Grain Shape Genes: Shaping the Future of Rice Breeding
Lu Xuedan, Li Fan, Xiao Yunhua, Wang Feng, Zhang Guilian, Deng Huabing, Tang Wenbang
Review
Grain Shape Genes: Shaping the Future of Rice Breeding
Lu Xuedan1, 2, Li Fan1, Xiao Yunhua1, 2, Wang Feng1, 2, Zhang Guilian1, 2, Deng Huabing1, 2, Tang Wenbang3, 4
(College of Agronomy, Hunan Agricultural University, Changsha 410128, China;Hunan Provincial Key Laboratory of Rice and Rapeseed Breeding for Disease Resistance, Changsha 410128, China; State Key Laboratory of Hybrid Rice, Changsha 410125, China; Hunan Hybrid Rice Research Center, Hunan Academy of Agricultural Science, Changsha 410125, China)
The main goals of rice breeding nowadays include increasing yield, improving grain quality, and promoting complete mechanized production to save labor costs. Rice grain shape, specified by three dimensions, including grain length, width and thickness, has a more precise meaning than grain size, contributing to grain appearance quality as well as grain weight and thus yield. Furthermore, the divergence of grain shape characters could be utilized in mechanical seed sorting in hybrid rice breeding systems, which has been succeeded in utilizing heterosis to achieve substantial increase in rice yield in the past decades. Several signaling pathways that regulate rice grain shape have been elucidated, including G protein signaling, ubiquitination-related pathway, mitogen-activated protein kinase signaling, phytohormone biosynthesis and signaling, microRNA process, and some other transcriptional regulatory pathways and regulators. This review summarized the recent progress on molecular mechanisms underlying rice grain shape determination and the potential of major genes in future breeding applications.
grain shape; grain quality; yield; mechanization of seed sorting; hybrid rice; molecular function; regulatory pathway
Rice is the staple food for more than half of the world’s population, and rice yield must be increased to meet the food demand due to an increasing population. Rice yield per plant is determined by the multiplicative interaction of three main components: panicle number per plant, grain number per panicle and 1000-grain weight (Sakamoto and Matsuoka, 2008). Among them, 1000-grain weight is contributed by grain shape and grain filling. Notably, grain shape was specified by three dimensions, including grain length (GL), grain width (GW) and grain thickness (GT).
The grain shape is determined by the shape of the glumes, which are derived from diploid maternal plants (Li and Li, 2016; Li N et al, 2019). The glumes are consisted of one lemma, one palea and two lodicules.With the progress of grain-filling, the glumes gradually dehydrate to form the spikelet hull. As many other monocot plants, rice grain grows inside the spikelet hull (the husk). The spikelet hull sets the volume of the cavity, within which the embryo and the endospermdevelop, and determines the final grain shape. Therefore, grain shape is controlled greatly by the maternal plant genotype, while the zygotic genotype also contributes a little. Grain growth can theoretically be stimulated by changes in cell elongation, cell expansion, cell division, or differentiation in spikelet hulls.
Rice grain quality and commodity value are also affected by the grain shape (Harberd, 2015). Additionally, rice grain appearance caters to the preferences of different consumers to a certain extent. For example, long and slender grain varieties are preferred by consumers in the United States, Western Europe and most Asian countries including China, India, Pakistan and Thailand, while short and bold grains are preferred by consumers in Japan, South Korea and Sri Lanka (Unnevehr et al, 1992). Despite the costumers’ preference, grain shape influences greatly on the endosperm chalkiness and eating and cooking quality (ECQ) (Tan et al, 2000). Chalkiness is a key parameter that can negatively affect rice milling quality, appearance quality and ECQ (Butardo et al, 2019). It is worth noting that grain shape affects the formation of chalky grain. Generally, slender rice grains with a large length-to-width ratio will decrease the chalkiness degree (Liu et al, 2018; Zhao et al, 2018). Overall, grain shape plays a vital role in the determination of rice grain quality (Li et al, 2022).
Furthermore, the grain shape characters have great potential to be utilized in the mechanization of hybrid rice seed production. Hybrid rice takes advantage of heterosis (hybrid vigor), and is a great achievement in agriculture, with a yield of 20%–30% higher than conventional rice (Ma and Yuan, 2015). Nowadays, there are two main kinds of mechanized seed production technology of hybrid rice in China. The separate planting method is widely adopted. The restorer lines and sterile lines are planted separately in different rows, and then hybrid seeds are mechanically harvested after the harvest of restorer self-pollinated seeds by hand. In contrast, the other production method is to mix the sowing of sterile and restorer lines, and then harvest together. Based on the great differences in grain shape between the female and male parents (Tang et al, 2020), mechanical separation is more convenient and labor-saving, contributing to the realization of the whole process mechanization of hybrid seed production (Zhou et al, 2022).
Many efforts have been devoted to mine key QTLs/ genes/alleles for grain shape, revealing the regulatory mechanism of grain shape and the utilization of the beneficial alleles. A considerable number of QTLs and genes have been identified (Li and Li, 2016; Li N et al, 2019). The precise molecular regulation of grain shape entails the G protein signaling pathway, the ubiquitination-related pathway, the mitogen-activated protein kinase (MAPK) signalingpathway, microRNA action, phytohormone homeostasisand signaling, and some other transcriptional regulatorypathways and regulators (Li and Li, 2016; Chen K et al, 2021). In this study, recent research progress on the molecular mechanisms underlying rice grain shape control is reviewed. Furthermore, this review summarized the preliminary attempts of rice breeding in recent years based on the application value of major genes in improving yield, rice quality and mechanized seed production of hybrid rice.
Molecular mechanisms underlying regulation of grain shape
G protein signaling pathway involved in grain shape determination
G proteins are key regulators of a multitude of signaling pathways in all eukaryotes (Ofoe, 2021). The G protein complex consists of three different subunits, Gα, Gβ and Gγ. The rice genome encodes one Gα subunit (RGA1), one Gβ subunit (RGB1), and five Gγ subunits (GS3, DEP1, GGC2, RGG1 and RGG2) (Table 1) (Duan and Li, 2021).
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
All the G protein subunits have been shown to regulate rice grain shape. Loss-of-function ofresults in small seeds, especially leading to dramatic decrease of grain length (Oki et al, 2005, 2009). In addition to,acts as a positive regulator of cellular proliferation, and knock-down ofin the background of(loss-of-function mutant of) causes shorter grains thanitself (Utsunomiya et al, 2011).positively controls grain length, width, thickness and weight through regulating auxin biosynthesis in rice endosperm (Zhang D P et al, 2021). Besides functioning together with RGA1, RGB1 forms a complex with atypical extra-large GTP-binding proteins,which also regulate grain length and weight (Biswal et al, 2022).
, encoding one atypical Gγ subunit, is the first reported major QTL for grain length and weight and also a minor QTL for grain width and thickness in rice (Fan et al, 2006). This tissue- and stage-specific expression ofsuggests that it regulates seed size through control of ovule development (Takano-Kai et al, 2009). There are at least four natural variants of, showing differences in the length of the C-terminal cysteine-rich domain (as ‘tail’ hereafter) and the grain length (Mao et al, 2010).allele, from the rice variety Zhenshan 97, encoding a protein with 231 aa (amino acids) in length, is probably the prototype for the majority of widely grownvarieties with medium grains.allele, from the rice variety Nipponbare (NPB, medium grain) with a 3-bp insertion, resulting in an in-frame addition of a serine residue, is the genotype for the most of medium grainvarieties.allele from ancultivar Minghui 63 large grainhas a C to A substitution at 165 bp, causing a premature termination and encoding a 55-aa protein, lacking the intact OSR (Organ Size Regulation) domain and the C-terminal tail. It contributes to the increases of grain length and thickness, and the decrease of grain width (Fan et al, 2006, 2009; Takano-Kai et al, 2009; Mao et al, 2010).allele is associated with enhanced grain length inbut not otherspecies. Haplotype analysis further indicated thatallele arises in aancestor and moves into thegroup through introgressive hybridization.allele, from ancultivar Chuan 7 small grain encoding a truncated protein with OSR domain but lacking the C-terminal tail, contributes to very short grains, which is extremely rare in rice germplasm collections. Variedgenotypes correspond to different grain lengths, which show that it is in the process of natural selection and artificial selection.
The OSR domain of GS3 is both necessary and sufficient for negatively regulating grain length formation, demonstrated by that theallele from Minghui 63 causes the absence of OSR domain, leading to alleviation of inhibitory effect of OSR and thus relatively long grains (Fan et al, 2006, 2009; Takano-Kai et al, 2009; Mao et al, 2010). Interestingly, its C-terminal TNFR/NGFR and VWFC domains exhibit an inhibitory effect on the OSR function, and the loss-of-function of these domains results very short grains, as demonstrated by the function ofallele (Mao et al, 2010). Moreover, the GS3-4 protein is more stable than GS3-1 protein, indicating that the C-terminal tail of GS3 is necessary for degradation of the protein (Sun et al, 2018). Overall,functions as a negative regulator for grain length-to-width ratio, grain thickness and grain weight (Fan et al, 2006, 2009; Takano-Kai et al, 2009; Mao et al, 2010).
Another atypical Gγ subunit DEP1is pleiotypically responsible for dense panicle, high grain number per panicle and erect panicle (Huang et al, 2009). Zhou et al(2009) also identifiedas a major gene, regulating the panicle erectness (PE) type of inflorescence. The dominate allele, with a premature stop codon and consequently a loss of 230 residues from the C terminus, confers PE type, increases nitrogen use efficiency, but reduces grain length formation (Sun et al, 2014; Sun et al, 2018). Theallele may have arisen from a naturally occurring mutation and was exploited due to its preferred phenotype by high-yieldingrice breeding programs (Huang et al, 2009; Zhou et al, 2009). Similar with GS3, DEP1 protein contains an N-terminal GGL domain, a putative transmembrane domain, and a cysteine-rich C-terminus with three VWFC (von Willebrand Factor Type C) domains (Li X B et al, 2019). DEP1 with a long tail of 305 aa in length produces long and heavy grains, while GGL domain alone or short-tailedproduces short and light grains (Li X B et al, 2019). It was indicated that the two or three C-terminal VWFC domains effectively repress the negative effects of the GGL domain on grain length formation. Manipulation of the C-terminal length of DEP1 could therefore be useful to generate varieties with various desired grain lengths according to different requirements in rice breeding.
Moreover, another geneencoding an atypical Gγ protein acts as a positive regulator of grain length additively with, butrepresses the effects ofandon grain size (Sun et al, 2018). The effects of,andon regulating grain length are dependent onand(Sun et al, 2018). GS3-1, DEP1 and GGC2 interact with RGB1 through the OSR domain. However, the RGB1- DEP1 or GGC2-RGB1 interaction was suppressed by GS3-1, suggesting a competition between GS3 and DEP1 or GGC2 in binding with RGB1. RGB1-DEP1 and RGB1-GGC2 dimers promote the formation of grain length, while occupation of RGB1 by the GS3 protein disrupts the dimerization of GS3 protein caused by deletion of C-terminal tail inmutant, and results in very short grains (Sun et al, 2018).
