Antibacterial Activity of 4-Hexylresorcinol against Three Spoilage Bacteria in Culture and Its Effect on the Quality of Pacific White Shrimp
QIAN Yunfang, YANG Shengping, XIE Jing*
(Shanghai Engineering Research Center of Aquatic Product Processing and Preservation, College of Food Science and Technology,Shanghai Ocean University, Shanghai 201306, China)
Antibacterial Activity of 4-Hexylresorcinol against Three Spoilage Bacteria in Culture and Its Effect on the Quality of Pacific White Shrimp
QIAN Yunfang, YANG Shengping, XIE Jing*
(Shanghai Engineering Research Center of Aquatic Product Processing and Preservation, College of Food Science and Technology,Shanghai Ocean University, Shanghai 201306, China)
The antibacterial activity of 4-hexylresorcinol against the spoilage bacteria Carnobacterium maltaromaticum,Shewanella putrefaciens and Aeromonas salmonicida from Pacific white shrimps and its effect on the quality of Pacific white shrimps was assessed in this study. The results of Oxford cup method and the growth inhibition curves for the bacteria indicated that 4-hexylresorcinol showed a strong inhibitory effect on the three spoilage bacteria. The determination of electrical conductivity and flow cytometric analysis showed that 4-hexylresorcinol led to increased permeability of the cytoplasmic membrane of S. putrefaciens cells. When 0.25 mg/mL 4-hexylresorcinol was applied in shrimps, melanization was inhibited according to the low melanosis score and high whiteness value during cold storage at 4 ℃. However, a significant inhibitory effect on the increase of psychrotrophic bacterial counts and total volatile basic nitrogen was only observed in the first 4 days in comparison with the control. At the end of storage, the bacterial count of 4-hexylresorcinoltreated samples was 0.14 (lg(CFU/g)), which was higher than that of the control. These results showed that though 4-hexylresorcinol was effective in inhibiting the growth of the three spoilage bacteria in vitro, it was preferred to be used as a melanosis inhibitor for shrimps rather than as an inhibitor against the growth of spoilage bacteria in shrimps.
Litopenaeus vannamei; 4-hexylresorcinol; antibacterial activity; Shewanella putrefaciens; Aeromonas salmonicida; Carnobacterium maltaromaticum
Melanosis (black spot formation) is a severe problem that occurs in crustaceans during post-harvest storage[1]. It is a biochemical process triggered by an enzymatic complex called polyphenoloxidase (PPO), tyrosinase or phenolase[2].In live crustacean, PPO exists in hemolymph and cuticle in an inactive state (proPPO), and can be activated by the response to immune system stimulation[3-4]. Several mechanisms are involved in this activation, such as microbial substances(carbohydrates and lipopolysaccharides)[5]and proteolytic enzymes[6-7]. Although melanosis causes no direct harm to consumers, it usually associates with the sensory features of crustaceans and decreases their commercial value.
To prevent the development of melanosis in crustacean,many melanosis inhibitors have been researched. Sulphites and their derivatives are used extensively. However, another compound named 4-hexylresorcinol (4-HR) is proved to be a good alternative[8]. 4-HR has a long history of human pharmaceutical used as an anthelmintic and antiseptic on minor skin infections or as an ingredient in throat lozenges[9].It is considered to be GRAS (generally recognized as safe) as a food additive (a maximum residue limit of 2 mg/kg)[10]. The effectiveness of 4-HR against melanosis of crustacean has been demonstrated both in laboratory and on board tests[11-15].
Besides melanosis problem, microbiological spoilage is considered to be another important issue that affects the quality deterioration of crustacean. A preservative which can act both as a melanosis inhibitor and as an antimicrobial is expected. 4-HR happens to be such kind of preservative,whose antiseptic properties have been well investigated in the pharmaceutical area[16], but studies carried out in the preservation of crustacean displayed quite different opinions on its antibacterial activity. Monsalve-Gonzalez et al.[17]noticed that the apple products treated by 4-HR were protected from bacterial contamination. López-Caballero et al.[15]found that a formula containing 0.05% and 0.10% (0.05 and 0.10 g of inhibitor per 100 g of shrimp) of 4-HR could inhibit the growth of bacteria of deepwater pink shrimp. However,others believed that 0.25% 4-HR did not play a role in preventing bacterial growth of Pacific white shrimp combined with modified atmosphere packaging and pyrophosphate[12].The European Commission also demonstrates that no disinfectant effects are expected against any potential contaminating microorganisms until the concentration of 4-HR in dipping solution exceeds 0.5%-1.0%[18].
These studies confused the researches whether 4-HR has contributed to inhibiting the growth of spoilage bacteria during storage, or at what circumstances the antibacterial activity of 4-HR can be concerned. This study is trying to evaluate the antibacterial activity of 4-HR against the dominant spoilage bacteria Carnobacterium maltaromaticum,Shewanella putrefaciens and Aeromonas salmonicida from Pacific white shrimp in culture and its effect on quality changes of Pacific white shrimp to confirm whether 4-HR can inhibit microbial growth and quality deterioration of Pacific white shrimp during storage.
1 Materials and Methods
1.1 Strains, materials and reagents
Carnobacterium maltaromaticum (accession No.:KF263652 in NCBI), Shewanella putrefaciens (accession No.: KF263653) and Aeromonas salmonicida (accession No.:KF263654) strains were isolated from spoiled Pacific white shrimps previously.
Pacific white shrimp: purchased from local market of Luchao port, Shanghai.
Trypticase soy broth (TSB), tryptose soya agar (TSA)and plate count agar (PCA) were obtained from Qingdao Hope Bio-Technology Co. Ltd., Qingdao, China; 4-HR and propidium iodide (PI) were purchased from Sigma-Aldrich Ltd., Shanghai, China; dimethyl sulphoxide (DMSO), NaCl,KCl, and other chemical reagents were purchased from Sinopharm Chemical Reagent Co. Ltd., China.
