嘧菌酯施药措施与最终残留量间的相关性分析及其膳食摄入风险评估
吴绪金 李通 马婧玮 汪红 张军锋 周玲 马欢
摘要:【目的】對嘧菌酯在花生植株、花生、花生壳及土壤中的最终残留及其消解动态进行分析,评价嘧菌酯在花生生产上的残留安全性。【方法】对不同施药次数、施药剂量及采收间隔期与花生植株、花生、花生壳及土壤中嘧菌酯最终残留量间的相关性进行分析,同时对嘧菌酯进行了膳食摄入风险评估。【结果】嘧菌酯在花生植株和土壤中的消解半衰期分别为7.24~12.07 d和5.57~13.48 d。嘧菌酯在花生植株、花生、花生壳和土壤中的最终残留量分别低于1.135、0.154、0.922和0.957 mg/kg,嘧菌酯残留量排序为花生<花生壳<土壤<花生植株。根据最终残留量试验结果,嘧菌酯在花生中的残留中值为0.05 mg/kg,普通人群嘧菌酯的国家估算每日摄入量为0.418785 mg/kg,占日允许摄入量的3.32%左右。采收间隔期为21和28 d时,在不同施药次数、施药剂量和采收间隔期条件下, 嘧菌酯在花生植株、花生、花生壳和土壤中的残留量差异均不显著(P>0.05)。【结论】按常规方式施用嘧菌酯通常不会对一般人群健康产生不可接受的风险,但采收间隔期为14 d时,施药剂量和施药次数对最终残留量有一定影响。
关键词:嘧菌酯;花生;残留;消解动态;风险评估
0 Introduction
【Research significance】Peanut is one of the most important leguminous plants in China which is mainly used for the production of nut fruit, peanut oil, peanut butter, dessert, etc. Peanuts are high in protein, unsaturated fatty acids, minerals, vitamins and anti-fibrinolysis enzymes(Zhang et al., 2012). The byproduct, peanut shell, contains rich nutrients and can be used as livestock and poultry roughages. As peanut production around the world is being affected by plant diseases, fungicide applications are currently necessary for disease control(Bagi et al., 2014). Azoxystrobin, methyl(E)-2-{2-[6-(2-cyanophenoxy) pyrimidin-4-yloxy] phenyl}-3-methoxyacry-
late(Aguilera et al., 2002;Flores et al., 2007), is a systemic fungicide which inhibits cell respiration by binding to the protein subunits in mitochondrial cytochrome bc1 complex. Broad-spectrum fungicide belongs to the class of methoxyacrylates, which is derived from the naturally-occurring strobilurins. It is absorbed through roots and translocated in xylem to the stems and leaves, or through leaf surfaces to leaf tips and growing edges. Azoxystrobin controled foliar and soil-borne diseases including downy and powdery mildew, early and late blight, and pathogens Sclerotinia, Alternaria, Ascochyta, Pythium, and Rhizoctonia on many crops(Jordan et al., 1999; Fisher and Meunier, 2008), and was also used to protect fungi from peanuts during growth. 【Research progresses】To the best of our knowledge, the residue analysis of azoxystrobin has been reported in various matrix such as maize(Liu et al., 2015), soybean(Yin et al., 2011),cucumber(Yin et al.,2006;Hong et al.,2012), strawberry(Yang et al., 2013), orange(Han et al., 2009), banana(Huan et al.,2013;Wang et al.,2013), mango (Liu et al.,2010), lychee(Wang et al., 2014) and ginseng(Wang et al., 2010). Moreover, techniques for the quantitative analysis of azoxystrobin residues include gas liquid chromatography(Bo et al., 2005; Ding et al., 2006; Li et al., 2008; Wu et al., 2012), high-performance liquid chromatography(Abreu et al.,2006; Shi et al., 2010; Abdelraheem et al., 2015), gas chromatography-mass spectrometry(Bo, 2007), high-performance liquid chromatography-tandem mass spectrometry(Wu et al., 2009) and direct competitive enzyme-linked immunosorbent assay(dc-ELISA)(Wa-
tanabe and Migake, 2013). 【Research breakthrough point】Currently, no research regarding the azoxystrobin dissipation and residues in peanut was available, neither were researches on the relation between application methods and residues of azoxystrobin in peanut and soil under field conditions, and risk assessment for dietary residue intake. Analytical methods for determining azoxystrobin residues have been described in the literature; however, analytical methods for azoxystrobin residues in peanut plant, peanut, peanut shell and soil have not been published. 【Solving problems】The present study developed a method to determine azoxystrobin based on high performance liquid chromatograph with photodiode array detector(HPLC-PDA). Price of HPLC-PDA is cheap, therefore it is widely used in analytical labs. Based on the available literature, the researchers investigated the dissipation dynamics and behavior of terminal residues of azoxystrobin in peanut and soil under field conditions,and risk assessment for dietary intake based on supervised residue trial data. The effects of application frequency and rate and the interval to harvest on terminal residues were studied. The purpose of this study was to determine whether azoxystrobin usage is safe under the recommended application methods. These results will help the authorities to provide guidance concerning the proper and safe use of azoxystrobin, and provide a reference for MRL setting in peanut.