Furthermore,(), encoding a MADS-domain transcription factor, acts downstream of the G protein βγ dimmers (Liu et al, 2018). GS3 and DEP1 can interact directly with OsMADS1/OsLG3b and act as cofactors to enhance OsMADS1 transcriptional activity to promote the co-operative transactivation of common target genes, thereby regulating grain shape (Liu et al, 2018). Biochemical analyses further demonstrated that GGC2, RGG1, RGG2 and RGB1 interact with OsMADS1 (Liu et al, 2018). RGB1-DEP1 (Gβγ dimer) interacts directly with OsMADS1/OsLG3b and then promotes the co-operative transactivation of common target genes, thereby regulating grain shape (Liu et al, 2018). Thus, the RGB1-DEP1-GS3-OsMADS1/OsLG3b module plays a key regulatory role in G protein signaling pathway to determine the grain shape. Haplotype analyses revealed that theOsMADS1haplotype is common withinaccessions andgermplasm, but is rare within the eliteandrice varieties (Liu et al, 2018). Another haplotypeOsLG3Bmight have arisen after domestication ofand spread to subspeciesorby natural crossing and artificial selection (Yu et al, 2018). Both of theOsMADS1andOsLG3Balleles encode the same alternatively spliced form of OsMADS1, which is associated with the grain length and thickness (slender grains) with improved grain quality (Liu et al, 2018; Yu et al, 2018). PyramidingOsMADS1orOsLG3Bwithand/alleles can promote grain length formation, thus enhancing yield and quality (Liu et al, 2018; Yu et al, 2018).
Two typical Gγ subunits RGG1 and RGG2 play important roles in grain shape regulation through integration with phytohormone signaling. By regulating cell division, over-expression ofsignificantly decreases grain length and grain width, andmight negatively regulate grain size through repressing cytokinin (CK) biosynthesis (Tao et al, 2020). By regulating the expansion of the outer epidermal cells of the spikelet hulls, over-expression ofleads to decreased grain length while gene- editing ofresults in increased grain length, grain width, grain thickness and grain weight (Miao et al, 2019).possibly acts as a negative regulator of grainsize via the gibberellin (GA) pathway (Miao et al, 2019).
Overall, key regulators in G protein signaling play important roles in grain shape determination. By combining different G-protein variants, grain length can be decreased by up to 35% or be increased by up to 19% (Sun et al, 2018), suggesting great potential of utilization of various genetic haplotypes of these key genes to manipulate desirable grain shape.
Ubiquitination-related processes involved in grain shape determination
Ubiquitination, one of key processes for the post-translational modification, precisely modulates the stability and trafficking of proteins, thus playing important roles in crop agronomic traits (Pan et al, 2019; Varshney and Majee, 2022). Ubiquitin (Ub) addition is catalyzed by an enzymatic cascade involving an E1 Ub-activating enzyme, followed by the sequential activities of E2 Ub-conjugating enzyme and E3 Ub ligase (Smalle and Vierstra, 2004). Ub-related modification is a dynamic and reversible process, as a result of deubiquitinating enzymes, rescuing target proteins from the degradative pathway (Reyes- Turcu et al, 2009). Key regulators in ubiquitination- related processes play important roles in rice grain shape determination (Table 2 and Fig. 1).
() encodes a RING protein with E3 ubiquitin ligase activity. It was firstly identified as a major QTL for grain width and weight through the population derived from the cross between WY3 (avariety with very large grains) and FAZ1 (Fengaizhan 1, a high-quality elitevariety with small grains). A 1-bp deletion in the coding region ofallele in WY3 causes a premature stop and loss-of-function of, resulting in grain width and weight increased (Song et al, 2007). NIL-WY3, containing a smallregion from WY3 on the FAZ1 background, exhibits a substantial increase in grain width and also a slight increase in grain thickness and grain length, and thus a significant increase in 1000-grain weight compared with FAZ1 isogenic control (Song et al, 2007).allele in WY3 increases the cell number of the outer parenchyma cell layer of spikelet hull, accelerates the grain milky filling rate and promotes the endosperm cell expansion, resulting in increased grain width, weight and yield (Song et al, 2007).Moreover, NIL-WY3shows no reduction in ECQ with little difference on appearance quality with FAZ1. Therefore, theWY3allele is a useful locus for high-yielding crop breeding without obvious deterioration in grain quality (Song et al, 2007). Haplotype analysis in a set ofand aromatic rice varieties reveals the absence of theWY3allele (Dixit et al, 2013), suggesting the great potential of introducing the allele intorice to increase the grain width. Down-regulation ofexpression by RNAi can increase the grain width and weight inrice (Verma et al, 2021).

Table 2. Main regulators in ubiquitination-related pathway for rice grain shape.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
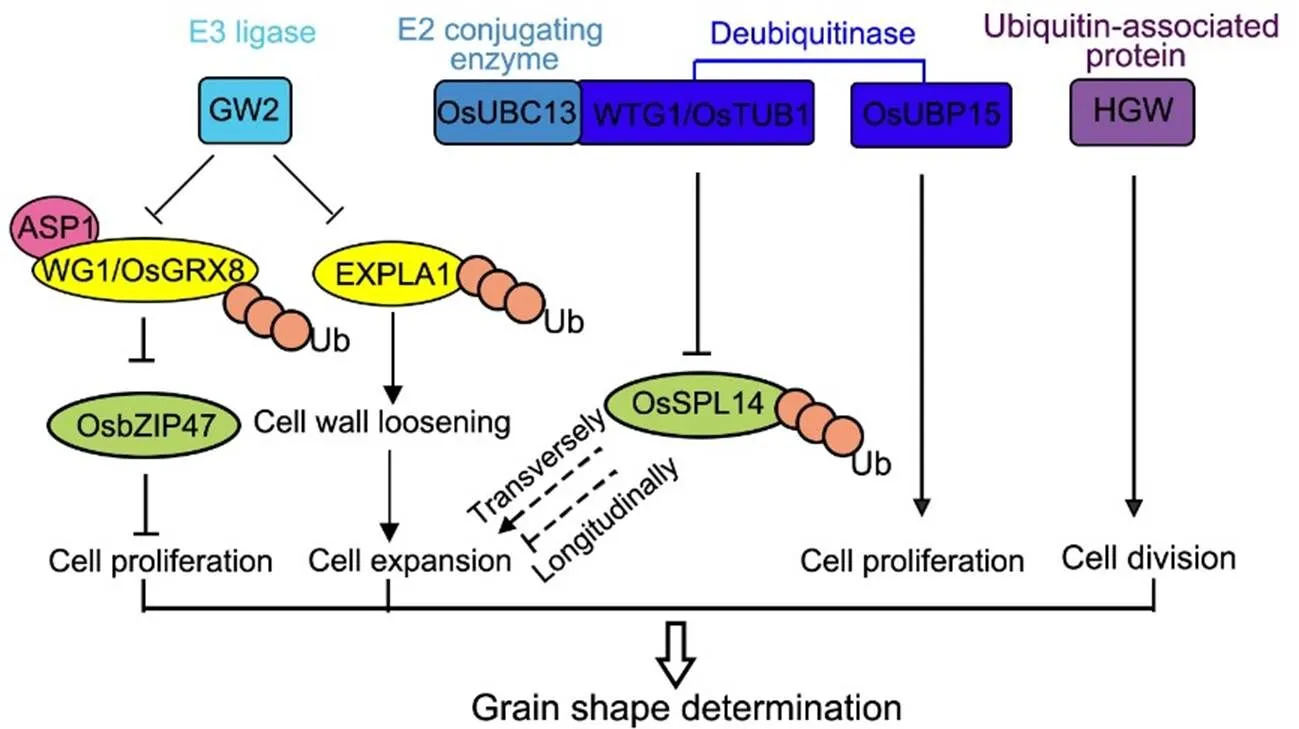
Fig. 1. Control of grain shape by ubiquitination-related pathway.
GW2, a RING protein with E3 ubiquitin (Ub) ligase activity, interacts and catalyzes the ubiquitination of EXPLA1 and WG1/OsGRX8. EXPLA1 is a cell wall-loosening protein that increases cell expansion, while WG1/OsGRX8 acts as an adaptor protein to recruit the transcriptional co-repressor ASP1 to repress the transcription activity of OsbZIP47. OsbZIP47 limits grain growth by inhibiting cell proliferation. The deubiquitinase WTG1/OsTUB1 forms complex with E2 conjugating enzyme OsUBC13 to regulate grain width, maybe through promoting the ubiquitination-dependent proteasomal degradation of OsSPL14, and thus longitudinally increases cell length and transversely decreases cell width. Another deubiquitinase OsUBP15 positively regulates cell proliferation to determine grain shape. Moreover, a novel plant-specific ubiquitin-associated domain protein HGW (heading and grain weight) promotes latitudinal cell division to play a role in grain shape determination.
Regular arrows indicate positive regulation, while block arrows indicate negative regulation.
GW2 interacts with and catalyzes the ubiquitination of EXPLA1 (EXPANSIN-LIKE 1), which is a cell wall-loosening protein that increases cell growth (Choi et al, 2018). Another target of GW2 is WG1/OsGRX8, a CC-type glutaredoxin that promotes cell proliferation and grain width formation through repressing the transcription factor OsbZIP47 (Hao et al, 2021). Over-expression ofresults in wide, slightly short and heavy grains as well as reduced plant height and panicle length (Hao et al, 2021). WG1/OsGRX8 may act as an adaptor protein to recruit the transcriptional co-repressor ASP1 to repress the transcription activity of OsbZIP47 (Hao et al, 2021).limits grain growth by inhibiting cell proliferation (Hao et al, 2021). The GW2-WG1/OsGRX8-OsbZIP47 regulatory modulecontrols grain growth and thus grain shape.
WTG1/OsOTUB1 is a deubiquitinase that forms a complexwith E2 ubiquitin- conjugating OsUBC13, regulating grainwidth and weight together(Huang et al, 2017; Wang et al, 2017). The loss-of-function mutant ofexhibits wide, thick, shortand heavy grains togetherwith an increased number of grains per panicle, while over-expression ofleads to narrow, thin and long grains.is a recessive mutant, which introduces a 4-bp deletion in the extron-intron junction region, resulting in altered splicing ofand a premature stop./mainly influences cell expansion as shown by wide and short cells in the mutant as well as narrow and long cells in over-expressors in the epidermis of spikelet hulls. By contrast, cell number in the grain-width direction inis slightly decreased, while that in the grain- length direction is slightly increased (Huang et al, 2017). It was suggested that there is a compensation mechanism between cell expansion and cell proliferation. Furthermore,plays a negative role in control of the NPT (new plant type) architecture characterized by fewer tillers, sturdier culms, larger panicles and higher grain yield in rice, through promoting the ubiquitination-dependent proteasomal degradation of OsSPL14 (Wang et al, 2017). However, whether the WTG1/OsOTUB1-OsSPL14 module modifies the grain shape through regulation of protein ubiquitination needs further investigation.
OsUBP15 acts as a positive regulator of grain width, grain length, grain thickness and grain weight in rice. The increased grain size in the dominant gain-of- function mutant ofnamedis caused by cell proliferation in the spikelet hull (Shi et al, 2019). Notably,is not independent onto negatively regulate grain size, implying that grain shape and yield can be improved by pyramidingand(Shi et al, 2019).
Despite the modules summarized, a novel plant- specific ubiquitin-associated domain proteinHGW (Heading and Grain Weight) promotes the grain width and grain weight in rice by increasing latitudinal cell number in the lemma and palea (Li et al, 2012).is co-expressed with genes encoding various components of ubiquitination machinery, suggesting the important role of the ubiquitination process in grain width and weight control (Li et al, 2012).
MAPK signaling in grain shape determination
MAPK cascades play crucial roles in diverse aspects of rice growth and development regulation. A classical MAPK cascade consists of a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK), and a MAPK, which transduces extracellular cues into cells, typically through linear and sequential phosphorylation and activation of the members of the signaling cascades (Ma et al, 2022). Among the 75 MAPKKKs, 8 MAPKKs and 15 MAPKs in rice, a considerable of them have been validated to regulate rice grain shape (Table 3) (Chen J et al, 2021).
