Research Progress of Genomes of Insect Pests in Paddy Field
Xu Hongxing, Zhao Xianxin, Lü Zhongxian, Li Fei
Letter
Research Progress of Genomes of Insect Pests in Paddy Field
Xu Hongxing1, Zhao Xianxin2, Lü Zhongxian1, Li Fei2
(State Key Laboratory for Managing Biotic and Chemical Treats to the Quality and Safety of Agroproducts, Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China; State Key Laboratory of Rice Biology and Breeding / Ministry of Agricultural and Rural Affairs Key Laboratory of Molecular Biology of Crop Pathogens and Insect Pests, Institute of Insect Sciences, Zhejiang University, Hangzhou 310058, China)
Innovations in sequencing technology and the development of bioinformatics have allowed for studies of the genomics of many rice pests. At present, draft genomes of rice pests including,,,,,,andhave been completed. The molecular mechanisms of biological characteristics of rice pests are gradually becoming clear with the help of genomics. We summarized the important progress in determining genome size of rice pests and the role of genomics in their biological characteristics as well as prospects for future research on rice pests combined with the development of omics.
Insects are the most abundant species on earth, which include insects that are beneficial to human life, such as bees, silkworms and ladybugs, and numerous pests that endanger food security. Rice, an important food crop globally, suffers from multiple pests. According to a survey about global burden of pathogens and pests on major food crops, global rice yield loss caused by pests and diseases reaches 24.6%–40.9% (Savary et al, 2019). With globalization, these pests and pathogens have spread to all parts of the world, and have had a negative impact on agricultural production. According to the method of feeding, the pests can be divided into four categories: borer pests [e.g., purple stem borer(Lepidoptera: Noctuidae), striped rice borer(Lepidoptera: Crambidae), yellow rice borer(Lepidoptera: Crambidae)], stinging and sucking pests [e.g., brown planthopper (BPH)(Homoptera: Delphacidae), small brown planthopper (SBPH)(Homoptera: Delphacidae), white-backed planthopper (WBPH)(Homoptera: Delphacidae)], root eating pests [e.g., rice water weevil(Coleoptera: Erirhinidae)], and leaf eating pests [e.g., rice leaffolder(Lepidoptera: Crambidae), rice leaf roller(Lepidoptera: Crambidae)]. A comprehensive and in-depth understanding of the genetic information of rice pests can help researchers understand their biological characteristics more clearly.
With the rapid development of high-throughput sequencing technology, a large amount of omics data have been generated. At present, hundreds of insect genomes have been sequenced, and more sequencing projects have been proposed and initiated with the wide use of genomics and other omics in contemporary insect research. For example, the Darwin tree of life project aims to sequence, assemble and annotate the genomes of all 70 000 eukaryotic species found in the UK and Ireland (Darwin Tree of Life Project Consortium, 2022). In 2018, the ‘Top 1000 Insect Genome Project’ was jointly launched by the Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences and multiple other Chinese scientific research institutions, including Chinese Academy of Sciences, Zhejiang University, China Agricultural University, Southwest University and other units, aiming to sequence and complete genome mapping to obtain high-quality reference genomes. With the advancement of large-scale sequencing plans and projects, the mining and analysis of insect genetic information will be more deeply. High- throughput sequencing technology can provide a good data foundation to prevention and control of pests, which fills the gap of species genetic information and interprets their habits based on molecular characteristics.Genome analysis of rice pests is still at the initial stages, and there are only eight rice pests with sequenced genomes (Table 1). Therefore, the research on rice pest genomes has broad prospects.
Rice planthopper, the most serious pest in rice, harms rice and other crops by sucking plant sap (Muduli et al, 2021). In China, BPH is the most serious rice planthopper, followed by WBPH and SBPH (Liu et al, 2016). BPH is an important migratory pest in rice, with wing dimorphism, and its genome was first sequenced in 2014. In the first version of genome, Xue et al (2014) sequenced the whole genome of 13th generations of BPH purified by single mating, and finally obtained a 1.14-Gb assembled genome. The scaffold N50 was 356.6 kb, and the gene set of 27 571 protein coding genes was annotated by genome annotation. This version of the genome is characterized by high content of repetitive sequences (48.6%) and many species-specific genes (59.2%). The results of comparative genomic analysis indicated that the characteristics of BPH feeding on rice alone may be related to the contraction of gene families of olfactory receptors and gustatory receptors (Xue et al, 2014). Furthermore, based on genomic data, the wing differentiation of BPH is closely related to rice nutrition. When rice nutrition is low, genes related to wing development increase in BPH.