1.2 Instruments and equipments
C MBR Bioscreen Oy Growth Curves Ab Ltd., Finland;DDS-307A conductivity meter Precision & Scientific Instrument Co. Ltd., Shanghai, China; MoFloXDP flow cytometer Beckman Coulter Inc., USA; CR-10 portable colorimeter Konica Minolta Investment Ltd., Japan;Kjeltec 2300 autoanalyzer Foss Tecator Ab, Sweden.
1.3 Determination of the antimicrobial activity of 4-HR
1.3.1 Bacterial strains and strain culture
Three bacterial strains Carnobacterium maltaromaticum,Shewanella putrefaciens and Aeromonas salmonicida isolated from spoiled Pacific white shrimps previously stored in sterilized TSB with 25% glycerine at -80 ℃. The strains were pre-cultured in TSB at 30 ℃ until the concentration reached 108CFU/mL approximately.
1.3.2 Antimicrobial evaluation of 4-HR
The antibacterial activity of 4-HR against the three specific spoilage microorganisms strains was evaluated by the Oxford cup method as described previously[19]. The culture was diluted with sterilized TSB to achieve an inoculum of approximately 106CFU/mL, and 0.1 mL of the dilution was spread on 15 mL TSA in a Petric dish. The 4-HR was dissolved in DMSO. A series of two-fold dilutions of 4-HR(200 μL) was added into an Oxford cup (Ф = 6 mm) which were placed on the agar surface. The plates were incubated for 24 h at 30 ℃. The size of the diffusion zone around the cup (including that of the Oxford cup) was measured and the results were expressed millimeters. DMSO was the negative control.
Growth experiments were performed on micro-well plates. 198 μL of the diluted inoculum (105CFU/mL) and a series of 4-HR dilutions (2 μL) with final concentrations of 0.25 and 0.50 mg/mL were mixed in the wells. Bacterial growth was monitored every 2 h until 35 h using the C MBR Bioscreen at 30 ℃. Growth was measured by recording at OD600nm.
Cellular leakage of the bacterial cells was determined by measuring electrolyte leakage into the incubation medium with a conductivity meter[20]. After the inoculum reached exponential phase, the bacteria were centrifuged at 8 000 × g for 5 min. The pellets were washed with triple 0.1 mol/L phosphate buffer saline (PBS) (pH 7.0) and diluted with the same buffer to approximately 106CFU/mL. 4-HR was then added to the suspension with final concentrations of 0.25 and 0.50 mg/mL, and then the mixtures were incubated with shaking (170 r/min) at 30 ℃. The conductivity was measured every 10 min.
1.3.3 Flow cytometric assessment
The cell suspensions (30 mL) were centrifuged at 8 000 × g for 5 min. The supernatant was discarded. The pellet was re-suspended in PBS (0.01 mol/L pH 7.4, containing 8.0 g NaCl and 0.2 g KCl). Serial mixtures of 4-HR dilution(2 μL) and the bacterial suspension (198 μL) were incubated at 30 ℃ for 1.5 h. The pellet was obtained by centrifugation at 8 000 × g for 5 min, and re-suspended in PBS and centrifuged as described above. The bacterial suspensions were then stained by propidium iodide (PI) stock solution (20 μg/mL)at 4 ℃ according to the method described by Ananta et al.[21]. The unstained cell suspension was served as background. The cell suspension in the absence of 4-HR was served as negative control, while the suspension incubated in 70 ℃ for 30 min was used as positive control[22]. The pretreated cell suspensions were filtered through 400-mesh filter and kept on ice until use.
Flow cytometric analysis was performed on a MoFloXDP flow cytometer equipped with 15 mW, 488 nm,air-cooled argon-ion laser. The flow rate was set at 400-600 events per second, up to a total of 105events per sample.Red fluorescence was collected in the FL2 channel (630-700 nm). Forward scatter (FSC), sideward scatter (SSC) and fluorescence signals of individual cells passing through the illuminated zone were collected as logarithmic signals. A gate in the dot-plot of FSC vs. SSC was preset. Data were analyzed with the software package Summit 5.2.
1.4 Inhibitory effect of 4-HR against spoilage bacteria attached to Pacific white shrimp
1.4.1 Preparation and treatment of Pacific white shrimp
The shrimp samples with average weight 12-16 g were purchased from local market and delivered to the laboratory alive within 30 min. Upon arrival, the shrimp were slaughtered by immersed into the ice-slurry and cleaned in the cold water from the tap. The shrimps were separated into 2 groups randomly (10 kg for each group), and one of them was immersed into the 4-HR solution (0.25 mg/mL) for 3 min. After then, all samples were stored at 4 ℃ for 10 days.Sampling was carried out every 2 days.
1.4.2 Melanosis assessment
The melanosis scores of shrimp were given by 10 trained panelists through visual inspection by 10-point hedonic scales from 0 (absent) to 10 (extremely heavy)[1]. The mean values of all scores given by panelists were calculated.
1.4.3 Colorimetric analysis
The whiteness values were calculated by L* (lightness),a* (redness and greenness) and b* (blueness and yellowness)recorded by a portable colorimeter according to Qian Yunfang et al.[23].
1.4.4 Microbial numeration
To determine total psychrotrophic bacteria count, 25 g of minced white shrimp samples were homogenized with 225 mL of sterilized saline water (0.85 g/100 mL NaCl).The homogenates were serially diluted logarithmically by sterilized saline water. 1 mL of an appropriate dilution was spread in plate count agar (PCA) with 0.5 g/100 mL NaCl.The bacterial colonies were counted after the plates were incubated at 4 ℃ for 10 days and reported as lg(CFU/g) of flesh weight[23-24].
