埃及产百里香和牛至精油、提取物的抗氧化和抗菌活性研究
Adel F.AHMEDGhada Abd-Elmonsef MAHMOUD马常阳康文艺*
1.河南大学 国家食用菌加工技术研发专业中心,河南 开封475004,中国;2.埃及农业研究中心园艺研究所药用植物和芳香植物研究系,吉萨12619,埃及;3.艾斯尤特大学科学院植物与微生物系,艾斯尤特71515,埃及;4.河南省药食两用资源功能研究国际联合实验室,河南 开封475004,中国
0 Introduction
Antioxidants are considered as radical scavengers,inhibiting lipid peroxidation and other free radical-mediated processes and can protect the human body as well as processed foods from oxidative damage attributed by the reaction of free radicals[1].The use of synthetic antioxidants in foods is discouraged due to their perceived carcinogenic possibility and safety concerns[2].Recently,there is a growing interest in assessing the antioxidant and antimicrobial properties of substances from safe natural sources that can potentially be used by the food and pharmaceutical industries.Herbs and spices are almost the most important sources of natural antioxidants and antimicrobials from the point of view of safety.
Aspergillus sp.,Fusarium sp.,Penicillium sp.,Bacillus sp.,Escherichia coli,Klebsiella sp.,Micrococcus sp.andSerratia sp.are common human and plant pathogens responsible for diseases ranging from mild to highly s evere with high spread pace in the environment,foodstuff and uncooked foods[3-4].To control these pathogens,antimicrobial agents can be considered as lifesaving compounds via killing the microbial pathogens or inhibiting their growth.The use of safe antimicrobial agents that are friendly to the environment is the purpose of many researches in food products and also a challenge in this filed.
There are many e xtracts and essential oils of aromatic plants with the abilities to suppress or kill the pathogenic microorganisms and they could be used as alternative antimicrobial agents[5-6].The advantages of using the plant extracts and/or their essential oils in human therapy and that they do not stimulate the antibiotic resistance by long-term use as is the case for synthetic antibiotics[7],although they have key constituents that work in a similar manner as commercial antibiotics[8].In the food industry,the addition of natural products against spoilage and pathogenic microorganisms is more acceptable than chemical agents by most consumers[9].
Although there are many advantages in therapeutics or food preservatives,the application of these products is still in a low level and their effects on more types of microorganisms need to be determined before their widespread use.The antimicrobial mechanisms of these natural products include attacking cell membrane through the phospholipid bilayer,disrupting enzyme systems,changing the genetic nature,and oxidizing unsaturated fatty acids[10-11].
Thyme(Thymus vulgarisL.)and oregano(OriganumvulgareL.)are aromatic herbs belonging to the family Lamiaceae.T.vulgarisis native to the southern Europe and the Mediterranean region.In folk medicine,it is used to treat alopecia,dental plaque,dermatophyte infections,bronchitis,cough,inflammatory skin disorders,and gastrointestinal distress[12].O.vulgareis commonly cultivated throughout Asia,Europe,and northern Africa.Traditionally,it is used to treat respiratory disorders,dyspepsia,painful menstruation,rheumatoid arthritis,scrofulosis and urinary tract disorders.Besides,it is also used as a culinary herb in gastronomy[13].
Many studies have focused mainly on the chemical composition of thyme and oregano essential oils from different regions of the world.The composition of essential oils varies widely according to the geographical area and growth conditions.Also,the essential oils and different extracts have been reported as antioxidant[14-17]as well as antimicrobial agents[11,3].However,few reports on chemical composition,antioxidant and antimicrobial properties of thyme and oregano essential oils cultivated in Egypt have appeared.There is also no information on the antioxidant and antimicrobial (human and plant pathogens)properties of ethanol extracts of these herbs.In addition,there is an urgent need to search for suitable alternative antimicrobial agents,especially of the natural origin,which are highly recommended for their healthy and eco-friendly properties.Therefore,this study aimed to identify the chemical composition of essential oils of thyme and oregano from Egypt and to evaluate the total phenolic content,antioxidant activity of the essential oils and ethanolic extracts as well as the effect of these oils and extracts on human and plant pathogenic microorganisms.
1 Materials and Methods
1.1 Plant Materials
Thyme (Thymus vulgarisL.)and Oregano(Origanum vulgareL.)were collected from Minia governorate,Egypt.The samples were collected during summer 2018 and identified by Dr.Atef A.Kader at the Department of Medicinal and Aromatic Plants Researches,Horticulture Research Institute,Agricultural Research Center.The current study was conducted during 2018 and 2019 years at National R&D Center for Edible Fungus Processing Technology,Henan University,Kaifeng,Henan,China.Antimicrobial activity experiments were carried out at the Department of Botany and Microbiology,Faculty of Science,Assiut University,Assiut,Egypt.
1.2 Extraction of essential oil
The essential oils(EOs)of thyme and oregano were obtained from dried (leaves and stems)samples(100 g)by hydro-distillation for 3 h with 1 000 m L distilled water in a Clevenger-type apparatus.The obtained EOs were dried over anhydrous sodium sulphate and stored at 4℃for further use.The yields of the EOs were 1.5%for thyme and 2.4%for oregano.