1 Materials and methods
1. 1 Experimental materials
Pesticide: azoxystrobin standard(99.0% purity) was purchased from Sigma-Aldrich Corporation, USA. Azoxystrobin 20% water dispersible granule(WG) was provided by Sipcam Agro China Co., Ltd, Shanghai, China.
Chemicals: acetonitrile, ethyl acetate and acetone of HPLC grade were procured from Fisher Chemi-
cals(USA). Deionized water was obtained from a Milli-Q water purification system(Millipore,USA). So-
dium chloride of analytical grade was purchased from Sinopharm Chemical Reagent(Beijing, China).
1. 2 Experimental design
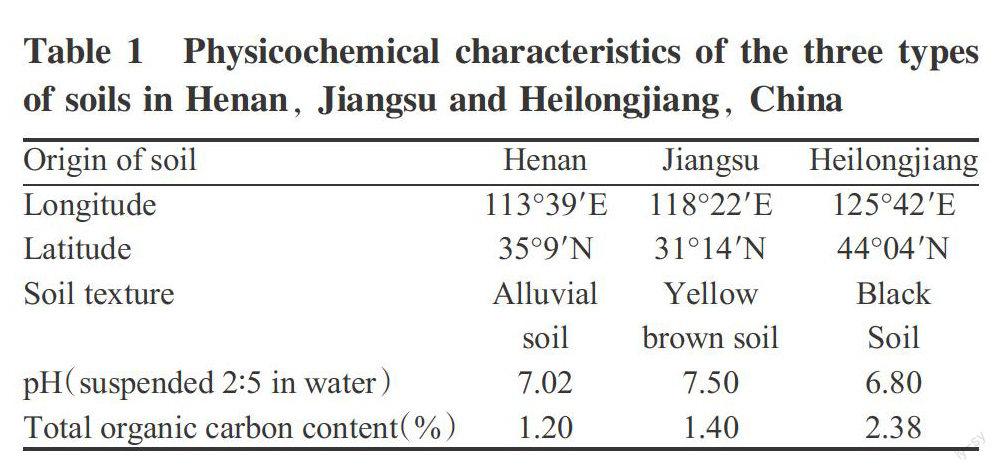
Field trials were conducted in a randomized block design in farmers fields, which were previously investigated to be free of the pesticide. The supervised field trials were carried out in Henan, Jiangsu and Heilongjiang, China, during 2012-2013. Three treatments were included in this study to(1)determine azoxystrobin dissipation,(2)analyze residues at high application rate and (3)analyze residues at low application rate. Each treatment had three replicated plots, and each plot was 30 m2. A 2-m-wide buffer area was used to separate different treatments in the field. None of the plots had been treated with azoxystrobin in the past. To ensure the reliability of the experimental results, field management was carried out in accordance with local methods. During the trial, the daily average temperatures in Henan, Jiangsu and Heilongjiang in the year of 2012 were 23.8, 24.8 and 17.4 ℃ respectively, and were 24.6, 25.6 and 17.2 ℃ respectively in the year of 2013. The rainfalls in Henan, Jiangsu and Heilongjiang in the year of 2012 were 303.25, 313.44 and 487.95 mm respectively, and were 125.45, 384.79 and 314.74 mm, respectively in 2013. Physico-
chemical characteristics of the three types of soils are presented in Table 1.