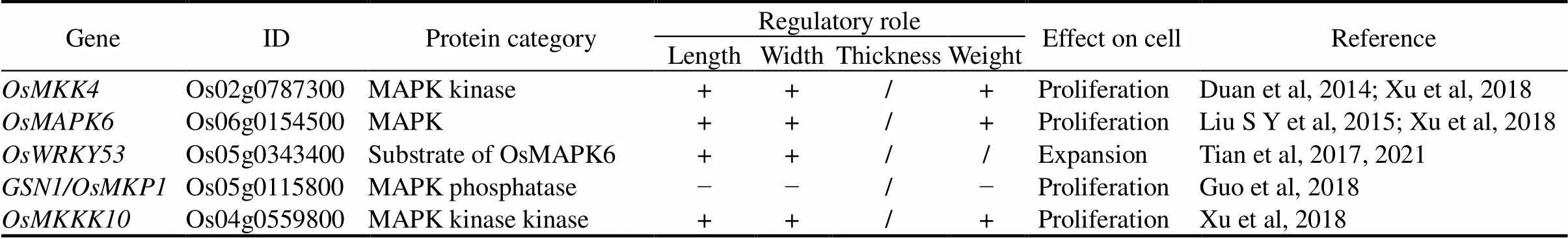
Table 3. Main regulators in mitogen-activated protein kinase (MAPK) signaling pathway for rice grain shape.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
Rice plants with loss-of-function oforproduce small grains due to the defect in cell proliferation (Duan et al, 2014; Liu S Y et al, 2015). OsMKK4 interacts strongly with and acts upstream of OsMAPK6, and both of them influence brassinosteroid (BR) homeostasis and signaling, indicating the crosstalk between MAPK transduction and BR regulation on grain shape (Duan et al, 2014; Liu S Y et al, 2015). OsMKKK10 sequentially phosphorylates and activates OsMKK4 and OsMAPK6(Xu et al, 2018). The OsMKKK10-OsMKK4-OsMAPK6 cascade acts to control grain length, grain width and grain weight (Fig. 2) (Xu et al, 2018).
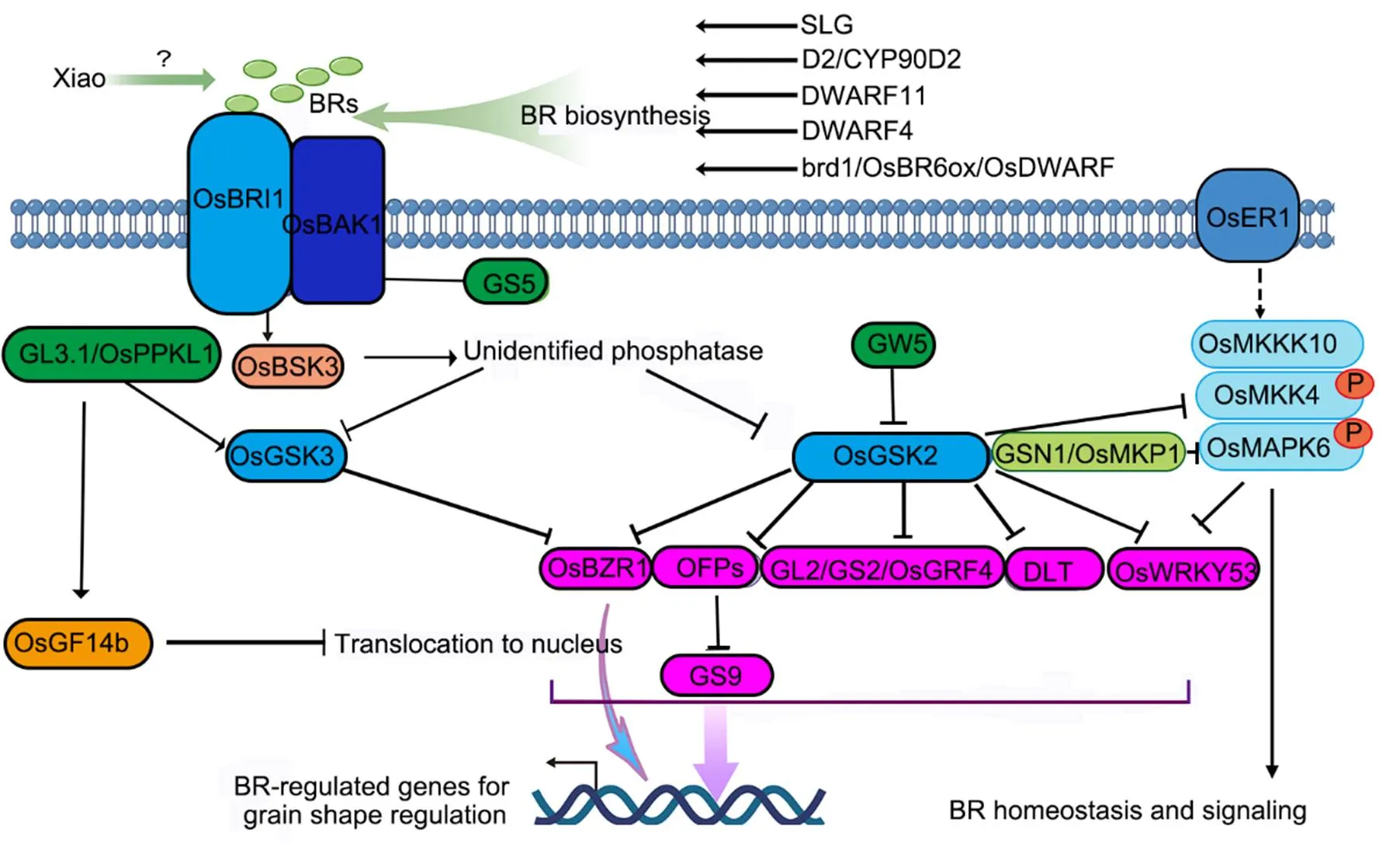
Fig. 2. Control of grain shape bybrassinosteroid (BR) and mitogen-activated protein kinase (MAPK) signaling pathway.
The rate limiting enzymes in BR synthesis, including SLG, D2/CYP90D2, DWARF11, DWARF4 and brd1/OsBR6ox/OsDWARF, are involved in grain shape determination through regulation of BR homeostasis. The BR signal is perceived by OsBRI1 and its co-receptor OsBAK1 and transduced to OsBSK3, which phosphorylates an unidentified phosphatase and further represses OsGSK2/OsSK22 and OsGSK3. OsGSK2 and OsGSK3 repress BR signaling by negatively regulating the transcription factors including OsBZR1, DLT, GL2/GS2/OsGRF4, OsWRKY53 and OFPs. OFPs repress the transcription activity of GS9. A protein phosphatase GL3.1/OsPPKL1 dephosphorylates but stabilizes OsGSK3, leading to accumulation of phosphorylated OsBZR1, which cannot activate BR-induced genes and thus suppressing BR signaling. GL3.1/OsPPKL1 also induces the phosphorylation of OsGF14b, which inhibits OsBZR1 to translocate to nucleus. OsGSK2 phosphorylates OsMKK4 to suppress OsMAPK6 activity, while the phosphorylation of OsWRKY53 by GSK2 lowers OsWRKY53 protein stability to regulate grain shape. GSN1/OsMKP1 inactivates OsMAPK6 via dephosphorylation and thus negatively regulates grain length and width formation.
Regular arrows indicate positive regulation, while block arrows indicate negative regulation.
One direct substrate of the MAPK cascade is the transcription factor OsWRKY53, which contributes to increase the grain length and grain width (Tian et al, 2017, 2021). The interaction and phosphorylation of OsWRKY45 by the MAPK cascade is required for the biological function of OsWRKY45 in grain size regulation (Tian et al, 2017). However, cytological observation showed thatpromotes the cell expansion but not cell proliferation as the MAPK components (Xu et al, 2018). OsGSK2 acts negatively in BR signaling and phosphorylates OsMKK4 to suppress MAPK6 activity, while the phosphorylation of OsWRKY53 by OsGSK2 lowers OsWRKY53 protein stability, indicating the sophisticated levels of interplay between BR signaling and MAPK cascade in grain shape determination (Fig. 2) (Tian et al, 2021).
() encoding a MAPK phosphatase OsMKP1, directly interacts with and inactivates OsMAPK6 via dephosphorylation.is a negative regulator of grain length and width, mainly through influencing cell proliferation (Guo et al, 2018).
, which encodes a receptor-like protein kinase, has been demonstrated to act upstream of the OsMKKK10-OsMKK4-OsMAPK6cascade to control spikelet number, and themutant has markedly longer and significantly wider grains compared with the wild type (Guo et al, 2020). However, howactsupstream of the MAPK cascade to regulate grain shaperemains largely unclear. Detailed investigation is needed to unveil the complete mechanisms of MAPK cascade in grain shape determination.
MicroRNA processes control grain shape by targeting gene expression of key regulators
MicroRNAs (miRNAs) are a class of 20- to 24- nucleotide endogenous small RNAs that repress gene expression. In plants, miRNAs control the expression of genes encoding transcription factors and other genesto affect a multitude of developmental and physiological processes by imparting sequence specificity to gene regulation (Rogers and Chen, 2013). Emerging evidence shows that the action of miRNA plays important roles in rice grain shape determination (Table 4 and Fig. 3).
OsmiR156 represses the transcription ofin young panicles (Xie et al, 2006).might promote latitudinal growth by increasing cell proliferation through its effect on the cell cycle machinery and inhibit longitudinal growth by repressing cell elongation (Wang et al, 2012).positively correlates with grain width and grain weight but negatively correlates with grain length (Wang et al, 2012). The critical polymorphism between Basmati and HJX74 is the 10-bp InDel in the promoter region. Haplotype analysis ofsuggested that Basmati haplotype is retained because of its association with better grain quality, whereas the HJX74 haplotype is selected for higher grain productivity in elitevarieties. Moreover, a 2-bp InDel at the miR156 target site infound in the Iranian rice cultivar Amol 3 causes alternation of the ratio of grain length and width, which may due to abolishment of the suppression of miRNA156 ongene expression (Wang et al, 2012). The elite alleles conferring slender grain shape are likely rare invarieties with higher grain productivity (Wang et al, 2012), suggesting a great potential to use the miR156-GW8/OsSPL16module for the improvement of grain appearance quality.
In addition, OsmiR156 regulates the gene expression ofby targeting its 3′-UTR (Si et al, 2016).is identified as the causal gene to explain the larger grain size ofrice varieties compared withvarieties (Khush, 1997; Ali et al, 2011). As a transcription factor, GLW7/OsSPL13 directly targets, which encodes an alpha-tubulin protein and promotes lemma cell elongation. Cytological observation indicated thatregulates grain shape through activation of cell expansion in the outer epidermis of grain hulls. The phenotypes of over-expressing lines, RNAi lines, T-DNA insertion mutants as well as complementary analysis demonstrated thatpositively increases grain length, grain thickness and grain weight, but does not affect grain width (Si et al, 2016). Interestingly, the 3′-UTR ofhas an OsmiR156 complementary site and OsmiR156 may be involved in grain shape regulation through targeting the gene expression ofdespite of.