1.3 Gas chromatography/mass spectrometry analysis of oils
GC-MS analysis was carried out by an Agilent 6890N gas chromatograph equipped with a capillary column DB-5MS(30 m×250μm×0.25 μm,Agilent Technologies(Palo Alto,CA,USA)and coupled with a 5975 B mass selective detector spectrometer from the same company.The front inlet was kept at 250℃in split mode.The temperature program was as follows:the initial column temperature was 60℃and kept there for 2 min,and then programmed to 120℃at a rate of 6℃/min and held for 2 min;finally it was ramped to 230℃at a rate of 4℃/min and held there for 5 min.Flow rate of split injection was 1.0 m L/min.Helium was used as the carrier gas at 1.0 m L/min.The MS detector was used in the EI mode with an ionization voltage of 80 eV.The ion source temperature was at 230℃.The transfer line was at 280℃.The spectra were collected over the mass range(m/z)30~1 000.Retention indices were calculated by the retention times of C6-C26nalkanes that were injected at the same chromatographic conditions.The volatile constituents were identified by comparison of their relative retention indices and their mass spectra with Nist08.L library of essential oil constituents.
1.4 Preparation of plants extracts(PEs)
Dried samples (leaves and stems)were crushed and extracted by using 70%ethanol at 50℃three times for 9 h in total.The extracts were filtered using a Buchner funnel and concentrated under reduced pressure at 40℃by using a rotary evaporator.The extracts were then kept at-40℃overnight,dried in a freeze dryer and subsequently collected and stored at-4℃until further analyses.
1.5 DPPH radical scavenging assay
The method of Kang et al.[18]was used to assay the DPPH radical scavenging activity.The stable free radical DPPH was dissolved in methanol to give a 200μM solution;10μL of the essential oil and extract samples in methanol(or methanol itself as blank control)was added to 175μL of the methanolic DPPH solution.For each test compound,different concentrations were used(20,10,5,2.5 and 1.25 mg/m L for extracts and 100,50,25,12.5 and 6.25 mg/m L for oils).After further mixing,the decrease of absorbance at 515 nm was measured after 20 min.The actual decrease in absorption induced by the test compound was calculated by subtracting from that of the control.The antioxidant activity of each test sample was expressed as an IC50value(the concentration in mg/m L that inhibits DPPH absorption by 50%)and was calculated from the concentration-effect linear regression curve.Butylated hydroxy toluene(BHT)was used as a positive control.The DPPH radical scavenging activity of each sample was calculated as given below.
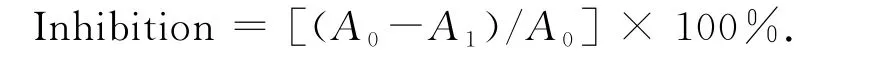
Where,A0is the absorbance of the DPPH itself,A1is the absorbance of sample or the positive control.
1.6 Determination of total phenolic contents(TPC)
The contents of total phenolic of thyme and oregano extracts and essential oils were determined spectrophotometrically according to the Folin-Ciocalteau method of Kang et al.[19]with slight modification.The diluted extract and oil(1.25 and 5 mg/m L methanol,respectively)were used in the analysis.A 0.2 m L aliquot of the diluted sample was mixed with 2.5 m L of 10%Folin-Ciocalteu's reagent in water.The mixture was covered and incubated for 2 min in the dark and then 2 m L 7.5%Na2CO3in water were added.The mixture was incubated for 1 h at room temperature.The absorbance was read at 765 nm against the blank.The blank had the same constituents except that the extract/oil sample was replaced by distilled water.Pyrocatechol was used as standard for preparing the calibration curve.The total phenolic content was expressed as mg pyrocatechol equivalents(PE)per g of extract.
1.7 Antimicrobial activity
1.7.1 Selected microorganisms
Antifungal characteristics of the plant extracts and essential oils were evaluated using human and plants pathogens.Human pathogenic yeasts and fungi isolated from air and food samples were used e.g.Aureobasidium pullulans Ap-34(de Bary)Arnaud,Aspergillus flavus Af-10 Link,Aspergillus niger An-14 van Tieghem,Candida albicans Ca-6 Robin(Berkhout),Cladosporium sphaerospermum Cs-78 Penzig andRhodotorula mucilaginosa Rm-32(Jorgensen)Harrison[20].Cellulitic hydrolyzing phytopathogenic fungi isolated from infected plants and contaminated soils were used e.g.A ochraceus Ao-15 Wilhelm,Fusarium oxysporum Fo-13(Schlechtendal),F verticilloides Fv-1(Saccardo)Nirenberg[21].Fincarnatum Fi-93(Desmazieres)Saccardo,Macrophomina phaseoli Mp-16(Tassi)Goidanich,and Trichothecium roseum (Persoon)Link Tr-18.Gram positive and negative pathogenic bacteria were used e.g.Bacillus cereus Bc-24,B subtilis Bs-62 andM luteus Ml-27,E coli Ec-25,K pneumonia Kp-76,Serratia marcescens Sm-26 andSerratiaplymuthica Sp-77[22].