To investigate the dissipation of azoxystrobin in peanut plant and in soil, azoxystrobin(WG, 25%) was dissolved in water and sprayed onto the surfaces of peanut plant using a manual sprayer(PS16-7, 40.5 cm×17.0 cm×57.0 cm, 16 L volume, max. pressure 1.0 MPa) at an application rate of 480 g a.i./ha(2.0× the highest recommended application rate), a plot of the same size but with no azoxystrobin application was compared simultaneously. For the soil treatments, soil with no plants was sprayed at an application rate of 1000 g a.i./ha(4×the highest recommended application rate). At 2 h and 1, 3, 5, 7, 10, 14, 21, 28, 35 and 45 days after spraying, approximately 200 g of peanut plants was randomly collected from several sites in each plot, and an approximately 1000 g soil sample was sampled randomly(at a depth of 0-10 cm) in each plot using a soil sampling apparatus(soil-sampling drill, i.d. 25 mm×height 20 cm) at 20 different sites. Control samples were obtained from the control plots.
To investigate the field residues, relation between pesticide application methods and the terminal residues of azoxystrobin in peanut plant, peanut,peanut shell and soil, two application rates were used: a low a-
pplication rate at 240 g a.i./ha(1.0×the highest recommended application rate) and a high application rate at 360 g a.i./ha(1.5×the highest recommended application rate). Both low and high application rates were used in three or four applications(7-day interval between treatments). The peanut plant, peanut, peanut shell and soil were sampled 14, 21 and 28 days after the last spraying, and the final residues were analyzed. For the control treatment, samples without pesticide were collected as an experimental treatment.
Immediately after picking, the samples were put into polyethylene bags, sealed and transported to the laboratory. The peanut samples were crushed and homogenized after being shed off the shells. The shell samples were mixed in a blender for homogenization after dried in shade. The soil samples were sifted through a 40-mesh sieve, and 100 g of the soil was concentrated using cone-and-quartering method. The peanut plants were cut into small pieces(approximately 0.5 cm×0.5 cm) prior to analysis. All samples were packed in polyethylene bags, labeled, stored at -20 ℃ until analysis.
1. 3 Analytical methods
20.00 g(5.00 g of the shell sample) previously homogenized samples were weighed into a 100 mL centrifuge tube, and 20 mL water and 50 mL acetonitrile were added, then the samples were mixed ultrasonically for 20 min. The upper layer was transferred to a measuring cylinder with 6 g NaCl after centrifuged, then was shaken for 1 min and placed to separate, 25 mL upper layer was transferred to a 100 mL beaker and heat to nearly dried at 45 ℃, then 5 mL ethyl acetate was added to elute the sample.
The neutral alumina column was successively prewashed with 5 mL of acetone: ethyl acetate(v/v=20/80) and 5 mL ethyl acetate to remove any impurities. Concentrated extracts were poured on top of the column, washed with 5 mL of acetone: ethyl acetate (v/v=20/80), and the procedure was repeated twice. The eluate was concentrated to dryness on a hot plate at 45 ℃ and re-dissolved with 2 mL acetonitrile for HPLC analysis.
HPLC separation was carried out using a Shimadzo LC equipped with a quaternary pump, an autosampler, a column oven and a PDA. Analytical separation of azoxystrobin was performed on a Agela Venusil XBP C18 reversed-phase column(150 mm×4.6 mm,5 μm), the column oven temperature was kept at 35 ℃, and the sample injection volume was 20 uL. The mobile phase was acetonitrile/water(60∶40) with the flow rate of 1.0 mL/min. Concentrations of azoxystrobin were determined by external calibration using peak area measurements at 204 nm. The retention time of azoxystrobin was 6.5 min.
1. 4 Statistical analysis
The dissipation of azoxystrobin was described using a first-order kinetic equation, Ct=C0×e-kt. The dissipation half-life(t1/2) of azoxystrobin in each experiment was obtained using the function t1/2=ln2/k, where Ct was the concentration(mg/kg) at time t(days) after application, C0 was the initial concentration(mg/kg), and k was the first-order rate constant(days-1)(Li et al., 2008; Chen et al., 2011; Zhang et al., 2012).