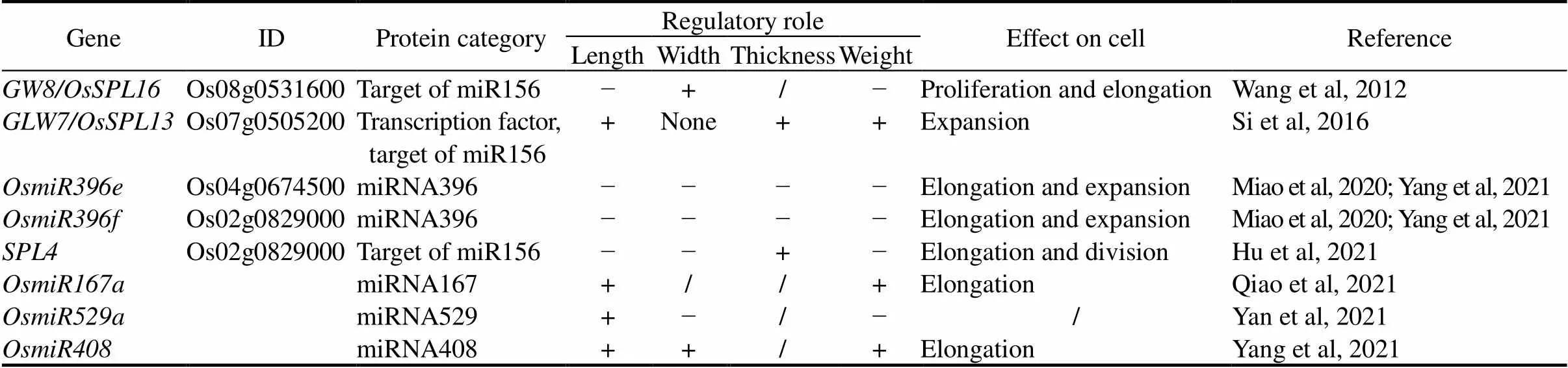
Table 4. Main regulators in microRNA process for rice grain shape.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
Moreover, down-regulation of another target of OsmiR156,, might contribute to the change of rice grain shape (Hu et al, 2021). Loss-of-function mutations ofthrough the CRISPR/Cas9 system result in grain length and width increased but in grain thickness decreased, eventually leading to grain weight and grain yield per plant increased (Hu et al, 2021).inhibits both the cell expansion and cell division in the longitudinal direction of the outer glumes. Overall, the OsmiR156-OsSPLs module plays important roles in rice grain shape determination (Wang et al, 2012; Si et al, 2016; Hu et al, 2021). Notably, the SPL family genes are not only downstream target of miR156 but also targeted by miR529a, which may promote grain length but inhibit grain width formation through down-regulation of six SPL protein-encoding genes, including,,,,and(Yan et al, 2021).
Despite the OsmiR156/OsmiR529a-OsSPLs modules, the miR396-GRFs module plays an important role in grain shape determination and grain yield. The rice genome contains 8 mIR396genes, and 11 of the 12 identified() genes (–) are targets for(Choi et al, 2004; Kim and Tsukaya, 2015). Hu et al (2015) suggested the positive role ofbeneficial allele on regulation of grain thickness formation. There is no doubt thatis important in regulating grain length, width and thickness formation (Che et al, 2015).regulates grain size by promoting both cell division and cell expansion in spikelet hulls (Che et al, 2015; Duan et al, 2015). Compared with small grain varieties such as Zhonghua 11 (ZH11), Bobai B and Xiushui 09, thealleles in large grain varieties such as Baodali, RW11 and Judali harbor a 2-bp TC/AA substitution in the miR396c target site. The 2-bp substitution perturbs the cleavage ofby miR396, resulting in elevated expression ofand thus the increase of grain size (Che et al, 2015; Duan et al, 2015; Hu et al, 2015). The beneficial allele is rare as demonstrated by the extensive sequence analysis of nearly thousand rice varieties, implying a tremendous potential forin hybrid breeding (Che et al, 2015; Li et al, 2016).
Besides that miR396c is involved in grain shape determination through regulatinggene expression,might be another mediator of OsmiR396especially for the ratio of grain length to grain width regulation, and the over-expression ofleads to great increase in the ratio of grain length to grain width (Yang et al, 2021). Furthermore, mutations ofand, together generated by a multiplex CRISPR/Cas9 system, significantly increase grain length, width and thickness, thus 40% increase of 1000-grain weight compared with the wild type (Miao et al, 2020). The epidermal cells ofspikelet hulls are longer and wider than those of wild type, resulting from the obviously enlarged cells (Miao et al, 2020). Down-regulating of allincreases grain length in bothandsubspecies (Yang et al, 2021).
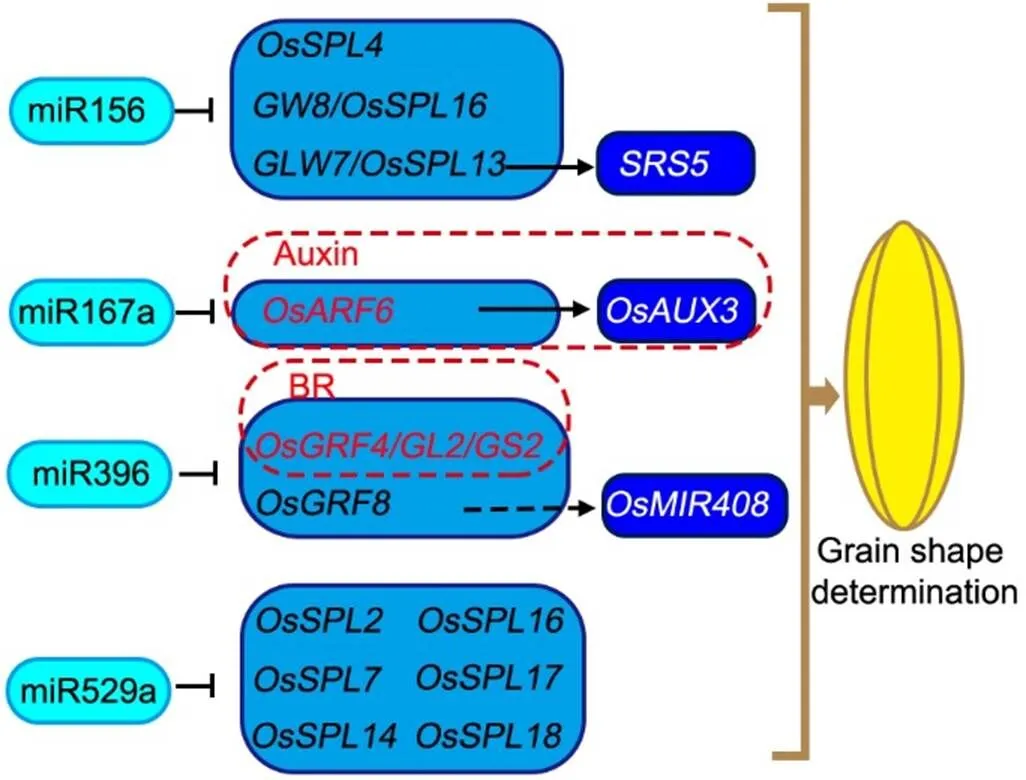
Fig. 3. MicroRNAs control rice grain shape by targeting key transcription factor-encoding genes.
MicroRNA156 (miR156) targets and down-regulates the genes encoding transcription factors,and.promotes the gene expression of. miR167a targets and inhibits, which encodes an upstream transcription factor promoting the expression ofin the auxin signaling pathway. miR396 directsandmRNA silencing.is an important cross-connect node of brassinosteroid (BR)signaling and miRNA regulatory pathway to determine the grain shape.directly binds to thepromoter and may promote the accumulation of. Moreover, miR529a is involvedin the grain shape determination by directing other OsSPL family genes.
Regular arrows indicate positive regulation, while block arrows indicate negative regulation.
, further filtered out by the miRNA microarray assays, also regulates grain shape (Yang et al, 2021).specifically expresses in embryoes around 10 d after ferterilization, suggesting its contributionto late-embryo development, starch content enrichment and grain enlargement at that to developmental stage (Yang et al, 2021). In contrast to the negative roles ofin grain size,positively regulates grain length and grain width by promoting the cell elongation in the husk (Yang et al, 2021). The large grainphenotype ofmutants can be complemented partially by the mutation of, and OsGRF8 binds directly thepromoter, suggesting thatacts downstream of the miR396-GRFs module in grain shaping (Yang et al, 2021).
Despite the regulating roles of,,,,and,increases grain length, grain weight and grain length/ width ratio, at least in part, through post-transcriptional suppression of(Qiao et al, 2021). OsARF6 is a transcription factor in the auxin signaling pathway, playing a negative role in grain length formation (Qiao et al, 2021). In conclusion, the microRNA metabolism is likely to act as a hub integrating hormone signals and the grain shape determination, needing comprehensive analyze in the future.
BR biosynthesis and signaling pathway involved in grain shape determination
BRs were first isolated and characterized in the 1970s and have been studied since then for their diverse biological functions such as regulating cell elongation, cell division and cell differentiation (Mitchell et al, 1970; Oh et al, 2020). In rice, it has long been known that the BR levels affect grain shape, especially grain length (Table 5). For example, loss-of-function mutants//(encoding BR-6-oxidase) produce extra-small grains (Mori et al, 2002). The mutations of(CYP90D2) (Hong et al, 2003) and(, encoding a cytochrome P450, CYP724B1) (Tanabe et al, 2005), result in decreased BR biosynthesis and significantly shorter grains than wild type. A single nucleotide polymorphism (deletion) in the promoter region ofreduces its expression level, leading to the inhibition of outer glume surface cell elongation and thus reduction of grain length, grain width and grain thickness dramatically (Zhou et al, 2017). The transgenic plants expressing another BR-biosynthetic genedriven by constitutive promoter or seed-specific promoter have significantly longer brown grains than the wild type, and the overall performance of seed-specific promoter driven lines is better than that of over-expressing lines, indicating the important roles of BR biosynthesis in the grain development (Li et al, 2018). Moreover,(), a BAHD acyltransferase-like proteingene, positively regulates endogenous BR levels, leading to the increase of cell length, thus the formation of long and narrow grains (Feng et al, 2016).
In addition to the effects of BR homeostasis on rice grain shape determination, BR signal transduction pathway also plays an important role (Fig. 2). The BR signal is perceived by OsBRI1 receptor and its co-receptor OsBAK1 and transduced to OsBSK3, which phosphorylates an unidentified phosphatase and further represses OsGSK1, OsGSK2 and OsGSK3 (Nakamura et al, 2006; Koh et al, 2007; Li et al, 2009; Tong et al, 2012; Zhang B W et al, 2016; Gao et al, 2019). OsGSK1, OsGSK2 and OsGSK3 are homologs ofBIN2 and repress BR signaling by negatively regulating the transcription factors including OsBZR1, DLT, OsGRF4/GL2/GS2, BU1, LIC, OFP1, OFP3, OFP8 and OFP19 (Bai et al, 2007; Tong et al, 2012; Zhang C et al, 2012; Che et al, 2015; Yang et al, 2016; Tong and Chu, 2018; Yang et al, 2018; Xiao et al, 2019; Xiao et al, 2020a).