1.7.2 Antimicrobial test
Fungal inoculums were prepared by growing the isolates on potato dextrose agar medium at(28±1)℃for 5 days,then homogenous spore suspensions were prepared by scraping the fungal growth and mixed it with sterilized saline water containing Triton×100(0.1%v∶v)as dispersal agent[4,23].For yeasts and bacteria,the isolates were cultivated in Czapek's dextrose broth medium and nutrient broth medium for 48 and incubated at(28±1)℃with shaking(150 r/min)24 h[24-25].The cells were harvested by centrifugation(4 000g for 10 min),washed twice with sterilized double distilled water,then re-suspended in sterilized saline water and used in 1×106CFU/m L[26].Plant extracts and essential oils were prepared in three concentrations(50,100 and 200μg/m L)diluted with ethanol and sterilized with membrane filtration(0.22 mm pore in diameter).These extracts were loaded over sterilized filter paper discs(5 mm)to obtain the final concentration,and 1 m L of the pathogen inoculum was loaded into sterilized Petri dish followed by 25 m L of milted Czapek's dextrose agar medium (45±1)℃.This was slowly homogenized and left to solidify.After that,the treated discs were loaded on the plates against the negative control of ethanol (plates with essential oils were closed tightly using para-film)and incubated at(28±1)℃with three replicates for two(bacteria)and over one week (yeasts and fungi).The clear zones of antimicrobial activity were measured in mm[27].
1.8 Statistical analysis
Data were analyzed statistically using Statistix Version 8.1 software.Differences between means were determined using the least significant difference test atP<0.05.The data were presented as().
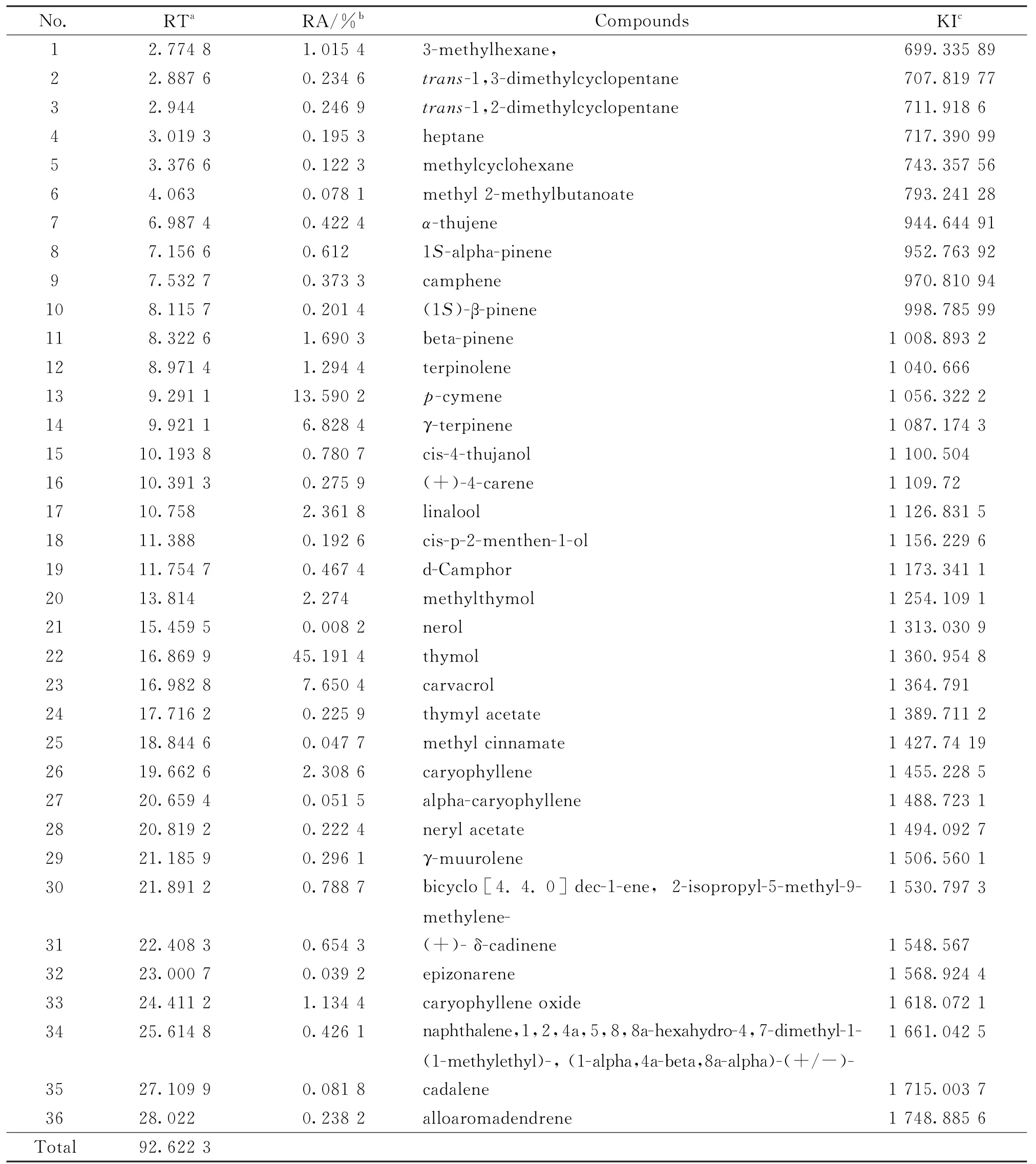
Table 1 Thyme essential oil composition(%),obtained by GC-MS
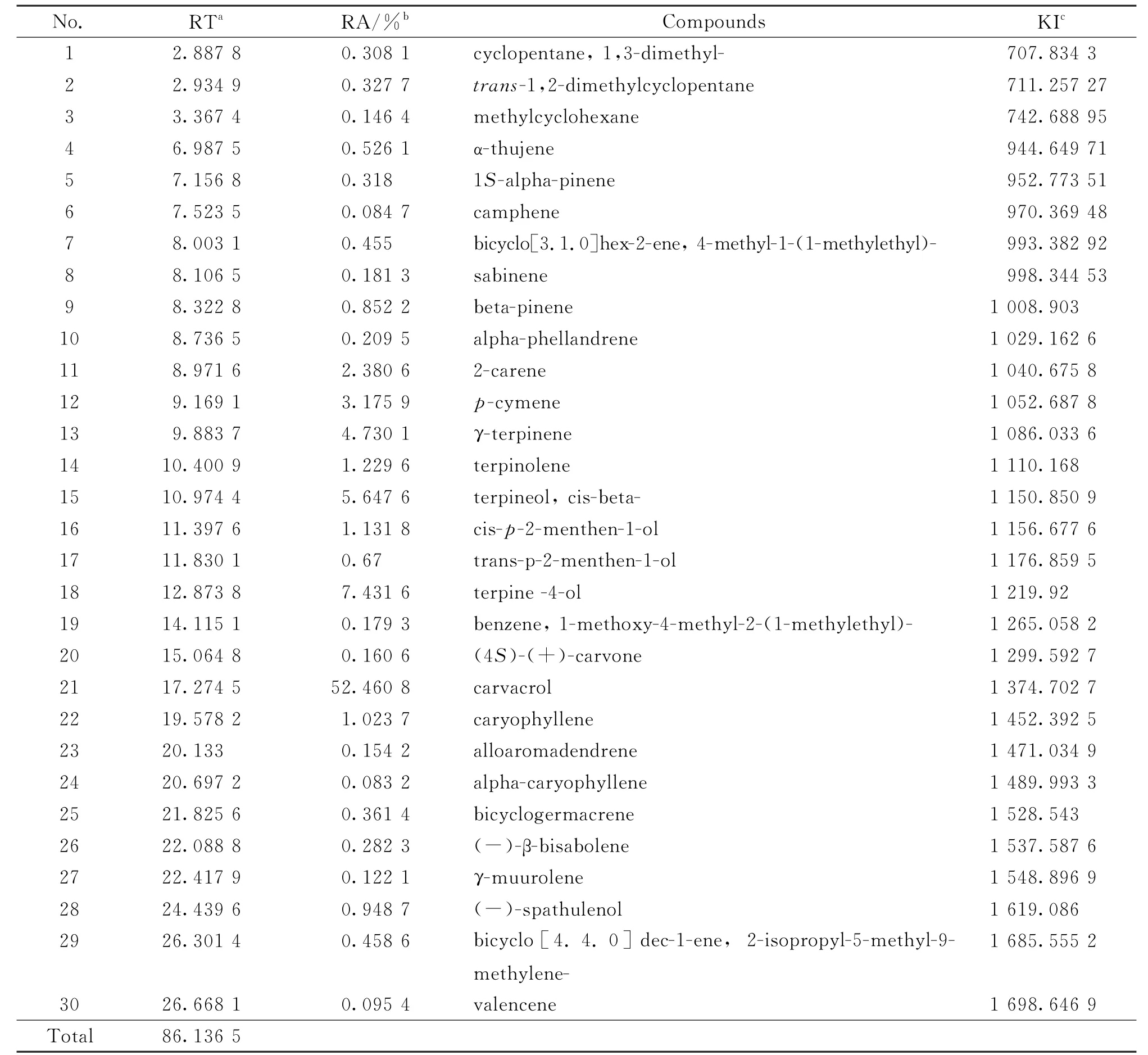
Table 2 Oregano essential oil composition(%),obtained by GC-MS
2 Results and Discussion
2.1 Chemical composition of Essential Oils