International estimated daily intake(IEDI) values provided a “best estimate” of dietary intake because these values were set based on residues in edible portions at the level of the median residue values from supervised trials. The IEDI was defined according to the formula IEDI=[STMRi(or STMR-Pi)×F]/bw, where IEDI was the international estimated daily intake(mg/kg), STMRi represented the supervised trial median residue(mg/kg), STMR-Pi was the supervised trial median residue in a processed commodity(mg/kg), Fi was the consumption of the food agro-product i(kg/d), and bw was body weight(kg)(Wang et al., 2012). Long-term dietary intake and chronic risk assessment of azoxystrobin based on the acceptable daily intake was compared against the international estimated daily intake. The ADI% was defined according to the formula ADI%=(IEDI×100%)/ADI, where ADI was the acceptable daily intake(mg/kg), and ADI% was the percentage of ADI. When ADI%≤100%, then the chronic risk of azoxystrobin was acceptable; the smaller the ADI%, the lower the chronic risk. When ADI%>100%, then the chronic risk of azoxystrobin was unacceptable; the higher the ADI%,the greater the chronic risk.
The effects of azoxystrobin application rate and application frequency and the interval to harvest on the terminal residues in peanut plant, peanut, peanut shell and soil were examined. The terminal residues in peanut plant, peanut, peanut shell and soil from each plot in Henan, Jiangsu and Heilongjiang were analyzed using the Statistical Package for Social Sciences (SPSS 22.0) program.
2 Results and analysis
2. 1 Method validation
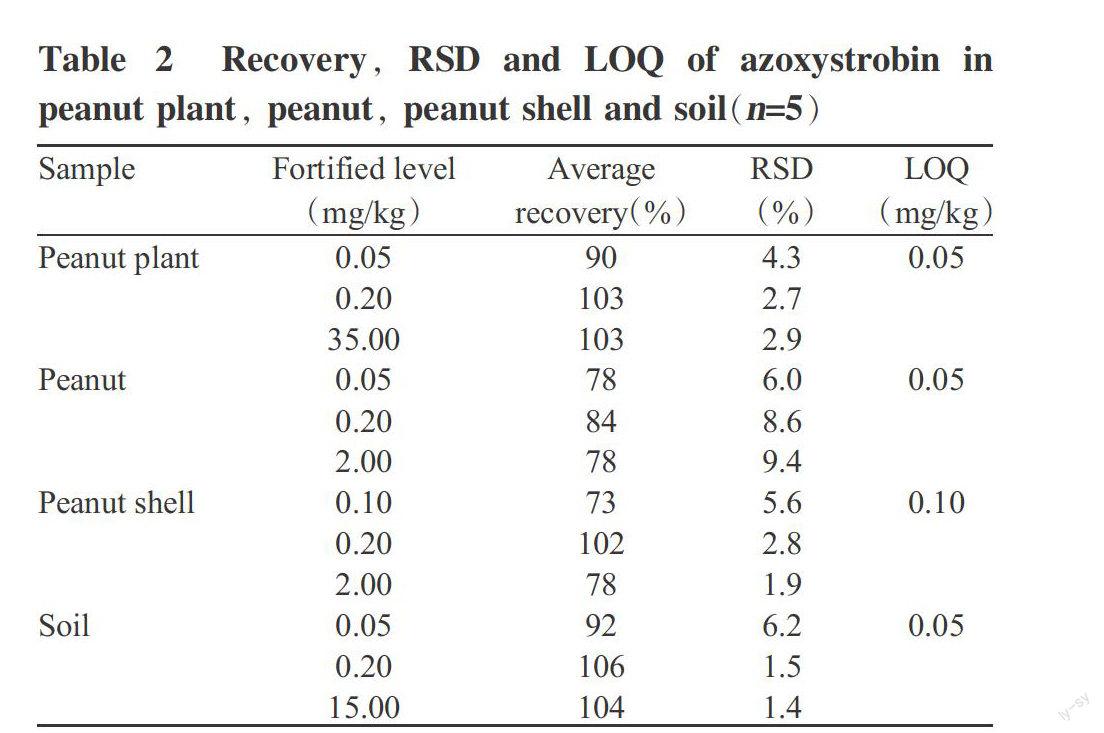
A standard calibration curve of azoxystrobin was constructed by plotting the analyzed concentrations against the peak areas. Good linearity(y=35018x+968.9) was achieved in the range of 0.1 to 5.0 mg/L, with a correlation coefficient of r2=0.9999. The recovery study was conducted for different matrixes at three different fortification levels(0.05,0.10,0.20,2.00,15.00 or 35.00 mg/kg); the average recoveries from fortified samples in five replicate experiments for each matrix were in the range of 73% to 106%, with a relative standard deviation(RSD) range of 1.4% to 9.4%(Table 2). The limits of quantification(LOQ) of peanut plant, peanut and soil were all 0.05 mg/kg, and that of peanut shell was 0.10 mg/kg. The LOQ and recovery obtained could meet Chinas national standards(Ministry of Agriculture of the Peoples Republic of China, 2004). This result suggested that azoxystrobin could be detected with good precision and had good recoveries using the extraction procedure adopted.