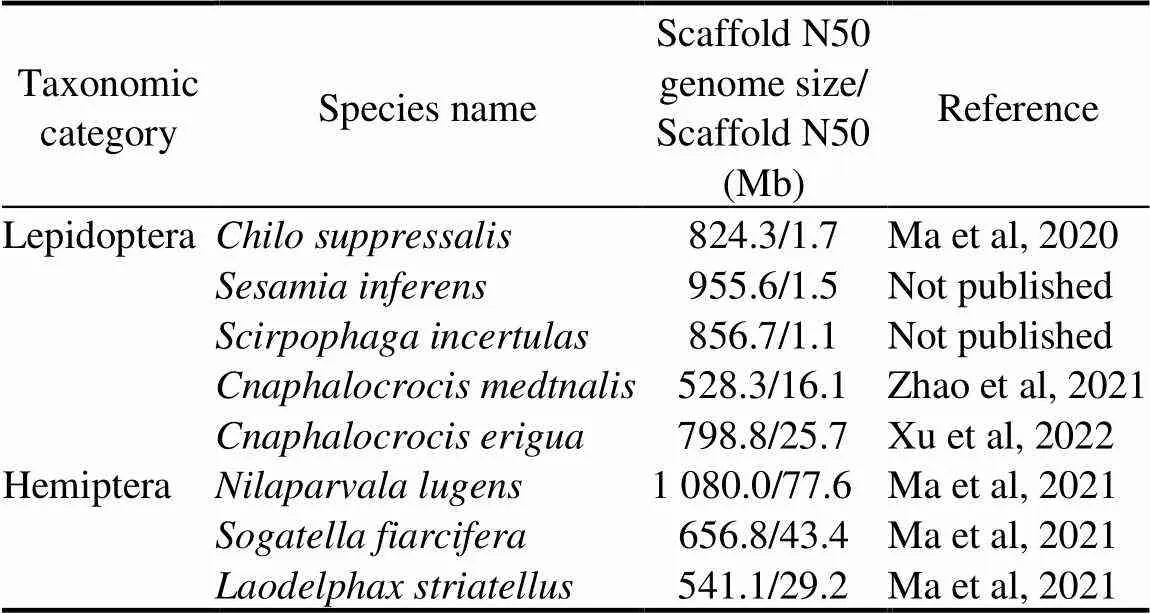
Table 1. Summarize of rice pest genomes.
The migration of BPH is mainly completed by long-wing form adults. Therefore, identifying the key regulatory genes or pathways of wing differentiation is necessary for effective control of BPH. The insulin receptor gene can control the wing type differentiation of BPH under the regulation of, and there are two highly homologous insulin receptor genes,and, in BPH. Among them,is the target gene of. When the expression ofis low, the content of NlInR1 in BPH is high, the insulin signal transduction pathway is opened, and BPH develops into the long-wing form. When the expression ofis increased, NlInR1 is inhibited, the content of NlInR2 is increased, the signal transduction pathway is closed, and the BPH develops into the short-wing form (Xu et al, 2015; Ye et al, 2019). With the gradual increase of extensive resequencing, the understanding of the migratory route of BPH will be improved, and a basis will be provided for monitoring the identification of different hazard levels caused by BPH, to reasonably control it (Luo et al, 2022).
In 2017, sequencing and assembly of WBPH and SBPH genomes were completed (Wang et al, 2017; Zhu et al, 2017). The sequencing strategy of the WBPH genome is similar to that of BPH. The selected sample was a pair of WBPH populations purified for 18th generations. Through Illumina sequencing, a 720.7-Mb genome assembly of WBPH was obtained. The scaffold N50 is 1 185 kb, which is about three times higher than that of the BPH genome. A total of 21 254 protein coding genes were annotated, and a high proportion of multi-copy genes existed. In addition, the differentially expressed genes and their expression patterns in WBPH at different developmental stages were also systematically analyzed (Wang et al, 2017).