The volatile compounds detected by GC-MSanalysis in the thyme and oregano EOs with their percentage composition are shown in Tables 1 and 2,respectively.T.vulgarisEO identified 36 constituents representing 92.62%of the total oil.Thymol (45.19%),p-cymene (13.59%),carvacrol(7.65%)and γ-terpinene (6.82%)were the major constituents of the essential oil tested.A little difference existed between our results and the pervious results obtained by Viuda-Martos et al.[28]who found that thymol(48.64%)p-cymene(22.94%)andα-terpinolene (6.49%)were the main components inT.vulgarisEO from Egypt.In that research the plants were cultivated at organic plantation and the samples were collected during the flowering period.These variations in the composition of the essential oil could be due to factors such as plant age,plant part development stage,growing place,harvesting period and adaptive metabolism of plants.
Many studies have focused on the chemical composition of the essential oil extracted from thyme.For example,Agili[29]found that thymol(54.26%),γ-terpinene (9.50%),p-cymene(7.61%)and carvacrol (4.42%)were the major constituents of essential oil extracted fromT.vulgariscollected from Saudi Arabian markets.Jamali et al.[30]reported the major constituents identified in the essential oil from Moroccan endemic thyme (T.maroccanusBall)as carvacrol(14.1%~77.6%),p-cymene (3.5%~7.9%),γterpinene(3.8%~6.6%)andα-pinene(1.2%~7.8%).Semeniuc et al.[15]reported that the major components identified in the essential oil extracted from dried leaves of thyme cultivated in Romania werep-cymene (49.8%),α-terpinene(18.6%),thymol(8.7%)andβ-myrcene(5.4%).However,Boruga et al.[31]identified thymol(47.59%),γ-terpinene(30.90%),and p-cymene (8.41%)as the major components in the essential oil of Romanian thyme.Soto-Mendívil et al.[32]reported that the main constituents identified in essential oil extracted fromT.vulgariscollected from Mexican markets were borneol (28.4%),thymol(16.6%),carvacrol methyl ether(9.6%),camphene(6.9%),α-humulene (6.4%)and carvacrol(5.0%).