2. 2 Dissipation of azoxystrobin in peanut plant and soil
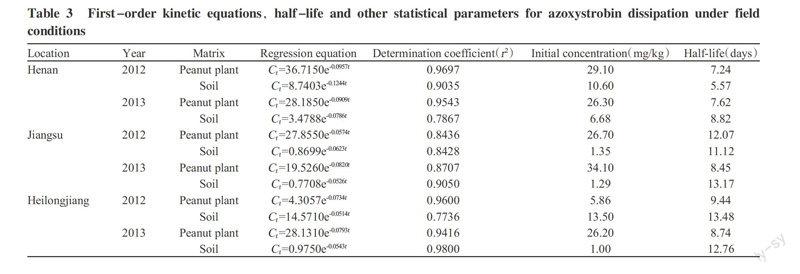
The half-lives and other statistical parameters of the azoxystrobin residue dissipation were calculated from the experimental data and were summarized in Table 3.
2. 3 Terminal residue of azoxystrobin in peanut plant, peanut, peanut shell and soil
The residues of azoxystrobin in peanut plant, peanut, peanut shell and soil under different application rates, different frequencies, and different intervals to harvest were presented in Table 4. The residue le-
vels in peanuts were <0.050-0.154 mg/kg 14 days after spraying,<0.050-0.101 mg/kg 21 days after spraying, and <0.050-0.057 mg/kg 28 days after spraying. The residue levels in peanut plants were 0.179-1.135 mg/kg 14 days after spraying, 0.055-0.200 mg/kg 21 days after spraying, and <0.05-0.194 mg/kg 28 days after spraying. The residue levels in peanut shells were <
0.100-0.922 mg/kg 14 days after spraying,<0.100-0.265 mg/kg 21 days after spraying, and <0.100-0.224 mg/kg 28 days after spraying. The residue levels in soil were 0.149-0.957 mg/kg 14 days after spraying,<0.050-0.523 mg/kg 21 days after spraying, and 0.050-0.338 mg/kg 28 days after spraying.
2. 4 Risk assessment for dietary intake based on supervised residue trial data
The supervised trial median residue(STMR) in peanut was 0.050 mg/kg according to Table 4, where the average body weight of a Chinese adult was 63 kg and the daily dietary intake of vegetable oil according to the nutrition and health status of the Chinese people by Ministry of Health, Peoples Republic of China was 0.0327(kg/Chinese adult). IEDI was defined according to formula and IEDI of azoxystrobin was 0.418785 mg/kg. ADI of azoxystrobin in China was established at 0.2 mg/kg(Ministry of Health of the Peoples Republic of China, Ministry of Agriculture of the Peoples Republic of China,2014), and ADI% was calculated according to the formula and found to be 3.32%.
2. 5 Relation between pesticide application methods and terminal residues
The comparison of means using a one-way ANOVA and Duncans test, showing the effects of azoxystrobin application rate, frequency and interval to harvest on the terminal residues in peanut plant, peanut shell, peanut and soil, were calculated from the experi-
mental data and were summarized in Table 5.
3 Discussion
Based on the dissipation data in 2012 and 2013, the different initial concentrations of azoxystrobin in peanut plants could be attributed to different plant sizes, planting locations and years. Differences in the dissipation rate and half-life of azoxystrobin in the peanut plants might have been caused by weather conditions(such as temperature and rainfall) after chemical application. The relationship between the rain-fastness of fungicides and their water solubilities revealed that the residue levels of fungicides rapidly decreased during the early stages of precipitation and the rate of decreasement in residue levels slowed down thereafter (Choi et al., 2009; Edwards et al., 2016). Other papers also reported a degradation of azoxystrobin with field studies, half-life in grapes and the soil were 5.4-11.2 and 8.1 days(Gajbhiye et al., 2011), in bananas and the soil were 7.8-11.6 and 11.9-16.1 days(Wang et al., 2013), in soybeans were 0.8-3.6 days(Yin et al., 2011).