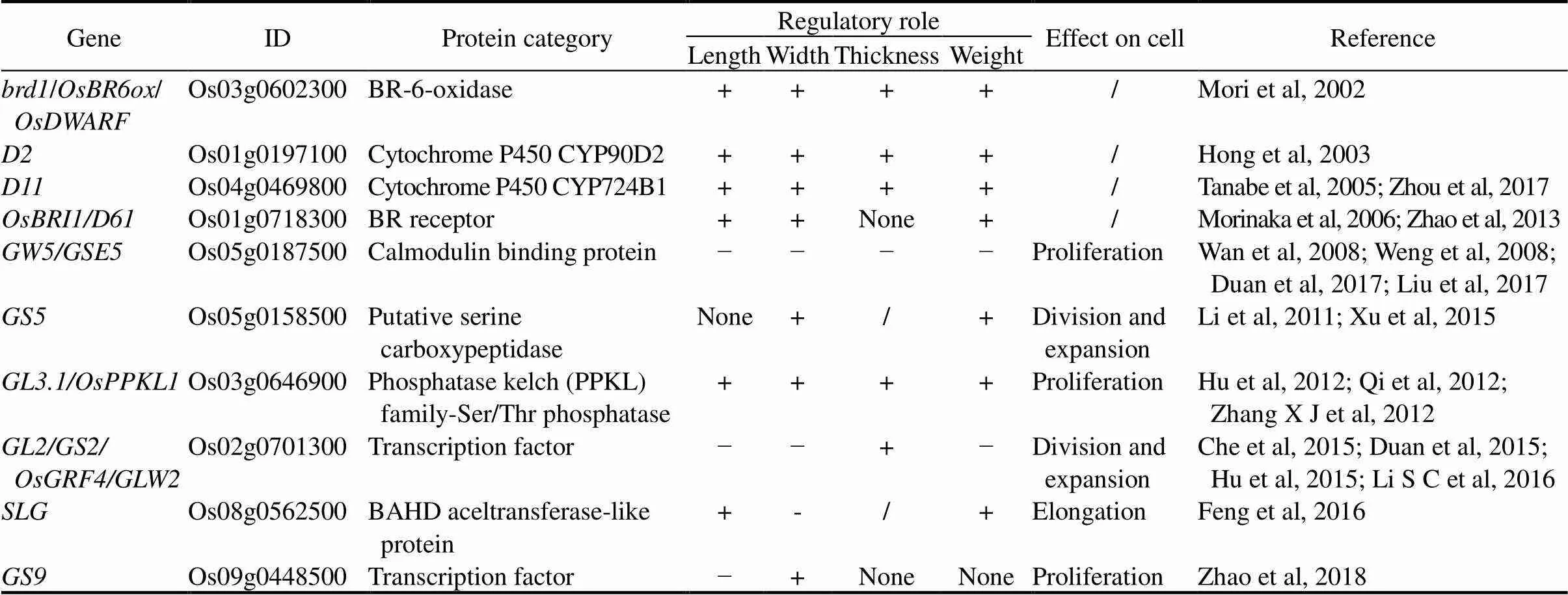
Table 5. Main regulators in brassinosteroid (BR) biosynthesis and signaling pathways for rice grain shape.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
The deficiency of BR receptor OsBRI1 significantly reduces grain length and width but not grain thickness(Morinaka et al, 2006). The loss-of-function of BR co-receptorleads to grain length and width reduced, indicating the major promoting role ofin forming of grain length (Yuan et al, 2017).promotes the cell division of inner and outer epidermal cells in the longitudinal direction (Yuan et al, 2017). Notably, OsBAK1 interacts with a key grain shape regulator, GS5 (Li et al, 2011). The OsBAK1-GS5 interaction leads to the arrest of OsBAK1 from endocytosis and thus enhanced BR signaling (Xu C J et al, 2015).encodes a putative serine carboxypeptidase belonging to the peptidase S10 family and the wide grain characteristic regulated byis fully dominant (Li et al, 2011).positively regulates grain width by increasing cell number of the inner parenchyma cell layer in the palea/lemma of spikelet possibly through activating the expression of putative G1/S-phase genes, while to some extent, increasing cell size of palea (Li et al, 2011). Bothandpossibly promote cell division (Li et al, 2011; Yuan et al, 2017). Sequence analysis of thepromoter regions conducted in 51 rice accessions from a wide geographic range across Asia identified three haplotypes to be associated with grain width: H94 type confers the narrow grain, and Zhenshan 97 type confers the medium grain while ZH11 type confers the wide grain (Li et al, 2011). Further study revealed that two single nucleotide polymorphisms (SNPs) located in the flanking sequence of a putative GA-responsive element in the promoter alter the response of thealleles to abscisic acid (ABA) suppression, resulting differential expression of thealleles in young panicles (Xu C J et al, 2015). It is worth to exploring whetherintegrates BR signals with other plant hormones such as ABA to regulate the grain shape in the future.
In addition to the BR receptor and co-receptor, the important negative regulator OsGSK2 in BR signal transduction pathway participates in the regulation of rice grain shape in at least three ways (Table 5 and Fig. 2) (Liu et al, 2017). Firstly, OsGSK2 directly interacts with and inhibits the transcription regulator OsGRF4/ GL2/GS2, which consequently activates BR responses by up-regulating a large number of BR-induced genes to promote grain length, width and weight (Che et al, 2015). Secondly, OsGSK2 phosphorylates the downstreamtranscription factor OsOFP8, which positively mediates BR signaling and confers a slender grain shape with high ratio of grain length to width (Yang et al, 2016; Zhao et al, 2018). Meanwhile, OsOFP8 together with OsOFP14 interacts directly with GS9, whose null mutant has slender grains (Zhao et al, 2018). However, OsGSK2 can alleviate the suppression on GS9 by inhibiting OsOFP8 function. The OsGSK2-OsOFP8-GS9 complex participates in the regulation of longitudinal cell division in spikelet hulls (Zhao et al, 2018). Thirdly, both autophosphorylation of OsGSK2 and trans-phophorylation of OsBZR1 and DLT by OsGSK2are repressed by GW5, thus leading to the accumulation of unphosphorylated OsBZR1 and DLT proteins in the nucleus to promote BR signaling and thus grain development (Liu et al, 2017). The important function ofin controlling rice grain width has been clarified more than ten years ago (Wan et al, 2008; Weng et al, 2008).encodes a calmodulin binding protein localized to the plasma membrane and regulates spikelet development via controlling cell number of the glumes of the rice flower (Wan et al, 2008; Weng et al, 2008; Liu et al, 2017). Two independent studies have carried out sequence analysis offrom 140 and 129 rice accessions collected from diverse geographical locations, respectively, and found that the 1 212-bp deletion in thepromoter region is selected duringdomestication and breeding for its effect on increased grain width and yield, most likely through down-regulatingexpression (Shomura et al, 2008; Liu et al, 2017). Moreover, Duan et al (2017) identified thatpromotes grain length formation but decreases grain width by influencing cell proliferation in spikelet hulls.is the same gene with. GSE5 protein interacts with rice calmodulin (OsCaM1-1), indicating that it may be involved in calcium signaling to regulate grain shape. Based on the deletion/insertion type in the promoter, three major haplotypes ofhave been identified and indicated to originate from differentaccessions during rice domestication. A 950-bp deletion (DEL1) and 367-bp insertion (IN1) invarieties carrying theGSE5haplotype and a 1 212-bp deletion (DEL2) invarieties carrying theGSEhaplotype are associated with decreased expression of, resulting in wide grains. Both the haplotype have been widely and successfully utilized by rice breeders (Duan et al, 2017). Notably, thedeletion is not only related to the increase of grain width, but also contributes to the increase of grain thickness and reduction of grain length (Weng et al, 2008). In brief,mediates BR signaling to control grain shape through the formation and dissociation of the following three complexes: OsGSK2-OsOFP8-GS9, OsGSK2-OsGRF4/GL2/GS2 and GW5- OsGSK2-OsBZR1-DLT (Table 5 and Fig. 2).
Moreover, the well-studied gene for grain length regulation,, is also involved in BR signaling (Table 5 and Fig. 2) (Hu et al, 2012; Gao et al, 2019)., which encodes a protein phosphatase kelch (PPKL) family-Ser/Thr phosphatase, was firstly cloned and characterized as the casual gene for the great difference in grain length between FAZ1 and WY3 (Qi et al, 2012).WY3differsFAZ1by two amino acid substitutions, inducing weaker dephosphorylation activity, thus accumulation of phosphorylated substrates such as Cyclin-T1;3, to promote cell proliferation during spikelet development (Qi et al, 2012). The Kelch domains in93-11play a negative role in cell density in the outer surface of glumes and thus forming grain length (Zhang X J et al, 2012).is a major grain length QTL, whichhas an additive or partial dominant effect indicated by grain shape trait value of F1plants (Hu et al, 2012). The single nucleotide substitution (C/A) at +1 092 bp results in replacement of aspartate in small grain varieties with glutamate in large grain varieties for the 364th amino acid, locating at the D position of the conserved sequence motif AVLDT of the Kelch repeat (Hu et al, 2012; Zhang X J et al, 2012). The beneficial allele ofnot only contributes to the forming of grain length, but also promotes the grain thickness and width. The association analysis of the sequence polymorphisms with grain length of 94 rice varieties indicated that thebeneficail allele with the C/A mutation is rare (Zhang et al, 2012). Application of the beneficial allele can significantly improve grain yield in both inbred and hybrid rice without sacrificing grain quality (Qi et al, 2012).
Recently,has been reported to participate in BR signaling pathway in at least three ways. Firstly, GL3.1/OsPPKL1 dephosphorylates and stabilizes OsGSK3, leading to accumulation of phosphorylated OsBZR1, which cannot activate BR- induced genes, thus suppressing BR signaling in rice (Gao et al, 2019). The negative role ofin BR signaling is abolished in extra-large grain variety and the grain length is therefore increased (Gao et al, 2019). Secondly, GL3.1/OsPPKL1 induces the phosphorylation of OsGF14b (Gao et al, 2022a), which modulated nucleocytoplasmic shuttling and transcriptional activation activity of OsBZR1, to eventually negatively regulate grain length. Mutation ofresults in longer grains whilemutant has shorter grains. Thirdly, GL3.1/OsPPKL1 protein interacts with an adenylate kinase OsAK3, which is involved in a variety of biological processes that regulate BR sensitivity and grain development (Zhang J Q et al, 2021). Loss-of-function ofresults in significantly decreased cell length and width of outer glume but remarkably increased cell number. Based on that the mutation ofcauses small grains, it was indicated thatpromotes rice grain size mainly by controlling cell expansion of glumes (Zhang J Q et al, 2021). In short,acts as an important regulatory node in BR signaling to determine the grain shape at least through the GL3.1/OsPPKL1-OsGSK3, -OsGF14b-OsBZR1 and -OsAK3modules (Gao et al, 2022b) (Fig. 2).
Despite GL2/GS2/OsGRF4, OsBZR1, OFP8 and OFP14 mentioned above, the transcription factor OFP1 exerts a positive role in regulating BR responses and obviously suppresses the grain width (Xiao et al, 2017). In contrast, its homolog OFP3 is a suppressor of both BR signaling and BR synthesis through interacting with many BR-related components (Xiao et al, 2020a). Consistent with its negative role in BR signaling, the over-expression ofleads to significant decrease of grain length (Xiao et al, 2020a). Additionally, the downstream transcription factor BU1 promotes the forming of grain length (Tanaka et al, 2009). The over-expression of, which encodes a transcription factor positively regulating BR responses, renders the increase of grain length but decreases grain width and thickness (Tong et al, 2012).
Besides the components in the classic BR signaling pathway, a rice T-DNA insertion mutantdisplays a tissue-specific enhanced BR response and greatly reduces BR content at the whole-plant level (Fig. 2). The decreased seed size in the mutant, especially lengthwise, maybe result from reduced cell division rate (Jiang et al, 2012).encodes a LRR kinase, however, the mechanism underlyingand BR signaling remains unclear (Jiang et al, 2012). Yuan et al (2022b) showed thatnegatively regulates grain size, but it is likely that the regulation is independent of BR signal transduction pathway. In conclusion, according to the current progress, BR is one of the most important hormones that can regulate the rice grain shape(Fig. 2).
Auxin signaling pathway involved in grain shape determination
Auxin, a well-known plant hormone, plays a vital role in nearly every aspect of plant growth and development (Zhao, 2010). Polar auxin transport is achieved by influx and efflux carriers that mediate auxin flow into and out of cells, respectively (Kerr and Bennett, 2007). AUX/LAX family members are responsible for auxin transport (Wang et al, 2019). Auxin signaling machinery contains three kinds of core components: the TIR1/AFB co-receptors, the Aux/IAA transcriptional repressors, and the ARF transcription factors. Auxin acts as the molecular glue bringing together the TIR1/AFB and Aux/IAA proteins, resulting in degradation of the Aux/IAAs and the release of ARF repression and thus regulating downstream target genes (Zhao, 2010; Leyser, 2018).