To eliminate the high heterozygosity of the genome, the 22nd generation offsprings incubated from a single pair of adults were selected for sequencing of SBPH genome. Sequencing and assembly were done using the strategy of combining the second generation with the third generation, resulting in an SBPH genome with a size of 540.9 Mb and a scaffold N50 of 1 085 kb. A total of 17 736 protein coding genes were annotated (Zhu et al, 2017). In 2020, chromosome level genomes of three species of planthopper were released publicly. Pac-bio third generation sequencing and high-throughput chromosome conformation capture (Hi-C) assisted assembly technology were used to optimize and upgrade the genomes of the three species of planthopper. In addition, using the resequencing data of male and female insects, the X chromosomes of the three species of planthoppers were successfully identified (Ma et al, 2021).
Rice stem borer is also an important pest that endangers rice production, of whichandare the most serious pests (Xiang et al, 2011). The larvae ofdrill into the rice stems for feeding and damage, resulting in withered young leaves, sheath and spikelet (Zheng, 2020; Gavara et al, 2021). After eating rice,induces rice to release high concentrations of volatile substances such asa-pinene and 2-heptanol, which attract BPH to feed and lay eggs. When BPH andharm rice together, it can significantly inhibit the rice defense response induced by. Jasmonic acid and other defense related genes are then down-regulated, and the content of protease inhibitors is also significantly reduced, completely eliminating the negative impact on the fitness of subsequentlarvae (Wang et al, 2018). Althoughis not as serious as, it also brings great loss to rice production in China (Sheng et al, 2003).is not the main pest in China, but with the change of farming methods, the occurrence of the giant borer has an upward trend in many regions of China, and the damage level has also risen in recent years (Li, 2018).
Liu et al (2014)reported the genome of, with a genome size of 824 Mb and a scaffold N50 of 5.2 kbGenome annotation was performed using the Optimized Marker- based Insect Genome Annotation platform, and 10 211 protein coding genes were obtained. At the same time, core genes related to the cytochrome P450, odor binding protein, and chemoreceptor genes as well as the RNAi metabolic pathway were systematically identified in thegenome. These data will provide references for studying the damage habits, drug resistance mechanism, growth and development ofSubsequently, in 2020, a team at Huazhong Agricultural University published the chromosome level genome of(Ma et al, 2020). This is the first time that a genome draft was constructed and obtained using Illumina combined with Pac-bio sequencing, and 99.2% of the bases were allocated to 29 chromosomes using Hi-C assisted assembly technology. Finally, a chromosome level genome assembly with a size of 824.3 Mb was obtained, and the scaffold N50 reached 1.7 Mb. Homology analysis of gene families related to animal cold tolerance revealed the genomic basis of cold tolerance of, and combined with transcriptome data revealed the expression of 14 cold tolerance related genes inunder different treatment conditions (including non-diapause, low-temperature diapause, low-temperature domestication and non-low-temperature domestication). These genes are involved in cold tolerance strategies mediated by glucose derived glycerol biosynthesis, triacylglycerol derived glycerol biosynthesis, fatty acid synthesis and trehalose transport (Ma et al, 2020).
The genomes ofandhave also been sequenced (data not published). Completed genomes of the three main rice borers help researchers to study their adaptability to the environment and the interaction through comparative genomics analysis in the future, providing a theoretical basis for the control of rice borers.
Rice leaf folder,,is an important agricultural pest with migratory habits. Its larvae damage the pale white stripes of the leaves of gramineous plants by folding the leaves and scraping the mesophyll inside, which has caused serious loss to rice planting areas with high temperature and humidity all over the world (Zhang et al, 1980; Yang et al, 2015)., a close relative of, is also widely distributed in rice planting areas of different countries. It has a very similar morphological phenotype and leaf rolling feeding behavior to. However, compared with the seasonal migration of,, another species of rice leaf folder, can overwinter in the rice regions of south and southwest China. Through different hybrid strategies combined with corresponding sequencing technologies, including Illumina, Pac-bio, 10× Genomics and Hi-C, genome assemblies ofandhave been completed with high quality, providing a basis for further understanding of the biological characteristics and ecological adaptability of the two pests (Zhao et al, 2021; Xu et al, 2022).