Differences in the dissipation rate and half-lives of azoxystrobin in the soil might have been due to local soil characteristics, farming methods, microbial activity and weather conditions. The azoxystrobin DT25(time to 25% dissipation), sorption and pH were significantly correlated(Bending et al.,2006). In this study, it was found that the dissipation of azoxystrobin in peanut plants was faster than that in soil. A growth dilution factor might play an important role in pesticide dissipation in plants, in addition to the effects of physi-
cal and chemical factors such as light, heat, pH and moisture(Tewary et al., 2005). The growth dilution factor was important in reducing azoxystrobin residue levels in peanut fields because the residue was expressed as a proportion of weight(mg/kg). The peanut plants were picked in different stages of growth in this study, and as the weight of peanut plant material increased, the proportion of residue decreased(Liang et al., 2012).
The terminal residues at harvest in peanuts plant, peanut, peanut shell and soil were lower than 0.154, 1.135, 0.922 and 0.957 mg/kg respectively. In this study, the residue levels increased, the increasements were peanut < peanut shell < soil < peanuts plant, these results could be attributed to the following conditions:(1)in the residue experiments, the azoxystrobin was sprayed directly on the peanuts plants and not on the soil, and although the dissipation of azoxystrobin in peanuts plants was faster than that in soil, the peanut plant moisture content was also greatly reduced at harvest, causing the azoxystrobin residues per unit weight to be higher than those in peanut, peanut shell and soil;(2)azoxystrobin was a systemic pesticide, so when it was sprayed on the peanut plants, it could be transferred to peanut; and(3)azoxystrobin residues in the soil were much higher in the top layer(0-20 cm) than in deeper layer(Herrero-Hernández et al., 2015). Pesticide residues in soils raised several potential risks, such as an adverse effect on subsequent crops and contamination of the groundwater(Zhang et al., 2012). In the final residue experiments, azoxystrobin residues in the soil were detectable at three locations, the potential risks of these residues remain undetermined.
The ADI% was less than 100%, indicating that the chronic risk of azoxystrobin was within acceptable limits. The results indicated that the potential health risk induced by azoxystrobin was not significant and that the recommended application methods were safe. When the interval to harvest was 21 or 28 days, there were no significant differences in the terminal residues in the peanut, peanut plants, peanut shell and soil. These results indicated that when the interval to harvest was 21 or 28 days, there was no effects of application rate or frequency on terminal residues; how-
ever, when the interval to harvest was 14 days, the application rate and frequency may have an effect on terminal residues.
4 Conclusion
A specific, sensitive and simple residue analytical method using LC-PDA for the detection and monitoring of azoxystrobin in peanut plant, peanut, peanut shell and soil was established. The half-life of azoxystrobin in peanut plants and soil were 7.24-12.07 days and 5.57-13.48 days respectively. When the application of azoxystrobin followed the recommended methods, the potential health risk was not significant. When the interval to harvest was 14 days, the application rate and frequency may have an effect on terminal residues.
References:
Abdelraheem E M H, Hassan S M, Arief M M H, Mohammad S G. 2015. Validation of quantitative method for azoxystrobin residues in green beans and peas[J]. Food Chemistry, 182:246-250.
Abreu S M, Caboni P, Cabras P, Garau V L, Alves A. 2006. Validation and global uncertainty of a liquid chromatographic with diode array detection method for the screening of azoxystrobin, kresoxim-methyl, trifloxystrobin, famoxadone, pyraclostrobin and fenamidone in grapes and wine[J]. Analytica Chimica Acta, 573-574:291-297.
Aguilera A,Valverde A,Camacho F,Boulaid M,García-Fuentes L. 2012. Effect of household processing and unit-to-unit variability of azoxystrobin, acrinathrin and kresoxim methyl resi-
dues in zucchini[J]. Food Control, 25(2):594-600.