In rice genome, there are 5 AUX/IAAfamily members and 25 ARFs (Auxin Response Factors)(Wang et al, 2018). Among them, OsARF4 is phosphorylated by OsSK41/OsGSK5 (Hu et al, 2018). Althoughbelongs to the same family with, there is limited evidence for its role in BR signaling pathway (Hu et al, 2018).is synonymous to, mapped through the recombinant inbred lines (RILs) and near isogenic lines (NILs) population derived from the cross between Zhenshan 97 and Nanyangzhan (avariety with extra-long grains) (Hu et al, 2018; Xia et al, 2018). Theallele from NYZ affects grain length and weight. Plants harboring the loss-of-function allele produce longer grains, indicating thatnegatively regulates the formation of grain length. There is an ~10% decrease in cell number and ~20% increase in cell length of longitudinal cells in spikelet hulls, leading to longer grains in NIL-NYZ(Xia et al, 2018). The mutation ofin NYZ is the same as that in CW23, and the NYZ allele named asJapallele is only 1% ofaccessions and only in thesubpopulation (Hu et al, 2018; Xia et al, 2018). The rare alleleJapmay have been selected together withunder artificial selection for extra-long grainvarieties (Xia et al, 2018). Another natural variant alleleAus(with C672A missense mutation) mainly exists in theaccession and greatly contributes to grain length variance inaccessions (Xia et al, 2018). Both theandpromoters are active in maternal tissues, and mutation of eitheroraffects both grain hull and endosperm development (Hu et al, 2018). Suppression ofresults in longer and wider rice grains. The OsSK41/OsGSK5-OsARF4 module possibly regulates rice grain size through the auxin signaling pathway (Table 6) (Hu et al, 2018).
Another ARF member OsARF6 binds directly to thepromoter to activate its expression (Qiao et al, 2021).is the causal gene for, a major QTL for grain length and grain weight, and encodes a transmembrane amino acid transporter (Table 6) (Qiao et al, 2021). Sequence analysis ofrevealed that G-1110in thepromoter is likely to confer the relatively shorter grain length, whereas T-1110confers longer grain length (Qiao et al, 2021). The expression level ofnegatively correlates with the grain length, grain weight and the ratio of grain length and width, but doesn’t significantly affect grain width (Qiao et al, 2021). The key SNP ofrelated to the grain shape diversity is covered in the AuxRE bound by OsARF6 (Qiao et al, 2021). Bothandinhibit cell expansion but not influence cell division of glume cells (Qiao et al, 2021). Moreover,mRNA can be silenced by miR167a, suggesting that the miR167a-OsARF6-OsAUX3 module integrates the miRNA process with auxin transport and signaling pathways to regulate grain shape (Qiao et al, 2021).
IAA, Indole-3-acetic acid; GSK, Glycogen synthase kinase; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
The transcription factor OsARF25 and its target geneplay a positive role in grain length formation, whereas OsIAA3 plays a negative role by interacting and repressing OsARF25 (Zhang et al, 2018)., encoding a RING-finger and WD40-associated ubiquitin-like domain-containing protein, promotes the formation of grain length by affecting cell expansion in the spikelet hulls (Zhang et al, 2018). Gnp4/LAX2 directly interacts with OsIAA3 and interferes with the OsIAA3-OsARF25 association, leading to the elevated expression of(Zhang et al, 2018). OsERF142/SMOS1 interacts with DLT (the key transcription factor in BR signaling pathway) to form a complex that promotes the expression of cell expansion related gene(Tong et al, 2012; Hirano et al, 2017). It is likely that the Gnp4/LAX2-OsIAA3-OsARF25-OsERF142/SMOS1- DLT module integrates auxin and BR signaling in the regulation of rice grain shape.
Besides the three main modules, an auxin- responsive genestimulates glume cell elongation and division, resulting in the increase of the organ volume (Liu L C et al, 2015). Moreover,, identified by high-resolution mapping using backcrossedinbred lines produced from NPB and the Indian landrace Kasalath, encodes a protein with indole-3-acetic acid (IAA)-glucose hydrolase activity (Ishimaru et al, 2013).in NPBseems to affect the timing of the transition from the syncytial to the cellular phase by controlling the supply of IAA and consequently limits cell number and grain length, resulting in reduced grain length and weight in NPB compared with Kasalath(Table 6) (Ishimaru et al, 2013). In short, loss-of-functionofenlarges grain size by inhibiting IAA-glucose hydrolysis and promoting cellularization during early endosperm development (Ishimaru et al, 2013). Moreover, haplotype analysis demonstrated that the superior Kasalath-typeis probably not a selection target during rice domestication, suggesting its great potential for further improvement of grain length and weight in modern cultivars (Ishimaru et al, 2013).
Other plant hormone signaling pathways involved in grain shape determination
Cytokinins (CK) is a class of phytohormone playing important roles in various biological processes including cell division and differentiation and thus regulating grain shape and size (Sakakibara, 2006). CKXs (cytokinin oxidase/dehydrogenases) can modulate CK homeostasis by degrading active CK. The activation of a CKX gene,, leads to grain length, grain width and grain weight decreased (Table 7) (Gao et al, 2014)., encoding a purine permease, acts as a putative CK transporter, and promotes the grain length, grain width, grain thickness and grain weight (Xiao et al, 2019)., the closest homolog of, also harbors the similar function (Xiao et al, 2019). However, over-expression of another homologleads to opposite phenotypes, including decreased grain width and weight, accompanying with decreased CK content in shoots and panicles, but increased in root (Table 7) (Xiao et al, 2020b). Furthermore,enhances grain length by activatingto modulate CK distribution (Table 7) (Yin et al, 2020). Thus, CK levels regulated by CK homeostasis and transported to shoots and panicles are closely related with grain length, width, thickness and weight.
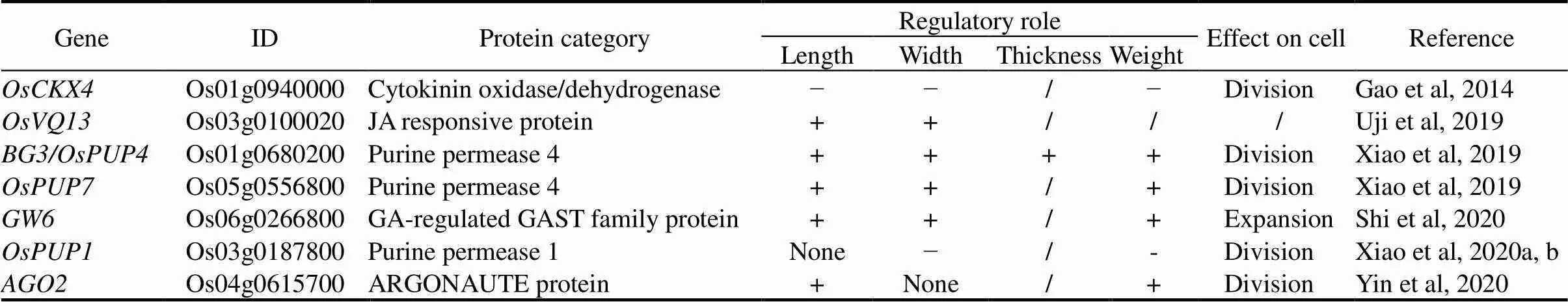
Table 7. Other plant hormones regulation for rice grain shape.
JA, Jasmonic acid; GA, Gibberellin; GAST, Gibberellic acid stimulated transcript.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
The plant hormone jasmonic acid (JA) plays an important role in the defense response. For instance, JA-responsive VQ-motif-containing protein OsVQ13 in JA signaling increases grain length and width (Table 7) (Uji et al, 2019). OsVQ13 associates with and influences the signaling pathway of OsWRKY45, which is phosphorylated by the defense response and grain size regulator OsMAPK6 (Uji et al, 2019). However, whether OsVQ13 acts with OsMAPK6- OsWRKY45 in a common pathway to regulate grain shape needs further investigation.
Gibberellins (GA) plays an important role in improving rice architecture for enhancing grain yield (Wu et al, 2021).encodes a GA stimulated transcript family protein, influencing GA response and biosynthesis (Shi et al, 2020).is a semi- dominant locus, which positively regulates grain width and weight by promoting cell expansion in the spikelet hull and grain filling rate (Table 7). A natural variation in the promoter ofis associated with its expression level and grain width. Sequence analysis revealed that allcultivars exclusively contain A allele (with high expression level) whereas both A and G (with low expression level) alleles exist incultivars. This variation in thepromoter has great potential to improve grain width and yield without other dramatic changes in agronomic traits (Shi et al, 2020).
Another plant hormone ABA also involves in the grain shape regulation. Two SNPs in the promoter region ofalter the responses to ABA treatments, resulting in higher expression in developing young panicles, suggesting that the ABA signaling may be involved in grain width regulation (Xu C J et al, 2015). Overall, it is important to further study the crosstalk among various plant hormones on the grain shape regulation to reveal a complete molecular regulatory network. From the perspective of practical application, inventing and applying cheap and pollution-free hormone analogues may provide a potential manner to make rice obtain the desired grain shape.
Other transcriptional regulatory pathways for grain shape determination
Despite the transcription regulators involved in the microRNA process and plant hormone regulatory network, there are other transcriptional regulatory pathways for grain shape determination (Table 8).
encodes a homolog of theTONNEAU1-recruiting motif protein with similarity to C-terminal motifs of the human centrosomal protein CAP350. Up-regulation ofpromotes the formation of more slender grains. GW7 interacts with OsTON1b and OsTON2, and the homologs are involved in preprophase band formation and the spatial control of cell division in plants (Spinner et al, 2013; Wang S K et al, 2015).is synonymous with(Wang S K et al, 2015; Wang Y X et al, 2015). NIL-, containing a chromosome segment from P13 in the background of NPB, has longer and narrower spikelet hull in comparison with NPB, resulting from the increase in cell length and the decrease in cell width for epidermal cells of the outer and inner glumes (Wang Y X et al, 2015).is a semi-dominant locus, regulating grain length/width ratio, the formation of starch granules in endosperm and grain chalkiness (Wang Y X et al, 2015). Interestingly, in addition to the InDel atlocus explaining the grain shape difference, copy number variation at thelocus also contributes to grain shape diversity (Wang S K et al, 2015; Wang Y X et al, 2015). Tandem duplication of a 17.1-kb segment at thelocus leads to elevated expression ofand down-regulation of its nearby negative regulator, and thereby increases the ratio of grain length to width and improves grain appearance quality (Wang Y X et al, 2015). The null mutation ofin long grain varieties such as 93-11 results in a truncated protein and elimination of the suppression on(Wang Y X et al, 2015). Thus, the GW8/OsSPL16-GW7/GL7 regulatory module plays an important role in rice grain shape determination. Moreover, the eliteallele responsible for slender grain shapes is common acrossgermplasm but unrepresented among high-yieldingcultivar, suggesting a great potential inrice breeding programs to induce custom preferable slender grains (Wang S K et al, 2015; Wang Y X et al, 2015).