1.4.5 TVB-N content
Total volatile basic nitrogen (TVB-N) content was determined by the Kjeldahl method[25]of via Kjeltec 2300 autoanalyzer. The results were recorded as mg N/100 g of flesh weight.
1.5 Statistical analysis
For the melanosis values, whiteness values, total psychrotrophic bacteria count and TVB-N content,3 replicates per samples of the samples were taken for tests every 2 days during storage. SPSS 15.0 software package was used to perform the analysis of variance (ANOVA) which was performed by Duncan’s multiple range tests, and P 〈 0.05 was considered as statistically different. OriginPro Version 8.5 was used to design the figures. Data were taken as
2 Results and Analyses
2.1 Antimicrobial activity of 4-HR in culture
2.1.1 Antimicrobial activities of 4-HR against the three spoilage bacteria
Table 1 showed the inhibition halos of serial dilutions of 4-HR against three tested spoilage bacteria C. maltaromaticum, S. putrefaciens and A. salmonicida. The diameters of the halos increased along with the increasing concentrations of 4-HR. When the concentration reached 0.1 mg/mL, the formation of the inhibition halos against C. maltaromaticum was significant (P 〈 0.05), and when the concentration was beyond 0.25 mg/mL, its antibacterial effect on S. putrefaciens and A. salmonicida was obvious. DMSO did not present an inhibitory activity on any of the tested microorganisms.
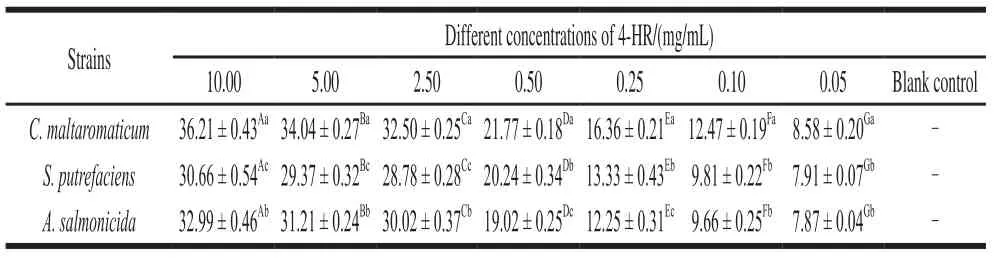
Table 1 Antibacterial activity of 4-HR against C. maltaromaticum, S. putrefaciens and A. salmonicida mm
2.1.2 Effect of 4-HR on the growth of the three spoilage bacteria
The growth patterns of the three bacteria treated with 4-HR were shown in Fig. 1. Lower OD600nmmaximums were observed in all bacterial samples compared to the control.From Fig. 1A and B, it was also found that the exponential phases of C. maltaromaticum and S. putrefaciens were shorter than the negative control. The most significant inhibitory effect on the bacterial growth was found in S. putrefaciens.
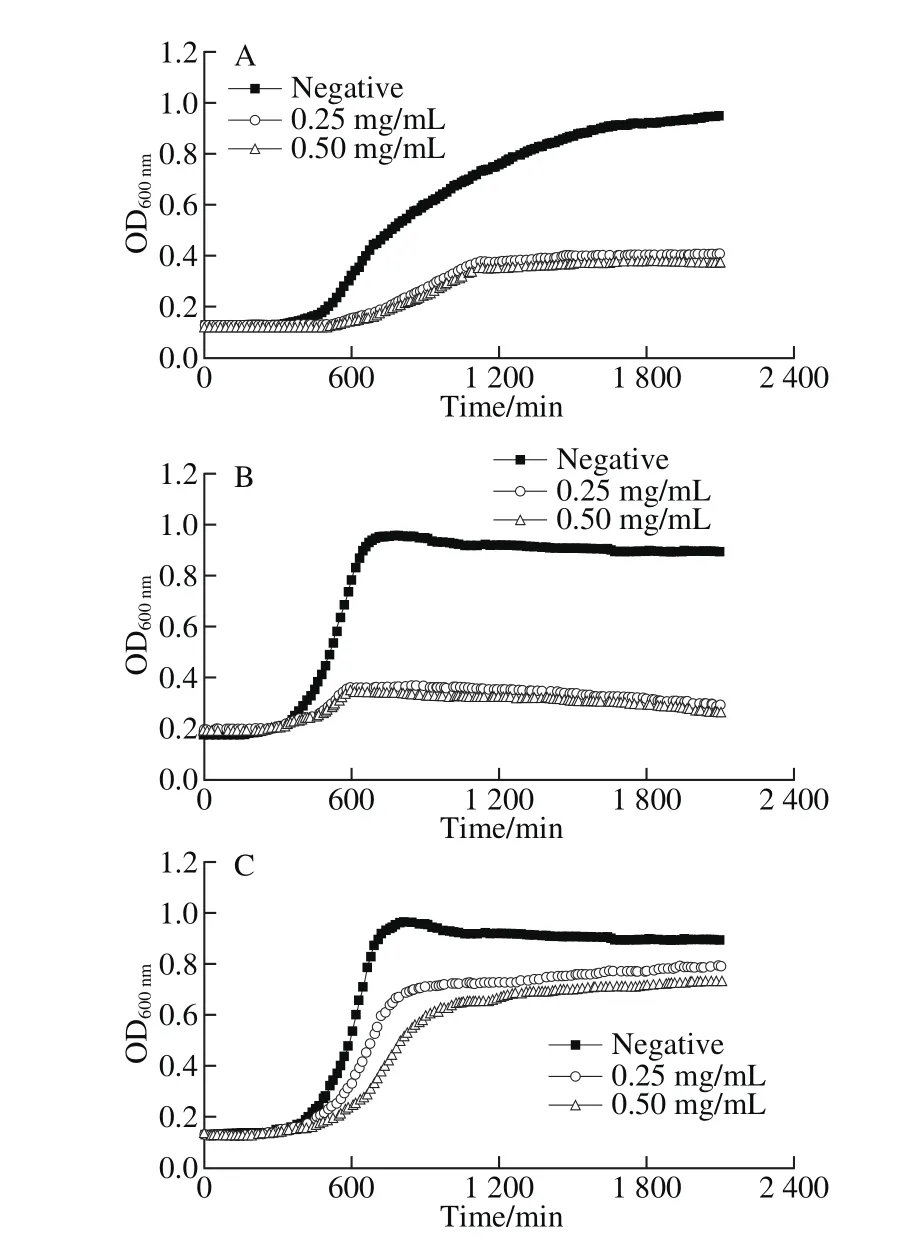
Fig. 1 Growth pattern and electrical conductivity of the three bacteria after being treated with 4-HR
2.1.3 Effect of 4-HR on the membrane permeability of the three spoilage bacteria
Electrical conductivities of the three spoilage bacteria after being treated with 4-HR were determined to evaluate the relationship between antibacterial activity of 4-HR and membrane permeability. The results were presented in Fig. 2.The initial electric conductivities of C. maltaromaticum,S. putrefaciens and A. salmonicida were around 11.66-11.68 mS/min. After adding 4-HR, the electric conductivities of the three bacteria grew up quickly in the first 20 min, but the differences between the control and the treated samples were not significant. After then, the electric conductivities of the control samples became stable, while those of the treated samples still increased steadily and became significantly higher than the negative samples.