Bagi F F, Budakov D B, Bursi■ V P, Stoj■in V B, Lazi■ S D, Vukovi■ S M. 2014. Efficacy of azoxystrobin for the control of cucumber downy mildew(Pseudoperonospora cubensis) and fungicide residue analysis[J]. Crop Protection, 61:74-78.
Bending G D, Lincoln S D, Edmondson R N. 2006. Spatial variation in the degradation rate of the pesticides isoprotu-
ron, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties[J]. Environmental Pollution, 139(2):279-287.
Bo H B, Bi Y, Chen L R. 2005. Determination of azoxystrobin residue in muskmelon and apple by gas chromatography[J]. Chinese Journal of Analytical Chemistry, 33(5):695-698.
Bo H B. 2007. Determination of azoxystrobin residues in fruits and vegetables by gas chromatography/mass spectrometry with solid-phase extraction[J]. Chinese journal of chromatography, 25(6):898-901.
Chen X X, Li W M, Wu Q, Zhi Y N, Han L J. 2011. Bromoxynil residues and dissipation rates in maize crops and soil[J]. Ecotoxicology and Environmental Safety, 74(6):1659-1663.
Choi Y K, Yu J H, Chun J C. 2009. Rainfastness of 5 fungicides on the leaf surface of hot pepper[J]. Journal of Applied Biological Chemistry, 52(3):126-132.
Ding R Y, Li H D, Du H X, Li M L, Ji J, Chen Z L. 2006. Determination methods of azoxystrobin residue in cucumber by gas chromatography[J]. Chemical Analysis and Meterage, 15(6):76-77.
Edwards P G, Murphy T M, Lydy M J. 2016. Fate and transport of agriculturally applied fungicidal compounds, azoxystrobin and propiconazole[J]. Chemosphere,146:450-457.
Fisher N, Meunier B. 2008. Molecular basis of resistance to cytochrome bc1 inhibitors[J]. FEMS Yeast Research, 8(2):183-192.
Flores J L, Díaz A M, Fernández de Córdova M L. 2007. Determination of azoxystrobin residues in grapes, musts and wines with a multicommuted flow-through optosensor implemented with photochemically induced fluorescence[J]. Analytica Chimica Acta, 585(1):185-191.
Gajbhiye V T, Gupta S, Mukherjee I, Singh S B, Singh N, Dureja P, Kumar Y. 2011. Persistence of azoxystrobin in/on grapes and soil in different grapes growing areas of India[J]. Bulletin of Environment Contamination and Toxicology, 86(1):90-94.
Han B, Yao A Q, Wu H M, Wang M C, Qin L. 2009. Degradation of azoxystrobin residue in orange and soil[J]. Agrochemicals, 48(12):899-901.
Herrero-Hernández E, Marín-Benito J M, Andrades M,Sánchez-
Martín M J, Rodríguez-Cruz S. 2015. Field versus laboratory experiments to evaluate the fate of azoxystrobin in an amended vineyard soil[J]. Journal of Environmental Management, 163:78-86.
Hong W Y, Wu Y J, Zhang H, Qian M R, Chen R. 2012. Degradation behavior and safely applying technology of azoxystrobin and pyraclostrobin in cucumber[J]. Acta Agriculturae Zhejiangensis, 24(3):469-475.
Huan Z B, Xu Z, Lü D Z, Xie D F, Luo J H. 2013. Dissipation and residues of difenoconazole and azoxystrobin in bananas and soil in two agro-climatic zones of China[J]. Bu-
lletin of Environmental Contamination and Toxicology, 91(6):734-738.
Jordan D B, Livingston R S, Bisaha J J, Duncan K E, Pember S O, Picollelli M A, Schwartz R S, Sternberger J A, Tang X S. 1999. Mode of action of famoxadone[J]. Pesticide Science, 55(2):105-118.
Li W, Qiu S P, Wu Y J. 2008. Triazophos residues and dissipation rates in wheat crops and soil[J]. Ecotoxicology and Environmental Safety, 69(2):312-316.
Li W, Wu Y J, Qin D M, Ma Y, Sun Y J, Qiu S P. 2008. A method for quantifying azoxystrobin residues in grapes and soil using GC with electron capture detection[J]. Chromatographia, 67(9-10):761-766.