In the essential oil ofO.vulgare,30 compounds were identified representing 86.13%of the total oil.The main constituents being carvacrol(52.46%),terpine-4-ol (7.43%),terpineol,cisbeta(5.64%)andγ-terpinene(4.73%).
The essential oil composition ofO genushas been extensively studied and show a wide variation between its different species.The variation in composition may even be present within the same species collected during different seasons or from different geographical areas.
Gong et al.[33]identified the major compounds in ChineseO.vulgareEO and reported presence of thymol (42.9%)andp-cymen-2-ol(37.5%),whileO.vulgareEO collected in Pakistan contained mainlyβ-citronellol(78.7%)and citronellol acetate (5.9%).Khan et al.[34]reported that the essential oil of Saudi ArabianO.vulgarecontained mainly carvacrol (70.2%)and γ-terpinene(5.6%).Hernández-Hernández et al.[35]found that thymol (66.3%)and γ-terpinene (9.6%)as the major constituents of the MexicanO.vulgareEO.Pradebon Brondani et al.[36]reported the BrazilianO.vulgareEO contained 4-terpineol(41.17%),thymol (21.95%),γ-terpinene(5.91%)and carvacrol(4.71%)as the major constituents.On the other hand,Fikry et al.[37]reported the major constituents of EO extracted from dried aerial parts (leaves,stems and flowers)ofO.vulgarecultivated in Egypt as terpinen-4-ol(38.35%),trans-sabinene hydrate (10.06%),αterpineol(7.32%),α-terpinene (4.51%),cissabinene hydrate (4.27%)and 4-terpinyl acetate(4.13%).
El Gendy et al.[38]reported the main compound in the EO of cultivatedO.syriacumplants from Egypt as carvacrol (81.38%),p-cymene (8.48%),γ-terpinene(1.98%),β-myrcene (1.32%),α-terpinene(1.24%),4-terpineol (0.89%),α-pinene(0.72%)and thymol (0.35%).However,Viuda-Martos et al.[14]found that the main components of EO isolated fromO.syriacumcultivated in Egypt were thymol(21.04%)andγ-terpinene(18.96%).
This difference in the EO composition may be attributed to the existing differences in growth conditions,origin of plant collection,stage of plant maturity,physiological modifications in response to various environmental factors and stresses,harvesting time,drying methods,method of essential oil isolation or even the solvents used for the GC/MS analysis[39-40].
2.2 Total Phenolic Content and Antioxidant Activity of Essential Oils(EOs)
Table 3 shows the total phenolic content(TPC)and DPPH radical scavenging activity of essential oils.There was a significant difference in TPC of essential oils.The highest TPC was found in thyme EO (228.78±1.24)mg/g followed by EO of oregano (129.43±0.64)mg/g.Both essential oils showed activity in DPPH radical scavenging but lower than that observed for BHT (IC50=6.80 mg/m L).The highest radical scavenging activity was recorded for thyme EO with IC50value of 68.95 mg/m L followed by EO of oregano with IC50of 96.47 mg/m L.Similar results obtained by Viuda-Martos et al.[14]who observed that EO ofT.vulgariscultivated in Egypt showed greater radical scavenging activity(IC50=4.50 g/L)than that of EO ofO.syriacum(IC50=6.66 g/L).This increment in antioxidant activity of thyme EO could be attributed to the phenolic content.Our finding is in agreement with Viuda-Martos et al.[28]who reported that EO of Egyptian thyme which has the highest content of total phenols(913.17 mg/L)showed high scavenging ability of DPPH radicals (IC50=7.57 g/L).In addition,Semeniuc et al.[15]reported the highest antioxidant capacity (197.0μM/1 m L)was noticed in essential oil extracted from Romanian thyme and this increment in antioxidant capacity was correlated with the contents of phenols(22.5 mg/100μL EO)which were recorded in the same tested EO.
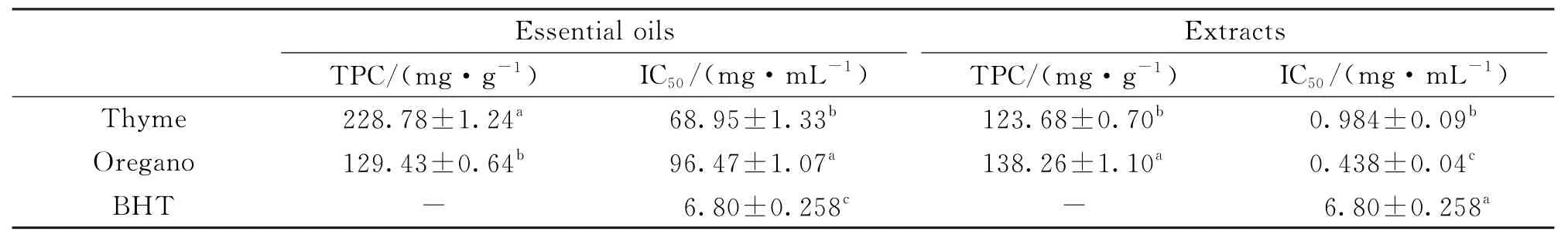
Table 3 Antioxidant assays of Thyme and Oregano essential oils and extracts
Furthermore,the highest antioxidant activity of thyme EO probably attributed to the high content of oxygenated monoterpene,thymol,which was present at a high concentration (45.19%)in EO of thyme but not in EO of,oregano.Thyme EO was able to reduce the stable DPPH radical[41]for which the compound most responsible for this activity was the oxygenated monoterpene,thymol as well as the mixture of mono-and sesquiterpene hydrocarbons.Thymol with greater steric hindrance of the phenolic group in comparison with carvacrol had a higher antioxidant activity[14].