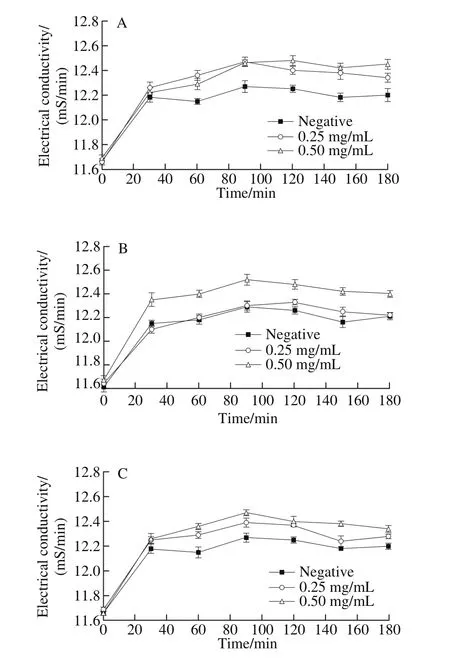
Fig. 2 Electrical conductivity of the three bacteria after being treated with 4-HR
2.1.4 Effect of 4-HR on the morphological alternations and membrane integrity of S. putrefaciens
As the best effectiveness of 4-HR was found against tested S. putrefaciens, further antibacterial mode of action of 4-HR against S. putrefaciens was confirmed in the application of PI staining and flow cytometry. The morphological alternations visualized by flow cytometry (FSC and SSC)(Fig. 3A-D). SSC of the bacterial cells increased as the increasing concentration of 4-HR, more close to that of the positive control in which the bacterial cells were killed in the hot water bath. Therefore, the application of 4-HR might cause an increase of the cell granularity or internal complexity[26]. PI can enter the cells whose membrane integrity are low and combine with nucleic acids providing fluorescence signals[27]. As shown in Fig. 3, PI-positive rate of the cell increased when cells were stained with higher concentration of 4-HR. The results indicated that 4-HR might also change the membrane permeability of the bacteria, in agreement with the results of electrical conductivity.
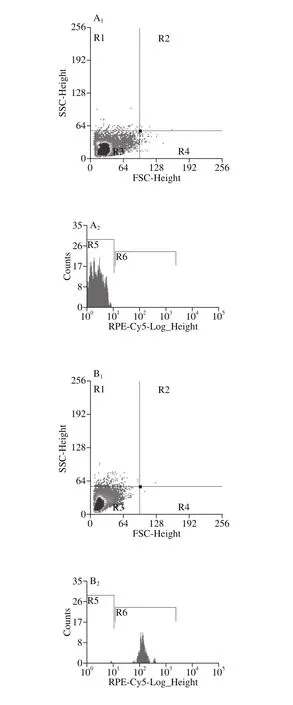

Fig. 3 Flow cytometry analysis of S. putrefaciens treated with 4-HR
2.2 Inhibitory effect of 4-HR against spoilage of Pacific white shrimp
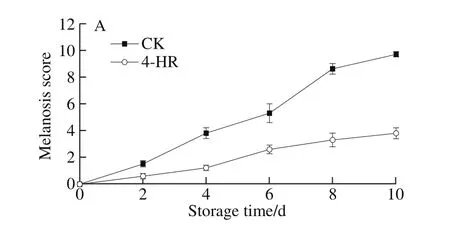
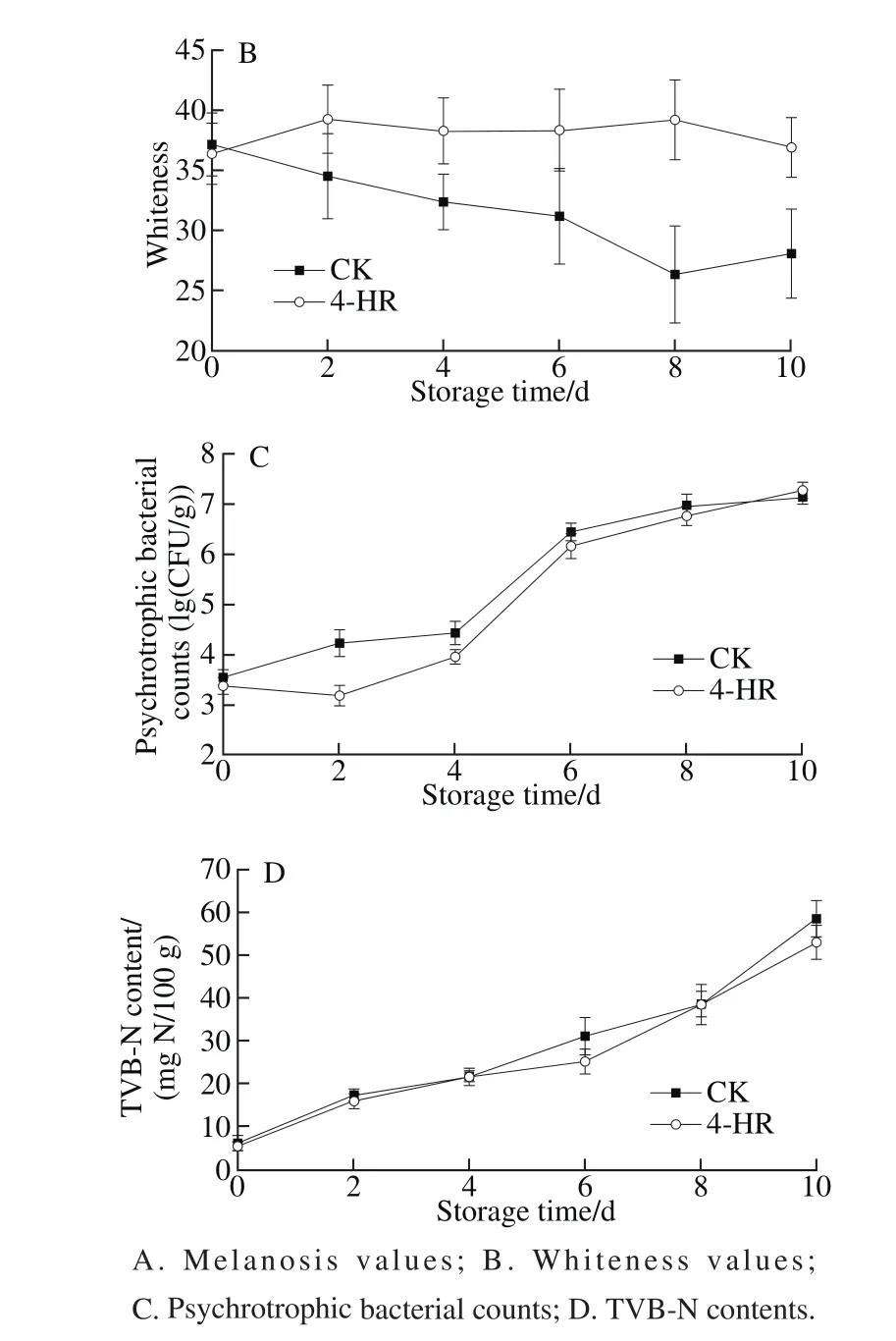
Fig. 4 Quality changes of Pacific white shrimps treated with 4-HR during storage at 4 ℃
The development of melanosis in white shrimp with or without 4-HR pretreatment stored at 4 ℃ was evaluated during 10-day storage (Fig. 4A). The melanosis scores of the samples without pretreatment of 4-HR increased rapidly during storage, and almost reached 10 on day 10. The shrimp treated with 4-HR had significantly lower melanosis scores throughout the storage period, which did not exceed 4 at the end of storage. No obvious black spots were observed in samples with 4-HR, though the whole shrimp appeared to be a little yellower after 8 days of storage.