The size difference betweenandgenomes is about 270 Mb, and high-quality genome assembly at the level of two chromosomes is generated by Hi-C assisted assembly technology. Among them, 3 248 scaffolds ofare successfully attached to 31 chromosomes, while 1 413 scaffolds ofare anchored to 32 chromosomes. Through the comparison and detection of the benchmarking universal single copy orthologs, the two genome assemblies show a high percentage (> 95%) of matching results, indicating that the genome assembly has high integrity. At the same time, using the resequencing data of male and female pupae, the sex chromosomes ofandare successfully identified. Finally, through the same gene annotation process, 15 765 protein coding genes ofand 14 922 protein coding genes ofare obtained. In addition, the repetitive sequences ofaccount for more than half (52%) of the genome size, and the transposon sequence has an amplification. Compared with the repetitive sequences of(39.5%), this may be related to the difference in genome size (Zhao et al, 2021; Xu et al, 2022).
In addition, phylogenetic and comparative genomics analyses show that although the biological characteristics of theandare similar and their genomes have high homology, they were differentiated into two species as early as 18 million years ago, and there are still differences in their genomic characteristics, gene family expansion and contraction, and positive selection pressure. For example, genes related to cold tolerance and resistance to exogenous infection show positive selection characteristics in. In, the signals of adaptive evolution mainly include genes related to energy storage, which may be adapted to the long-distance migration ability of the species (Zhao et al, 2021; Xu et al, 2022). Chromosome level genome assembly and comparative genome analysis ofandare helpful to clarify the genetic and molecular mechanisms of long-distance migration,toxin resistance, cold tolerance, sex determination, and the unique evolutionary trajectories of the two closely related insect species. These assemblies provide resources and new insights for studying the evolution and ecology of rice pests, which is beneficial to assist the management and implementation of relevant pest control.
With the improvement of sequencing and assembly technology, genomic data has been better applied in pest control. Sequencing and assembly technology always has data polymorphism. It is difficult to culture insects with homozygous genes and the individual size is small, so it is impossible to collect high- quality DNA samples. This combination of factors makes it difficult to easily assemble insect genomes (Rong et al, 2016). Using Nanopore and short-read sequencing, Ye et al (2020) completed the genome assembly of the cocoon peak of wheat moth with an ultra-low starting amount (20 ng DNA), which provides a good example for the genome assembly of species with small individuals, rare samples and only a small amount of DNA. In addition, with the rapid development of artificial intelligence (AI), the analysis of various omics will also enter a more intelligent stage, with research conclusions becoming more reliable with the optimization of training sets and algorithms. In addition, there are some other omics methods, such as metabolomics, the ‘chemical fingerprint’ of living cell metabolism, and transcriptome analysis methods such as full-length transcriptome, spatial transcriptome and single-cell transcriptome. In the future, the development and optimization of the AI image data acquisition system of insect pest control facilities is likely to promote the development of radiomics for insects. Moreover, a series of functionally targeted databases have also become favorable tools for researchers, such as the image database for agricultural diseases and pests.
Integrated pest management (IPM) has been the top priority in modern agricultural production. In recent decades, the pest species in China have increased significantly, and the degree of harm has become more serious. At the same time, with the development of global trade, the invasion of pests has become an extremely serious problem. With the mining of genomic data, IPM omics was proposed. IPM strategies, combined with genome technology, can be used to discover polymorphism in insect populations, and then explain the important problems of insect community changes. For example, rice insect-resistant genes are mainly distributed on several chromosomes, and BPH can inhibit the defense response of rice by salivary protein in saliva. The discovery of these mechanisms can help us select more resistant rice varieties and improve the control effect of BPH (Xu et al, 2021). Through the analysis of genomic data, the molecular mechanisms of pest species and drug resistance will be revealed. These molecular biological characteristics can be used to develop detection kits to achieve rapid detection of quarantine pests. Molecular markers designed for resistance genes can easily and quickly monitor the resistance frequency of pests in the field (Xiao et al, 2019). After comprehensive comparison of the obtained information with other existing data, the best pest management scheme can be formulated using the results and existing knowledge. Finally, information and communication technology can be used to effectively spread and develop strategies for broad pest control (Margam et al, 2011). Peng et al (2015) have proposed pest management strategies based on genomics, such as optimizing pest management structure in combination with landscape genetics, research on molecular identification, species identification and food web reconstruction based on DNA barcode technology, pest control through the establishment of a complete RNA interference technology system, and pest control supported by insect transgenic technology.