2.3 Total Phenolic Content and Antioxidant Activitiy of plants extracts(PEs)
The total phenolic content(TPC)and DPPH radical scavenging activity of ethanol extracts of thyme and oregano are presented in Table 3.In contrast to essential oils the highest content of phenolics (138.26 mg/g)was in oregano extract followed by thyme extract (123.68 mg/g)with statistically significant difference between them.
The ethanolic extracts exhibited very strong radical scavenging activity was higher than that of BHT.In general,the free radical scavenging activity of ethanolic extracts was superior to that of essential oils.Our results concluded that there was a significant difference in free radical scavenging activities of extracts with oregano extract having a higher radical scavenging activity with IC50value(0.438 mg/m L)than that of thyme extract(IC50=0.984 mg/m L).The greater antioxidant activity of oregano extracts could be attributed to their higher content of phenolics.These results indicate that phenolic compounds made a major contribution to the antioxidant capacity of herbal extracts tested.The antioxidant activity of phenolic is mainly due to their redox properties,which allows them to act as reducing agents,hydrogen donors,and singlet oxygen quenchers.They may also have metal chelating potential,if having the required structural features[42].
The results of our work reported here agree with those of Khouya et al.[16]who reported total polyphenol contents in the aqueous extracts of three Moroccan thyme varieties were between 475.00 and 495.47 mg caffeic acid eq/g of extract.These aqueous extracts displayed potent activity against DPPH radical with IC50between 0.12 and 0.44mg/m L of extract.Teixeira et al[17]reported that ethanolic extract ofO.vulgarefrom Portugal had a high content of phenolic(13.5 mg gallic acid/1 g sample)with strong antioxidant activity(EC50=64.1μg/m L).Likewise,our previous studies reported a linear relationship between total phenolic content and antioxidant activity of plant extracts[43-45].
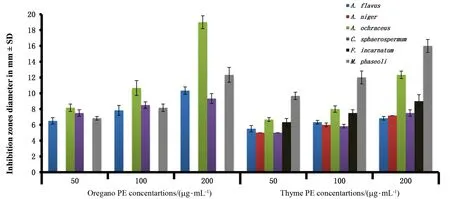
Figure 1 Antimicrobial activity of thyme and oregano extracts(PEs)and essential oils(EOs)against human pathogenic yeasts Aureobasidium pullulans (Ap-34)and Rhodotorulamucilaginosa (Rm-32)
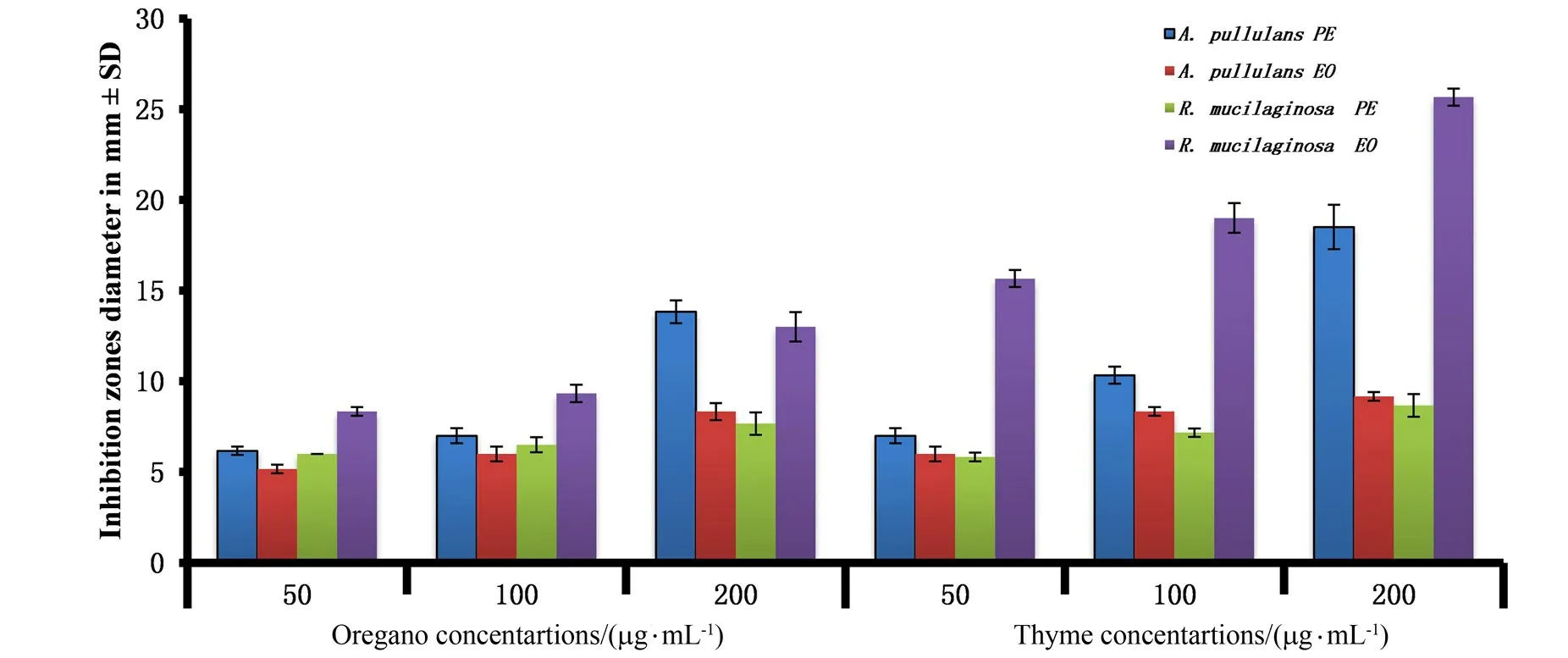
Figure 2 Antifungal activity of thyme and oregano extracts(PEs)against human and plant pathogenic fungi
2.4 Antimicrobial activity
Figure 1 shows that the oregano and thyme plant extracts possessed antimicrobial activity which increased in with concentration increase and reached its highest value at 200μg/m L PEs against three types of the pathogenic microorganisms(yeasts,fungi and bacteria).In pathogenic yeasts,oregano and thyme extracts displayed the highest antimicrobial activity inA.pullulans Ap-34 with(13.8±0.36)and (18.5±0.71)mm (diameter zones of inhibition)followed byR.mucilaginosa Rm-32 with (7.7±0.36)mm (oregano extract)and(8.7±0.36)mm in thyme extract,howeverC.albicans Ca-6 showed resistance against both extracts.Oregano and thyme EOs showed the highest antimicrobial activity c ompared to PEs inR.mucilaginosa Rm-32 with (13±0.47)and(25.7±0.27)mm followed byA.pullulans Ap-34 with(8.3±0.27)and (9.2±0.14)mm,whileC.albicans Ca-6 did not display any response against both PEs and EOs.
Oregano and thyme extracts exhibited the highest antifungal activity (Figure 2)againstA.flavus Af-10 with(10.37±0.2)and(6.8±0.14)mm (diameter zones of inhibition),A.ochraceus Ao-15 with(9±0.47)and (12.3±0.27)mm,C.sphaerospermum Cs-78 with(9.3±0.36)and(7.5±0.25)mm andM.phaseoli Mp-16 with(12.34±0.5)and(16±0.47)mm at 200μg/m L Pes,respectively.Remarkably,only thyme extract showed antifungal properties against(7.1±0.14)mm andF.incarnatum Fi-93(9±0.47)mm.F.verticilloides Fv-1 did not display any response against both PEs and EOs.In agreement with these results,Davidson and Naidu[46]and Musyimi et al.[47]showed suppression of growth ofA.niger(ochratoxins producing)andA.parasiticus(cause mycotoxicosis)by alcoholic extracts of thyme.
Oregano EOs showed positive antifungal properties againstA.niger An-14(6.8±0.14)mm while its alcoholic extract had a negative effect.Thyme EOs inhibitedF.oxysporum(7.3±0.27)mm andF.incarnatum(7.8±0.14)mm growth while its alcoholic extracts had a negative effect,as shown in Figure 3.Also,both EOs inhibitedT.roseumwith(7.3±0.14)and (16.5±0.4)mm inhibition by oregano and thyme EOs,respectively,but their extracts exerted a negative effect.The antifungal activity of oregano and thyme EOs were(9.3±0.27)and (12±0.47)mm forA.ochraceus Ao-15 and (10±0.47)and (16±0.47)mm forM.phaseoli Mp-16,respectively.A.flavus Af-10 andsphaerospermum Cs-78 were sensitive to oregano EOs by (16.5±0.24)and (12.3±0.27)mm and with thyme EOs by (11±0.47)and(8.8±0.36)mm,respectively.High phenolic contents of EOs correlated with antimicrobial properties[48].Essential oils demonstrated fungicidal effects against post-harvest plant pathogens,especiallyA.sp.andFusarium sp.[49].The growth ofA.flavus(cause human Mycotoxicosis)was inhibited,using oregano and thyme[50].