The changes of whiteness values of shrimp cephalothorax during storage are displayed in Fig. 4B. The whiteness values of the control sample (without 4-HR)decreased continuously from 37.15 to 28.04 (0-10 days),while the whiteness of samples with 4-HR remained stable at high level (36.89-39.24) throughout the storage period.
As the shrimp were usually stored at 4 ℃, the growth pattern of psychrotrophic bacterial counts was studied(Fig. 4C). The initial psychrotrophic bacterial counts of the shrimp immersed in distilled water or 4-HR distilled water solution were 3.54 and 3.37 (lg(CFU/g)), respectively. The bacterial counts of control samples (without 4-HR) began to rise rapidly since day 4 of storage, and the growth rate slowed after 6 days of storage. At the end of storage, the bacterial counts of control samples reached 7.13 (lg(CFU/g)).For the shrimp samples pretreated with 4-HR, a slight decrease in psychrotrophic bacterial counts was noticed in the first 2 days, and the counts were significantly lower than those of the control samples from day 0 to day 4.Interestingly, the bacterial counts of the samples pretreated with 4-HR increased rapidly since then, and even reached 7.27 (lg(CFU/g)) at the end of storage.
The accumulation of TVB-N contents was observed in both control sample and sample treated with 4-HR (Fig. 4D).The initial content of TVB-N of fresh shrimp was about 6 mg N/100 g. TVB-N accumulated in shrimp muscle quickly during storage. The contents of the CK and 4-HR treated samples reached 58.5 and 53.1 mg N/100 g on day 10, respectively. The samples with 4-HR were determined to have TVB-N content as much as the CK.
3 Discussion
In this study, both the in vitro antibacterial activity of 4-HR against the dominant spoilage bacteria from Pacific white shrimp (C. maltaromaticum, S. putrefaciens and A. salmonicida) and its effect on the quality deterioration of shrimp were researched.
