基因工程关键技术与支撑平台(2016YFB0700600)资助
© Editorial office of Acta Physico-Chimica Sinica
——李振声