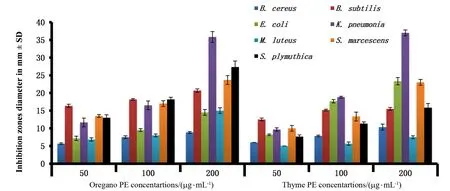
Figure 3 Antifungal activity of thyme and oregano essential oils(EOs)against human and plant pathogenic fungi
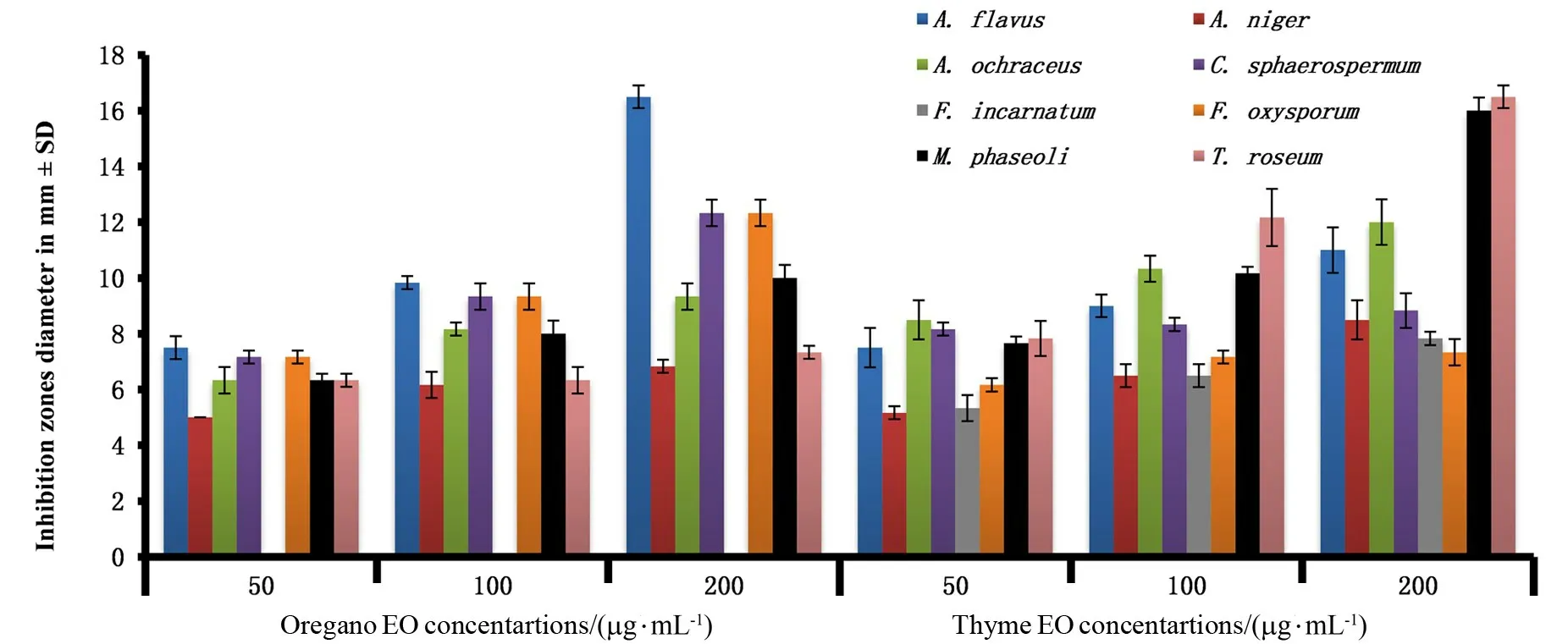
Figure 4 Antibacterial activity of thyme and oregano plant extracts(PEs)against human and plant pathogenic bacteria
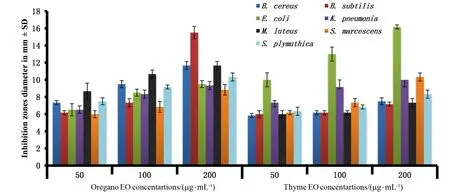
Figure 5 Antibacterial activity of thyme and oregano essential oils(EOs)against human and plant pathogenic bacteria
In Figures 4 and 5,oregano and thyme extracts showed highest antibacterial activity at 200 μg/m L PEs againstB.subtilis Bs-62((20.7±0.27)and (15.5±0.24)mm,respectively)followed byK.pneumonia Kp-76(35.8±0.89)and(37±0.47)mm.S.plymuthica Sp-77,S.marces-cens Sm-26,M.luteus Ml-27,E.coli Ec-25,B.cereus Bc-24 inhibited by oregano PEs with(27.3±0.98),(23.7±0.72),(15±0.47),(14.5±0.47),(8.8±0.14)mm and by thyme PEs with(15.8±0.68),(23±0.47),(7.5±0.24),(23.3±0.59),(10.3±0.49)mm,respectively.Oleszek[51]revealed that Gram-positive bacteria were sensitive to thyme extract at 0.3~1.25 mg/m L,while Gram-negative bacteria were sensitive in range of 1.25~5 mg/m L.Oregano alcoholic extract(40 mg/m L)inhibited the growth ofEscherichia coliandS.aureus[52-53].
The highest antibacterial activity of oregano EOs was observed inB.subtilis Bs-62 with(15.5±0.41)mm,while in thyme EOsE.coli Ec-25 showed the highest sensitivity by(16.2±0.14)mm inhibition zone.B.cereus Bc-24,M.luteus Ml-27,S.plymuthica Sp-77,K.pneumonia Kp-76,andS.marcescens Sm-26 inhibited by oregano extract with (11.7±0.27),(11.7±0.2),(10.3±0.27),(9.3±0.27),(8.8±0.3)mm and by thyme extract with(7.5±0.24),(7.3±0.27),(8.3±0.27),(10±0.47),(10.3±0.27)mm,respectively.Al-Bayati[54]and Burt and Reinders[55]reported that thyme EOs at 625 mg/m L inhibitedE.coligrowth,and at 500 mg/m L inhibitedK.pneumoniagrowth.Moreover,the antibacterial effective concentration of oregano EOs againstK.oxytocaranged from 0.90 to 2.11 mg/m L and 207.4~240 mg/m L inK.pneumoniae[3]and 100~250 mg/m L inE.coli[56].
3 Conclusions
This work clearly demonstrated that there were variations in chemical composition of essential oils of thyme (Thymus vulgarisL.)and oregano(Origanum vulgare L.)from Egypt.The essential oils of thyme and oregano contained appreciable levels of phenolic compounds and showed good DPPH radical scavenging capacity.The extracts had a lower amount of phenolics than that of essential oils but exhibited very strong DPPH radical scavenging capacity.Thyme and oregano extracts and essential oils represent good natural sources of antioxidant.In addition,they offer strong antimicrobial properties against human and plant pathogenic microorganisms and hence could be used as safe alternative antimicrobial agents.