The experiment of Oxford cup and the inhibited growth curves of the bacteria showed that 4-HR showed a strong inhibitory effect on S. putrefaciens, which played the most important part in the spoilage of the shrimp as we discussed in previous study[28]. To assess the mode of action of 4-HR on S. putrefaciens, the determination of the electrical conductivity and flow cytometric assessment was conducted. Both the higher electric conductivity of cell suspensions and PI-positive staining rate indicated that the cytoplasmic membranes became more permeable after the treatment of 4-HR. This phenomenon should contribute to the amphipathy of 4-HR, which has phenolic group. Other antibacterial compounds containing phenolic groups such as essential oils are also reported to have a rigorous effect on cell membrane[29-31].
To estimate the effect of 4-HR on the quality of Pacific white shrimp, the melanosis scores, whiteness values,psychrotrophic bacterial counts and TVB-N contents were determined. Both the whiteness values and the melanosis scores indicated that 0.25 mg/mL 4-HR could prevent the development of melanosis effectively, which was quite lower than the minimum dose (0.5 mg/mL) for deepwater pink shrimp (Parapenaeus longirostris)[32].Lower bacteria counts of 4-HR treated samples were observed in the first 4 days, even though the residue of 4-HR was lower than 0.25 mg/mL according to the ratio of the dipping solution and residue content reported previously[32].However, the antibacterial activity of 4-HR did not last long,the bacterial counts of samples exposed by 4-HR became even higher than the control at the end of storage. The TVB-N is commonly used as a reliable index quality decay of aquatic products, whose increase is usually associated with bacterial and enzymatic activity[33]. From the study, no significant differences of the TVB-N contents between the treated samples and the control were found during storage,which indicated that the effect of 4-HR on preventing the spoilage of Pacific white shrimp could be ignored, confirmed by the previous study[12].
To understand the effect of 4-HR on the bacterial growth and spoilage of shrimp, the mechanisms of the formation of melanin and the impact of phenoloxidase should be referred.It is believed that melanosis is triggered by a special kind of enzyme namely phenoloxidase[2]. This kind of enzyme can oxidize phenols to quinones[34]. The colorless quinones subsequently undergo nonenzymatic polymerization, forming black insoluble high molecular-weight pigment, as well as antibacterial compounds[35]. These enzymes usually exist in hemolymph and cuticle in an inactive state (proPPO).The proPPO would be activated by microbial substances(carbohydrates and lipopolysaccharides) and the proteolytic enzymes leaching from the digestive tract[6-7]. The increasing bacterial counts in shrimp treated by 4-HR might be contributed by two factors. For one thing, as a melanosisinhibitor, 4-HR could combine to the active PPO in shrimp,and then fewer doses of 4-HR were left to inhibit the bacteria.For another thing, as the melanization cascade could produce antibacterial compounds[36-37], the possible side effect of 4-HR by inhibiting melanosis may be reduce the production of the antibacterial compounds derived from the melanization,leading to the faster growth of bacteria thereafter.
In summary, this study shows an interesting finding about the antibacterial activity of 4-HR on spoilage bacteria from Pacific white shrimp both in vitro and in vivo. It was found that 4-HR had a strong inhibitory effect on spoilage bacteria C. maltaromaticum, S. putrefaciens and A. salmonicida in vitro. The possible antimicrobial mode of action of 4-HR against tested strains was due to its effect on the permeability and integrity of the membrane. The usage of 4-HR could also lower the bacterial counts in Pacific white shrimp in the first 4 days, although its effect would not last very long. Therefore, more other antibacterial preservatives or technology should be applied to retard quality deterioration.
[1] NIRMAL N P, BENJAKUL S, AHMAD M, et al. Undesirable enzymatic browning in crustaceans: causative effects and its inhibition by phenolic compounds[J]. Critical Reviews in Food Science and Nutrition, 2015,55(14): 1992-2003. DOI:10.1080/10408398.2012.755148.
[2] BENJAKUL S, VISESSANGUAN W, TANAKA M. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn(Penaeus japonicus)[J]. Journal of Food Biochemistry, 2005, 29(5):470-485. DOI:10.1111/j.1745-4514.2005.00042.x.
[3] HUANG J W, YANG Y, WANG A L. Reconsideration of phenoloxidase activity determination in white shrimp Litopenaeus vannamei[J]. Fish and Shellfish Immunology, 2010, 28(1): 240-244.DOI:10.1016/j.fsi.2009.10.010.
[4] MARTÍNEZ-ALVAREZ O, GÓMEZ-GUILLÉN C, MONTERO P.Presence of hemocyanin with diphenoloxidase activity in deepwater pink shrimp (Parapenaeus longirostris) post mortem[J].Food Chemistry, 2008, 107(4): 1450-1460. DOI:10.1016/j.foodchem.2007.09.078.
[5] AMPARYUP P, CHAROENSAPSRI W, TASSANAKAJON A.Prophenoloxidase system and its role in shrimp immune responses against major pathogens[J]. Fish and Shellfish Immunology, 34(4):990-1001. DOI:10.1016/j.fsi.2012.08.019.
[6] GONÇALVES A A, DE OLIVEIRA A R M. Melanosis in crustaceans:a review[J]. LWT-Food Science and Technology, 2016, 65: 791-799.DOI:10.1016/j.lwt.2015.09.011.
[7] ZAMORANO J P, MARTÍNEZ-ALVAREZ O, MONTERO P, et al.Characterisation and tissue distribution of polyphenol oxidase of deepwater pink shrimp (Parapenaeus longirostris)[J]. Food Chemistry,2009, 112(1): 104-111. DOI:10.1016/j.foodchem.2008.05.061.
[8] MARTÍNEZ-ÁLVAREZ Ó, LÓPEZ-CABALLERO M E, MONTERO P,et al. A 4-hexylresorcinol-based formulation to prevent melanosis and microbial growth in chilled Tiger prawns (Marsupenaeus japonicus) from aquaculture[J]. Journal of Food Science, 2005, 70(9):M415-M422. DOI:10.1111/j.1365-2621.2005.tb08327.x.
[9] WILSON C O, GISVOLD O. Textbook of organic medicinal and pharmaceutical chemistry[M]. New York: Lippincott-Raven, 1956:237-262.
[10] FRANKOS V H, SCHMITT D F, HAWS L C, et al. Generally recognized as safe (GRAS) evaluation of 4-hexylresorcinol for use as a processing aid for prevention of melanosis in shrimp[J].Regulatory Toxicology and Pharmacology, 1991, 14(2): 202-212.DOI:10.1016/0273-2300(91)90007-I.
[11] FOSSATI A A N, BERGMANN G P, RIBEIRO L A O, et al. Effects of different additives on colorimetry and melanosis prevention of Atlantic seabob shrimp (Xyphopenaeus kroyeri) stored under refrigeration[J]. International Journal of Fisheries and Aquaculture,2016, 8(8): 74-80. DOI:10.5897/IJFA2016.0564.
[12] THEPNUAN R, BENJAKUL S, VISESSANGUAN W. Effect of pyrophosphate and 4-hexylresorcinol pretreatment on quality of refrigerated white shrimp (Litopenaeus vannamei) kept under modified atmosphere packaging[J]. Journal of Food Science, 2008, 73(3): 124-133. DOI:10.1111/j.1750-3841.2008.00674.x.
[13] MARTÍNEZ-ALVAREZ O, GÓMEZ-GUILLÉN C, MONTERO P.Effect of different chemical compounds as coadjutants of 4-hexylresorcinol on the appearance of deepwater pink shrimp(Parapenaeus longirostris) during chilled storage[J]. International Journal of Food Science and Technology, 2008, 43(11): 2010-2018.DOI:10.1111/j.1365-2621.2008.01810.x.
[14] MONTERO P, MARTÍNEZ-ÁLVAREZ O, GÓMEZ-GUILLÉN M.Effectiveness of onboard application of 4-hexylresorcinol in inhibiting melanosis in shrimp (Parapenaeus longirostris)[J]. Journal of Food Science, 2004, 69(8): 643-647. DOI:10.1111/j.1365-2621.2004.tb09913.x.
[15] LÓPEZ-CABALLERO M E, MARTÍNEZ-ALVAREZ O, GÓMEZGUILLÉN M C, et al. Quality of thawed deepwater pink shrimp(Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage[J]. International Journal of Food Science and Technology, 2007, 42(9): 1029-1038. DOI:10.1111/j.1365-2621.2006.01328.x.
[16] MUELLER J F, GOODMAN L S, GILMAN A. The pharmacological basis of therapeutics[M]. New York: The Macmillan Company, 1970: e2.[17] MONSALVE-GONZALEZ A, BARBOSA-CANOVAS G V,MCEVILY A J, et al. Inhibition of enzymatic browning in apple products by 4-hexylresorcinol[J]. Food Technology, 1995, 49(4): 110-118.
[18] European Commission. Opinion of the Scientific Committee on food on 4-hexylresorcinol[A/OL]. (2005-11-17)[2016-03-14]. http://europa.eu.int/comm/food/fs/sc/scf/out170_en.
[19] YI S M, ZHU J L, FU L L, et al. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane[J]. International Journal of Food Microbiology, 2010, 144(1): 111-117. DOI:10.1016/j.ijfoodmicro.2010.09.005.
[20] LI Y Q, HAN Q, FENG J L, et al. Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus[J]. Food Control, 2014, 43: 22-27.DOI:10.1016/j.foodcont.2014.02.023.
[21] ANANTA E, HEINZ V, KNORR D. Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry[J]. Food Microbiology, 2004, 21(5): 567-577. DOI:10.1016/j.fm.2003.11.008.
[22] BUNTHOF C J, ABEE T. Development of a flow cytometric method to analyze subpopulations of bacteria in probiotic products and dairy starters[J]. Applied and Environmental Microbiology, 2002, 68(6):2934-2942. DOI:10.1128/AEM.68.6.2934-2942.2002.
[23] QIAN Y F, XIE J, YANG S P, et al. Study of the quality changes and myofibrillar proteins of white shrimp (Litopenaeus vannamei) under modified atmosphere packaging with varying CO2levels[J]. European Food Research and Technology, 2013, 236(4): 629-635. DOI:10.1007/s00217-013-1918-9.
[24] QIAN Y F, YANG S P, XIE J, et al. Impact of the O2concentrations on bacterial communities and quality of modified atmosphere packaged Pacific white shrimp (Litopenaeus vannamei)[J]. Journal of Food Science,2013, 78(12): M1878-M1884. DOI:10.1111/1750-3841.12305.
[25] MACÉ S, JOFFRAUD J J, CARDINAL M, et al. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon(Salmo salar) fillets stored under modified atmosphere packaging[J].International Journal of Food Microbiology, 2013, 160(3): 227-238.DOI:10.1016/j.ijfoodmicro.2012.10.013.
[26] CHEN J, YAN W, SETTON L A. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus[J].European Spine Journal, 2006, 15(3): 303-311. DOI:10.1007/s00586-006-0088-x.
[27] SILVA F, FERREIRA S, QUEIROZ J A, et al. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry[J]. Journal of Medical Microbiology,2011, 60(10): 1479-1486. DOI:10.1099/jmm.0.034157-0.
[28] QIAN Y F, XIE J, YANG S P, et al. In vivo study of spoilage bacteria on polyphenoloxidase activity and melanosis of modified atmosphere packaged Pacific white shrimp[J]. Food Chemistry, 2014, 155(5): 126-131. DOI:10.1016/j.foodchem.2014.01.031.
[29] BURT S. Essential oils: their antibacterial properties and potential applications in foods: a review[J]. International Journal of Food Microbiology, 2004, 94(3): 223-253. DOI:10.1016/j.ijfoodmicro.2004.03.022.
[30] LAMBERT R J W, SKANDAMIS P N, COOTE P J, et al. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol[J]. Journal of Applied Microbiology, 2001, 91(3): 453-462. DOI:10.1046/j.1365-2672.2001.01428.x.
[31] LI C M, YU J P. Chemical composition, antimicrobial activity and mechanism of action of essential oil from the leaves of Macleaya Cordata (Willd.) R. Br[J]. Journal of Food Safety, 2015, 35(2): 227-236. DOI:10.1111/jfs.12175.
[32] MONTERO P, MARTÍNEZ-ÁLVAREZ O, ZAMORANO J P, et al.Melanosis inhibition and 4-hexylresorcinol residual levels in deepwater pink shrimp (Parapenaeus longirostris) following various treatments[J]. European Food Research and Technology, 2006, 223(1):16-21. DOI:10.1007/s00217-005-0080-4.
[33] ERKAN N, ÖZDEN Ö. Quality assessment of whole and gutted sardines (Sardina pilchardus) stored in ice[J]. International Journal of Food Science and Technology, 2008, 43(9): 1549-1559. DOI:10.1111/j.1365-2621.2007.01579.x.
[34] KIM J, MARSHALL M R, WEI C. Polyphenoloxidase[M]. New York: Marcel Dekker, 2000: 271-315.
[35] ADACHI K, ENDO H, WATANABE T, et al. Hemocyanin in the exoskeleton of crustaceans: enzymatic properties and immunolocalization[J]. Pigment Cell Research, 2005, 18(2): 136-143.DOI:0.1111/j.1600-0749.2005.00217.x.
[36] LIU H, JIRAVANICHPAISAL P, CERENIUS L, et al. Phenoloxidase is an important component of the defense against Aeromonas hydrophila infection in a crustacean, Pacifastacus leniusculus[J].Journal of Biological Chemistry, 2007, 282(46): 33593-33598.DOI:10.1074/jbc.M706113200.
[37] PANG Z G, KIM S K, YU J P, et al. Distinct regulation patterns of the two prophenoloxidase activating enzymes corresponding to bacteria challenge and their compensatory over expression feature in white shrimp (Litopenaeus vannamei)[J]. Fish and Shellfish Immunology,2014, 39(2): 158-167. DOI:10.1016/j.fsi.2014.04.026.
4-己基间苯二酚对凡纳滨对虾3 种腐败菌的抑菌活性及虾品质的影响
钱韻芳,杨胜平,谢 晶*
(上海海洋大学食品学院,上海水产品加工及贮藏工程技术研究中心,上海 201306)
为研究4-己基间苯二酚(4-hexylresorcinol,4-HR)的抑菌活性及对虾品质的影响,通过体外及接菌实验分别评价了其对来源于凡纳滨对虾的麦芽糖肉食杆菌(Carnobacterium maltaromaticum)、腐败希瓦氏菌(Shewanella putrefaciens)和杀鲑气单胞菌(Aeromonas salmonicida)的抑菌活性及其对凡纳滨对虾冷藏期间品质变化的影响。牛津杯法和抑菌生长曲线显示4-HR能够显著抑制这3 种腐败菌的生长。通过对比处理前后腐败希瓦氏菌的电导率和流式细胞术分析,发现其抑菌机理可能与增强细菌膜通透性有关。将0.25 mg/mL的4-HR溶液涂膜于凡纳滨对虾上,发现在冷藏期间(4 ℃)凡纳滨对虾的黑变程度较低而白度值较高,表明0.25 mg/mL 4-HR能有效抑制凡纳滨对虾的黑变。但涂膜4-HR的凡纳滨对虾适冷菌总数和挥发性盐基氮值仅在贮藏的前4 d低于未涂膜的对照组;而到贮藏终点时,涂膜4-HR的凡纳滨对虾适冷菌总数较对照组高0.14(lg(CFU/g))。结果表明尽管4-HR对3 种腐败菌具有良好的体外抑菌活性,但在凡纳滨对虾贮藏过程中4-HR优先作为防黑变剂抑制对虾黑变。
凡纳滨对虾;4-己基间苯二酚;抑菌活性;腐败希瓦氏菌;杀鲑气单胞菌;麦芽糖肉食杆菌
TS254.4
A
1002-6630(2017)21-0021-09
2016-08-19
国家自然科学基金青年科学基金项目(31501551);上海市科委重点项目(14dz1205101);上海市高校青年教师培养资助计划项目(ZZZZhy15010);上海海洋大学博士科研启动基金项目(A2-0302-14-300076)
钱韻芳(1985—),女,讲师,博士,研究方向为水产品加工及贮藏工程。E-mail:yfqian@shou.edu.cn
QIAN Yunfang, YANG Shengping, XIE Jing. Antibacterial activity of 4-hexylresorcinol against three spoilage bacteria in culture and its effect on the quality of Pacific white shrimp[J]. 食品科学, 2017, 38(21): 21-29. (in English with Chinese abstract)
10.7506/spkx1002-6630-201721004. http://www.spkx.net.cn
10.7506/spkx1002-6630-201721004
*通信作者:谢晶(1968—),女,教授,博士,研究方向为食品冷冻冷藏。E-mail:jxie@shou.edu.cn
QIAN Yunfang, YANG Shengping, XIE Jing. Antibacterial activity of 4-hexylresorcinol against three spoilage bacteria in culture and its effect on the quality of Pacific white shrimp[J]. Food Science, 2017, 38(21): 21-29. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201721004. http://www.spkx.net.cn