Liang H W, Qiu J, Li L, Li W, Zhou Z Q, Liu F M, Qiu L H. 2012. Stereoselective dissipation of epoxiconazole in grape (Vitis vinifera cv. Kyoho) and soil under field conditions[J]. Chemosphere, 87(8):982-987.
Liu Y P, Sun H B, Zeng F J, Liu J M. 2010. Study on residual dynamics of azoxystrobin in mango and soil[J]. Guangdong Agricultural Sciences, 37(10):106-107.
Liu Y T, Wang J P, Guo Y Z, Chen Q S, Zhang Q, Yin P. 2015. Residue and degradation behavior of propiconazole and azoxystrobin in maize[J]. Tianjin Agricultural Sciences, 21(12):43-47.
Ministry of Agriculture of the Peoples Republic of China. 2004. NY/T 788-2004, Guideline on pesticide residue trials[S]. Beijing:Ministry of Agriculture of the Peoples Republic of China.
Ministry of Health of the Peoples Republic of China, Ministry of Agriculture of the Peoples Republic of China. 2014. GB 2763-2014, National food safety standard maximum residue limits for pesticides in food[S]. Beijing:Ministry of Health of the Peoples Republic of China, Ministry of Agriculture of the Peoples Republic of China.
Shi F, Zhao K H, Che J, Huang A T. 2010. Determination of residual azoxystrobinin in citrus shatangju by HPLC[J]. Journal of Hebei Agricultural Sciences, 14(2):161-163.
Tewary D K, Kumar V, Ravindranath S D, Shanker A. 2005. Dissipation behavior of bifenthrin residues in tea and its brew[J]. Food Control, 16(3):231-237.
Wang L, Zhao P Y, Zhang F Z, Li Y J, Du F P, Pan C P. 2012. Dissipation and residue behavior of emamectin benzoate on apple and cabbage field application[J]. Ecotoxicology and Environmental Safety, 78:260-264.
Wang S W, Hou Z G, Zou J, Lu Z B. 2010. Residual decline of azoxystrobin 25% SC in ginseng environment[J]. Agrochemicals, 49(6):436-438.
Wang S W, Sun H B, Liu Y P. 2013. Dissipation and residue of azoxystrobin in banana under field condition[J]. Environmental Monitoring and Assessment, 185(9):7757-7761.
Wang S W, Liu Y P, Sun H B. 2014. Dissipation and terminal residue of azoxystrobin in lychee under field condition[J]. Fujian Journal of Agricultural Sciences, 29(2):168-171.
Watanabe E, Miyake S. 2013. Quantitative analysis of fungicide azoxystrobin in agricultural samples with rapid, simple and reliable monoclonal immunoassay[J]. Food Chemistry, 136(2):695-702.
Wu Y X, Lin F, Lin H D, Shao L Z, Jiao H, Li X. 2009. Determination of azoxystrobin residues in Legume using high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry[J]. Journal of Instrumental Analysis, 28(5):617-620.
Wu J L, Wang H C, Wu X T, Zhao J, Yang N N. 2012. Determination of azoxystrobin residue in Panax ginseng and Panax quinquefolium and dietary intake risk assessment[J]. Chinese Journal of Pesticide Science, 14(1):67-73.
Yang Z H, Wei C J, Jia L F, Liang D, Zhao J Z. 2013. Residual dynamics of azoxystrobin in strawberry and soil[J]. Journal of Agro-Environment Science, 32(4):697-700.
Yin F P, Li X S, Huang H Y. 2006. Residual dynamics and final residues of azoxystrobin in cucumber and soil[J]. Journal of Agro-Environment Science, 25(S):590-594.
Yin L D, Hou Z G, Chen C, Ji H M, Wei X H, Lu Z B, Wang Y. 2011. Residues and decline study of azoxystrobin in soybean[J]. Chinese Journal of Pesticide Science, 13(3):304-309.
Zhang F Z, Wang L, Zhou L, Wu D, Pan H J, Pan C P. 2012. Residue dynamics of pyraclostrobin in peanut and field soil by QuEChERS and LC-MS/MS[J]. Ecotoxicology and Environmental Safety, 78:116-122.
Zhang X, Shen Y, Yu X Y, Liu X J. 2012. Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions[J]. Ecotoxicology and Environmental Safety, 78:276-280.
(責任编辑 陈德元)