Current genomics research focuses on single species, but each organism in the ecosystem does not exist independently. It is thus difficult to have a comprehensive understanding of a species only by studying its genome. In the future, numerous insect genomes will be sequenced and assembled, providing a lot of new information. Based on comparative genomic analysis, the genetic sequence information of some important pest related species and their natural enemies may also provide data for solving the evolution and mutual restraint relationships of species. Based on multi-omics data, through the research and mining of gene functions and the application of CRISPR/Cas9- mediated genome site-specific editing and other technical means, there will be revolutionary changes in the control of pests and the utilization of resource insects.
ACKNOWLEDGEMENTS
This study was supported by the Zhejiang Provincial Key Research and Development Plan, China (Grant Nos. 2020C02001 and 2022C02034), and the National Natural Science Foundation of China (Grant No. 31672022).
Darwin Tree of Life Project Consortium. 2022. Sequence locally, think globally: The Darwin tree of life project., 119(4): e2115642118.
Gavara A, Vacas S, Primo J, Navarro-Llopis V. 2021. Mating disruption of striped rice stem borer: Importance of early deployment of dispensers and impact on airborne pheromone concentration., 28(6): 525–528.
Li Q C. 2018. Present situation and control measures of corn borer in northern Henan., 6(12): 84–85. (in Chinese with English abstract)
Liu J D, Xiao H M, Huang S Q, Li F. 2014. OMIGA: Optimized marker-based insect genome annotation., 289(4): 567–573.
Liu W C, Liu Z D, Huang C, Lu M H, Liu J, Yang Q P. 2016. Statistics and analysis of crop yield losses caused by main diseases and insect pests in recent 10 years., 42(5): 1–9.
Luo J, Tang J, Wang A Y, Yang B J, Liu S H. 2022. A rapid detection assay ofbased on recombinase aided amplification-lateral flow dipstick technology., 36(1): 96–104. (in Chinese with English abstract)
Ma W H, Zhao X X, Yin C L, Jiang F, Du X Y, Chen T Y, Zhang Q H, Qiu L, Xu H X, Joe Hull J, Li G L, Sung W K, Li F, Lin Y J. 2020. A chromosome-level genome assembly reveals the genetic basis of cold tolerance in a notorious rice insect pest,., 20(1): 268–282.
Ma W H, Xu L, Hua H X, Chen M Y, Guo M J, He K, Zhao J, Li F. 2021. Chromosomal-level genomes of three rice planthoppers provide new insights into sex chromosome evolution., 21(1): 226–237.
Margam V M, Coates B S, Hellmich R L, Agunbiade T, Seufferheld M J, Sun W L, Ba M N, Sanon A, Binso-Dabire C L, Baoua I, Ishiyaku M F, Covas F G, Srinivasan R, Armstrong J, Murdock L L, Pittendrigh B R. 2011. Mitochondrial genome sequence and expression profiling for the legume pod borer(Lepidoptera: Crambidae)., 6(2): e16444.
Muduli L, Pradhan S K, Mishra A, Bastia D N, Samal K C, Agrawal P K, Dash M. 2021. Understanding brown planthopper resistance in rice: Genetics, biochemical and molecular breeding approaches., 28(6): 532–546.
Peng L, He W Y, Xia X F, Xie M, Ke F S, You S J, Huang Y P, You M S. 2015. Prospects for the management of insect pests in the genomic era., 52(1): 1–22. (in Chinese with English abstract)
Rong C H, Li W D, Wang S J, Shen X Z. 2016. Integrated pest management in the era of gene: A review of ‘IPM-Omics’., 45(2): 159–163. (in Chinese with English abstract)
Savary S, Willocquet L, Pethybridge S J, Esker P, McRoberts N, Nelson A. 2019. The global burden of pathogens and pests on major food crops., 3(3): 430–439.
Sheng C F, Wang H T, Gao L D, Xuan W J. 2003. Current situation, loss estimation and control countermeasures of rice borer in China., 29(1): 37–39. (in Chinese)
Wang L, Tang N, Gao X L, Chang Z X, Zhang L Q, Zhou G H, Guo D Y, Zeng Z, Li W J, Akinyemi I A, Yang H M, Wu Q F. 2017. Genome sequence of a rice pest, the white-backed planthopper ()., 6(1): 1–9.
Wang X Y, Liu Q S, Meissle M, Peng Y F, Wu K M, Romeis J, Li Y H. 2018.rice could provide ecological resistance against nontarget planthoppers., 16(10): 1748–1755.
Xiang Y Y, Zhang F, Xia B W, Guo R. 2011. Occurrence and control of rice borers in China., 31(11): 20–23. (in Chinese)
Xiao Y T, Wu C, Wu K M. 2019. Agricultural pest control in China over the past 70 years: Achievements and future prospects., 56(6): 1115–1124. (in Chinese with English abstract)
Xu H J, Xue J, Lu B, Zhang X C, Zhuo J C, He S F, Ma X F, Jiang Y Q, Fan H W, Xu J Y, Ye Y X, Pan P L, Li Q, Bao Y Y, Nijhout H F, Zhang C X. 2015. Two insulin receptors determine alternative wing morphs in planthoppers., 519: 464–467.
Xu H X, Zhao X X, Yang Y J, Chen X, Mei Y, He K, Xu L, Ye X H, Liu Y, Li F, Lu Z X. 2022. Chromosome-level genome assembly of an agricultural pest, the rice leaffolder(Crambidae, Lepidoptera)., 22(1): 307–318.
Xu X R, Tang M, Chen L. 2021. Research progress on the interaction between rice and brown planthopper,., 40(4): 1574–1583.
Xue J, Zhou X, Zhang C X, Yu L L, Fan H W, Wang Z, Xu H J, XiY, Zhu Z R, Zhou W W, Pan P L, Li B L, Colbourne J K, Noda H, Suetsugu Y, Kobayashi T, Zheng Y, Liu S L, Zhang R, Liu Y, Luo Y D, Fang D M, Chen Y, Zhan D L, Lv X D, Cai Y, Wang Z B, Huang H J, Cheng R L, Zhang X C, Lou Y H, Yu B, Zhuo J C, Ye Y X, Zhang W Q, Shen Z C, Yang H M, Wang J, Wang J, Bao Y Y, Cheng J A. 2014. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation., 15(12): 521.
Yang Y J, Xu H X, Zheng X S, Tian J C, Lu Y H, Lü Z X. 2015. Progresses in management technology of rice leafrollers in China., 42(5): 691–701. (in Chinese with English abstract)
Ye X H, Xu L, Li X, He K, Hua H X, Cao Z H, Xu J D, Ye W Y, Zhang J, Yuan Z T, Li F. 2019. miR-34 modulates wing polyphenism in planthopper., 15(6): e1008235.
Ye X H, Yan Z C, Yang Y, Xiao S, Chen L F, Wang J L, Wang F, Xiong S J, Mei Y, Wang F, Yao H W, Song Q S, Li F, Fang Q, Werren J H, Ye G Y. 2020. A chromosome-level genome assembly of the parasitoid wasp., 20(5): 1384–1402.
Zhang X X, Lu Z Q, Geng J G, Li G Z, Chen X L, Wu X W. 1980. Study on the migration route ofGuenee., 23(2): 130–140. (in Chinese with English abstract)
Zhao X X, Xu H X, He K, Shi Z M, Chen X, Ye X H, Mei Y, Yang Y J, Li M Z, Gao L B, Xu L, Xiao H M, Liu Y, Lu Z X, Li F. 2021. A chromosome-level genome assembly of rice leaffolder,., 21(2): 561–572.
Zheng W Y. 2020. Damage characteristics and control measures of., 13(10): 33–35. (in Chinese with English abstract)
Zhu J J, Jiang F, Wang X H, Yang P C, Bao Y Y, Zhao W, Wang W, Lu H, Wang Q S, Cui N, Li J, Chen X F, Luo L, Yu J T, Kang L, Cui F. 2017. Genome sequence of the small brown planthopper,., 6(12): 1–12.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.03.013
Lü Zhongxian (luzxmh@163.com);
Li Fei (lifei18@zju.edu.cn)
4 January 2023;
9 March 2023
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa