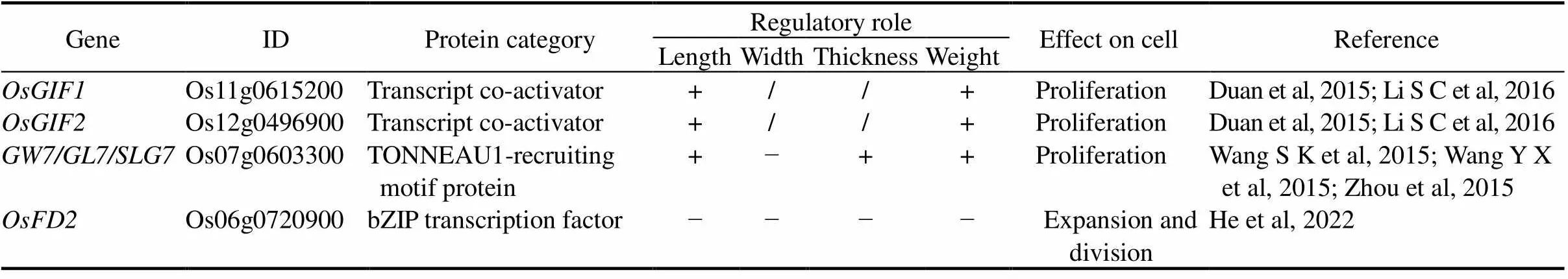
Table 8. Main regulators in other transcriptional regulatory pathway for rice grain shape.
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
, a new allele of, was identified using backcross introgression lines derived from 93-11 and Azucena.allele from Azucena causes longer and thinner grains, but without significant influence on grain weight and yield production (Zhou et al, 2015).encodes a protein homologous toand, both of which increase organ length in.longitudinally increases cell length and transversely decreases cell width (Zhou et al, 2015). Researchers found thatrice variety Jiaxingchangyungeng with good grain appearance quality and ECQ harbors the beneficial allele of(Zhang H G et al, 2021).
Moreover, transcription co-activators OsGIF1 and OsGIF2 directly interact with OsGRF4/GL2/GS2/GLW2, while the elevated expression levels ofandimprove grain size (Duan et al, 2015; Li S C et al, 2016).
Other regulators involved in grain shape determination
, encoding a multidrug and toxic compound extrusion transporter, was identified through QTL mapping method using an RIL population constructed by crossing 93-11 and Changui 121. Gene editing ofin ZH11 leads to the decrease of grain width and weight (Table 9) (Du et al, 2021).
The short-chain dehydrogenases/reductases use NAD(H) or NADP(H) as a cofactor and broad substrate spectra involved in primary and secondary metabolisms.encodes a SDR with atypical catalytic site. Disruption ofleads to significant reduction in grain thickness and grain width, as well as reduction in seed-setting rate, 1000-grain weight, grain weight per plant, panicle length and plant height, but no change in grain length (Table 9) (Zheng et al, 2022).
Genomic DNA is wrapped around the histone octamer to form a nucleosome, which is the subunit of chromatin. The assembly of DNA into chromatin allows the storage of genetic information but prevents the transcription machinery from getting access to the DNA template and thereby initiating transcription (Workman and Kingston, 1998). The state of histone regulated by a series of enzymes and other chromatin remodeling complexes (remodelers) affects plant growth and development (Shang and He, 2022). The() acts as the crucial subunit of chromatin-remodeling complexes and regulates gene expression (Table 9).influences BR homeostasis and signaling, and plays positive roles in grain length and grain width formation via regulating cell proliferation (Guo et al, 2022).
A novel QTL, a semi-dominant locus mainly increasing grain length and grain width but decreasing grain number, encodes a P-type pentatricopeptide repeat protein and regulates the development of the spikelet hull by affecting cell proliferation (Yuan et al, 2022a).may be a suppressor of. Notably, when crossing the SSSL-(a single segment substitutionline harboring the5 locus in the 93-11 background) with two male sterile lines, Y58S and Lu56S, the hybrid progeny exhibits significantly increased grain length and weight compared with Y58S/93-11 and Lu56S/93-11, respectively (Yuan et al, 2022a).
‘+’ means a positive regulatory role; ‘−’ means a negative regulatory role; ‘/’ means not applicable.
An evolutionarily conserved gene, encoding a NHL (NCL-1, HT2A and Lin-41) domain-containing protein, restricts excessive cell division in inflorescences (Chen et al, 2015). The loss-of-function ofleads to increased grain width, grain thickness and grain weight as well as decreased grain length (Table 9) (Chen et al, 2015).encodes a receptor-like kinase CR4 (Chun et al, 2020). The mutation disturbing MIS2 subcellular localization leads to reduced grain length, width and thickness along with wrinkled surface (Table 9) (Chun et al, 2020). The 18-bp InDel in the 5′-UTR causes different expression level ofin haplotypes by coordinately regulating epidermal cell size and cell number (Chun et al, 2020)., encoding a MYB-like protein, acts as a negative regulator of grain length and grain weight but a positive regulator of grain number per panicles (Table 9) (Li et al, 2022). It mainly regulates grain shape by inhibiting cell expansion and cell proliferation (Li et al, 2022). He et al (2022) reported that OsCEN2 negatively controls grain length, width, thickness and thus grain weight (Table 9), and restricts cell expansion in the spikelet hull and grain filling. OsCEN2 interacts directly with a G-box factor 14-3-3 homolog, GF14f, which interacts with a bZIP transcription factor OsFD2 (He et al, 2022). Both GF14f and OsFD2 proteins negatively regulate grain size by controlling cell expansion and division in the spikelet hull (Table 9) (Zhang et al, 2019). Overall, the OsCEN2-GF14f-OsFD2 regulatorymodule determines the grain shape from three dimensions and thus affects seed size and weight (He et al, 2022).
Although at present, many grain shape regulatory genes have been identified mainly based on positive genetics by using various populations, in view of the important value of grain shape regulatory genes in improving rice varieties, more genes need to be further explored and the molecular regulation network needs to be clarified in the future.
Implications of grain shape-related gene in modern rice breeding
The mining of rice grain shape regulating genes and the analysis of genetic mechanism provide important information for improvement of grain shape traits. Especially, the utilization of natural genetic variations based on haplotype analysis greatly contributes to rice breeding practice. The application of beneficial haplotypes or pyramiding of different genes with elite haplotypes can be used to obtain desired grain shape to improve rice yield and grain quality, as well as traits suitable for mechanized seed production of hybrid rice.
Grain shape-related gene pyramiding to enhance grain yield
, as the first major QTL identified for grain length, has been combined with many grain shape-related QTLs including,,and, to investigate the effect of QTL pyramiding on the improvement of yield.
Firstly, a QTL pyramiding combining elite alleles ofandshowed that the two loci have an additive effect on cell elongation and grain length (Che et al, 2015). It is likely that the introduction of the elite allele oftogether with other grain shape controlling QTL can significantly enlarge grain size, enhance grain weight and increase grain yield in high-yielding varieties. Secondly, the combination ofandgenerates significant genetic effects, which leads to larger grain sizes than either of the parents (Zhang Y D et al, 2016). The rare beneficial allele ofhas greater positive effects on the grain length and grain weight than that of(Zhang Y D et al, 2016). Moreover,increases grain width whereasdecreases grain width (Fan et al, 2006; Zhang Y D et al, 2012). Ashas been widely used in mostvarieties, it is believed that introduction of eliteallele into existing varieties will further increase the grain yield (Takano-Kai et al, 2009; Zhang Y D et al, 2016). Thirdly,regulates grain length and thickness independent onlocus, suggesting that preferable grain shape can be achieved by pyramiding the elite alleles ofandin rice breeding (Si et al, 2016). Fourthly, four contrasting allelic combinations ofandwere assembled into HJX74 (Huajingxian 74, a high-yieldingvariety with short and wide grains) background (Wang et al, 2012). NIL-/plants harboring theallele from Basmati 385 produces longer and heavier grains than those from NIL-/plants. Meanwhile, NIL-/plants possessingandalleles from Basmati 385 have narrower and slender grains than those from NIL-/plants (Wang et al, 2012).
Grain shape-related gene utilization for grain quality improvement
The simultaneous improvement of grain quality and yield of cereal crops is a major challenge for modern agriculture. Fortunately, recent studies have revealed some grain shape-determining genes which can improve rice grain quality without sacrificing rice yield. For instance, it is still, which can pyramid withandgene to improve the quality of hybrid rice (Saichompoo et al, 2021). Moreover, a series of grain shape-determining genes, such as,,and, also show their great potential in improving grain quality (Nayak et al, 2022).
Thebeneficial allele is a major determinant of grain length in TFA (rice variety with superior grain quality and slender grain shape), while up-regulation ofconfers slender grains (Fan et al, 2006; Wang S K et al, 2015). Meanwhile, up-regulation ofslightly decreases the grain-filling rate, induces the expression of several starch synthesis genes in developing rice endosperm and thus enhances grain appearance quality with decreased chalkiness (Wang S K et al, 2015). NILs carrying various combinations of alleles at theandloci on the high-yielding variety HJX74 background are more productive and show improved grain quality compared with other matching NILs (Wang S K et al, 2015). Through QTL pyramiding of theTFAandalleles, researchers developed new high-yieldinghybrid rice such as Taifengyou 55 and Taifengyou 208 with substantially improved grain quality (Wang S K et al, 2015). Moreover, the beneficial allele of/also improves grain quality revealed by decreased chalkiness and larger tightly packed-starch granules in endosperm without affecting grain yield per plant, head rice rate, amylose content, gel consistency or protein content (Wang Y X et al, 2015). By crossing high-quality variety P13 with a relatively high-yielding restorer line T461X, the introduction of/can significantly improve grain quality without compromise of grain yield (Wang Y X et al, 2015). The combination of the beneficial/from Yuefeng (ansterile line widely used in southeast China for its excellent appearance quality) and eliteallele from Xieqingzao (a formerly widely used high-yielding sterile line), significantly improves grain appearance quality and grain weight (Wang Y X et al, 2015). Moreover, the-related molecular marker-assisted selection results in a new photo-thermo-sensitive sterile line Shenhuaxiang 15S, with grain length increased by 29% and the length-width ratio increased by 51.7%, compared with the recurrent parent Y58S (Hou et al, 2019). These studies suggest that utilization of,/or pyramiding themwill be likely to contribute to the breeding of new elite rice varieties with improved grain quality (Wang Y X et al, 2015).
elite allele (leading to premature of protein) increases the cell length but decreases the cell number at the longitudinal direction, whileelite allele increases cell number to promote the grain length formation (Mao et al, 2010; Xia et al, 2018). Based on this, researchers combined the elite alleles ofandinbackground Nanyangzhan and facilitated to breed rice with extra-long grains (Xia et al, 2018). According to the distinct function of genes, whether they can regulate cell division or cell expansion, different genes with beneficial alleles can be aggregated to make up for each other’s functional deficiencies, so as to promote the formation of grain length by promoting the number and size of cells at the same time.
Besidestogether with its partners, some other major genes not only significantly change the grain shape but also affect grain quality. For example,encodes a protein without known conserved functional domain and interacts with the transcription factors OsOFP14 and OsOFP8 in BR signaling as summarized before. A total of five haplotypes, H1–H5, were classified based on the natural variation analysis ongene. Mostcultivars belong to H1 haplotype, while mostcultivars belong to H5. However, it is implied that natural variation ingenes has no direct correlation with the grain shape determination. The rare allele ofconferring slender grains and good appearance quality may have occurred spontaneously during CSSL (chromosome segment substitutional lines) construction (Zhao et al, 2018). Loss-of-function ofby constructing NIL-or using CRISPR/Cas9 approach leads to a sharp increase of grain length/width ratio, whileover-expression lines are rounder than the background variety without obvious difference in grain thickness or any other agronomic traits (Zhao et al, 2018). Introgression of the nullallele into twocultivars, Wuyunjing 27 and Wuyunjing 8, with high yield and widely grown in lower reaches of Yangtze River in China, significantly increases grain length/ width ratio but not affects grain thickness or weight (Zhao et al, 2018). Most importantly, the nullallele causes decreased chalkiness of milled rice but doesn’t alters the brown and milled rice rate, apparent amylose and protein contents, gel consistency or gelatinization properties of starch (Zhao et al, 2018). Construction of homologous nullmutant in the high-yielding cultivar Yandao 8 through the CRISPR/ Cas9 system leads to slender grains and significant decrease of chalkiness (Zhao et al, 2018). The application of nullallele to improve grain appearance quality in high-yielding but poor appearance rice cultivars is of great potential, especially for the null allele with rare and hardly used in breeding (Zhao et al, 2018).
, the major QTL for determining grain length/width ratio, is also related to milled rice quality (Wang et al, 2012). Down-regulation ofexpression produces better appearance quality in terms of slender grain shape, higher endosperm transparency and lower grain chalkiness degree (Wang et al, 2012). Consistent with the lower extent of grain chalkiness, the endosperm of NILs with eliteallele comprises largely sharp-edged, compactlyarranged polygonal starch granules (Wang et al, 2012).
Constitutive expression and seed-specific promoter- driven expression ofnot only result in longer brown rice but also change the viscosity of rice flour and thus improve ECQ, with unaltered chalkiness degree but elevated chalky grain percentage (Li et al, 2018).
allele encodes a plastid-localized pyruvate dehydrogenase complex E1 component subunit α1, which plays a key role in amyloplast development and starch biosynthesis by regulating the glycerolipid biosynthesis in rice endosperm (Lei et al, 2022). Themutant shows opaque of the interior endosperm with abnormal compound starch grains in the endosperm cells, while the mutation also reduces the grain length, grain width, grain thickness, 1000-grain weight as well as plant height, tiller number, panicle length, seed-setting rate, grain yield per plant (Lei et al, 2022).may promote the gene expression of,and some cell cycle-related genes to possibly increase cell division rate (Lei et al, 2022). Importantly, it is also suggested that the natural variation in the promoter sequence affects its expression level and contributes to variation in grain length among rice varieties (Lei et al, 2022). Based on these findings, it is possible that the sequence polymorphism ofpromoter can be utilized for germplasm improvement through either genome editing or traditional selective breeding (Lei et al, 2022).
Application of grain shape characters in mechanized seed production of hybrid rice
The continuous selection for large grains to obtain high yield during early domestication has led to the accumulation of alleles promoting grain size and weight in current cultivated varieties. Small seeds are usually favored by natural selection, as small seed size is frequently associated with more seeds per plant, earlier maturity, and wider geographic distribution (Harlan, 1992). Nowadays, when selecting hybrid rice parents, however, parents’ seeds are not always the bigger the better. Not all parent materials are the bigger the better. The diversity of grain shape characters can be very useful for the mechanization of hybrid rice seed production.
The deployment of heterosis in hybrid rice has boostedgrain yield, but the inefficiency of distinguishing hybrid seeds from self-pollinated restorer lines restricts the mechanization of hybrid rice seed production. Three kinds of mechanical separation systems to separate hybrid seeds from self-bred seeds of restorer lines have been invented on the grain shape characters by breeders: (1) the separating cylinder of pocket hole separating machine based on grain length difference, (2) the gravity separation system based on grain weight difference, and (3) the sieving sorting system based on grain thickness difference (Wu et al, 2010; Xu E B et al, 2015; Tang et al, 2020; Wu et al, 2021) (Fig. 4).
Fig. 4. Main genes regulating three-dimension of grain shape and their potential value in mechanized production of hybrid seeds.
Genes with regulatory roles in the formation of grain length, grain width and grain thickness are highlighted in green, orange and purple, respectively. Red arrows indicate positive regulation, and blue block arrows indicate negative regulation. Red rectangles frame the genes with positive roles while blue rectangles frame genes with negative roles. The cylinder sorting, sieve sorting, gravity sorting systems can be used to separate hybrid rice seeds in dependence of grain length, grain thickness, and grain weight differences, respectively.
Notably, the grain thickness is the smallest of the three parameters of grain shape, which determines whether the grain can pass through the sieve. Recently, a series of small grain sterile lines such as Zhuo 234S (grain thickness as 1.71 mm, 1000-grain weight as 13.8 g) and Zhan 998S (grain thickness as 1.73 mm, 1000-grain weight as 13.7 g) have been created (Tang et al, 2022). The characteristic of small grain sterile lines is that the grain thickness is extremely small, but the ratio of grain length to grain width is good, and the grain size is appropriate to ensure rice appearance quality and grain weight of the offspring. On the other hand, large grain restorer lines such as Xiangnonghui 1484 (grain thickness as 2.33 mm, 1000-grain weightas 31.3 g) and Xiangnonghui 0985 (grain thickness as 2.35 mm, 1000-grain weight as 32.1 g) as well as R141, R581, R2115 and Xinhui 1998 with grain thickness ≥ 2.20 mm have been created (Tang et al, 2022).
The grain shape of the F1hybrid seeds is similar to that of the female sterile line with small grains. Based on the significant difference of grain shape between small grain F1hybrid seeds and large grain restorer line self- pollinated seeds, a special narrow and long sieve was designed with grain thicknessas the limiting factor (Tang et al, 2022). Through mechanical sorting of mixed seeds, hybrid rice combinations, such as Zhuo-liangyou 141 (ZLY141), was prepared. The loss percentage of self-bred restorer seeds is 0% while hybrid seed loss percentage is 2.31%, and the purity of hybrid rice seeds reaches the production standard (Tang et al, 2022).
Although the hybrid F1seeds are very small, they carry possibly some semi-dominant or dominant genes promoting grain size provided by the large grain male parent, leading to desired grain weight and thus high yield of F2seeds (Zhou et al, 2022). For example, besidesZLY141, a series of combinations, including ZLY581, ZLY1998, ZLY0985 and ZLY1126, have been approved by the state and widely promoted with a yield increase of 4% over the control variety and excellent grain quality (Tang et al, 2022). The sieving sorting system based on the grain thickness together withsupporting cultivation techniques is a major breakthrough in whole mechanized production of hybrid rice (Tang et al, 2022). Firstly, the advantages of the sieve sorting system based on grain thickness lie in that it can help realize the whole process of mechanized seed production and reduce the cost of seed production. Secondly, there are more hybrid seeds per kilogram, which greatly reduces seed consumption per unit area and improves the propagation coefficient.
Perspectives
Although rice breeders in China have succeeded in using grain shape difference to create novel varieties with desired characters, the major causal genes of grain shape difference between parents remain largely unclear. Most breeders still use the traditional method for screening numerous hybrid offspring based on observation of agronomic traits to create new varieties. Although new varieties such as Yangnonggeng 1030, Huabiao 1, Taifengyou 55, Taifengyou 208 and Shenhuaxiang 15S were created based on known genes by using molecular marker-assisted gene pyramiding or constructing introgression lines, they have only passed the variety approval of the respective provinces, and the large-scale promotion still needs to be strengthened (Wang et al, 2012; Wang S K et al, 2015; Hou et al, 2019; Zhang H G et al, 2021).
The known major genes and the related molecular markers may assist breeders to cultivate hybrid rice parents with distinct grain shape and therefore create hybrid rice combinations with lower seed production cost as well as excellent yield and grain quality (Fig. 4). However, to achieve the ultimate goal in breeding practice, the regulatory roles of valuable QTLs need detailed investigation in the commercial rice varieties widely planted. Moreover, it needs careful assessment of the value of grain shape-related genes to avoid side effect on panicle architecture, plant architecture or otheragronomic characters, in the consideration of pleiotropism effect. For instance, the simultaneous improvement of seed size and number is a major challenge for modern agriculture because of a genetic trade-off between these two traits (Sadras, 2007). Decreased seed-setting rate needs careful avoidance when grain shape-related genes are implicated in breeding.
In the future, different methods can be adopted to accelerate the utilization of favorable alleles of grain shape determining genes. Gene pyramiding is a practicable manner to balance the grain yield and grain quality (Lee et al, 2015). However, gene pyramiding usually costs a lot of time. Marker-assisted breeding has been successfully used in acceleration of the introgression of genes during the gene pyramiding process. Recently, 21 PCR-gel-based markers were developed to genotype the functional nucleotide polymorphism of 14 major grain shape determining genes (Zhang et al, 2020). The functional markers may speed up the gene pyramiding further and facilitate the rational design of grain shape in future rice breeding (Zhang et al, 2020). Moreover, the multi-gene editing system provides another rapid and efficient method to obtain non-transgenic genetic germplasm with preferred grain shape (Wang S X et al, 2020; Liu X Z et al, 2022).
Finally, it is still worth emphasizing that the application of grain-shape determining genes is based on the full-acknowledgement of gene function and genetic mechanisms. Therefore, further gene mining and investigation of regulatory network integrated by distinct signaling pathways are still necessary.
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 32100257, 32172078, 31871599 and 31901528), Hunan Science and Technology Innovation Program, China (Grant Nos. 2021NK1001, 2021NK1003 and 2021NK1011), Key Research and Development, Projects in Hunan Province, China (Grant No. 2020NK2054), and the Open Programs of the State Key Laboratory of Hybrid Rice, Changsha, China (Grant No. 2020KF03).
Ali M L, McClung A M, Jia M H, Kimball, J A, McCouch S R, Eizenga G C. 2011. A rice diversity panel evaluated for genetic and agro-morphological diversity between subpopulations and its geographic distribution., 51: 2021–2035.
Bai M Y, Zhang L Y, Gampala S S, Zhu S W, Song W Y, Chong K, Wang Z Y. 2007. Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice., 104: 13839–13844.
Biswal A K, Wu T Y, Urano D, Pelissier R, Morel J B, Jones A M, Biswal A K. 2022. Novel mutant alleles reveal a role of the extra- large G protein in rice grain filling, panicle architecture, plant growth, and disease resistance., 12: 782960.
Butardo V M J, Sreenivasulu N, Juliano B O. 2019. Improving rice grain quality: State-of-the-art and future prospects., 1892: 19–55.
Che R H, Tong H N, Shi B H, Liu Y Q, Fang S R, Liu D P, Xiao Y H, Hu B, Liu L C, Wang H R, Zhao M F, Chu C C. 2015. Control of grain size and rice yield by-mediated brassinosteroid responses., 2: 15195.
Chen J, Gao H, Zheng X M, Jin M N, Weng J F, Ma J, Ren Y L, Zhou K N, Wang Q, Wang J, Wang J L, Zhang X, Cheng Z J, Wu C Y, Wang H Y, Wan J M. 2015. An evolutionarily conserved gene,, plays a role in determining panicle architecture, grain shape and grain weight in rice., 83(3): 427–438.
Chen J, Wang L H, Yuan M. 2021. Update on the roles of rice MAPK cascades., 22(4): 1679.
Chen K, Łyskowski A, Jaremko Ł, Jaremko M. 2021. Genetic and molecular factors determining grain weight in rice., 12: 605799.
Choi B S, Kim Y J, Markkandan K, Koo Y J, Song J T, Seo H S. 2018. GW2 functions as an E3 ubiquitin ligase for rice expansin- like 1., 19(7): 1904.
Choi D, Kim J H, Kende H. 2004. Whole genome analysis of thegene family encoding plant-specific putative transcription activators in rice (L.)., 45(7): 897–904.
Chun Y, Fang J J, Zafar S A, Shang J Y, Zhao J F, Yuan S J, Li X Y. 2020.() encodes a receptor-like kinase that controls grain size and shape in rice., 13(1): 7.
Dixit N, Dokku P, Amitha Mithra S V, Parida S K, Singh A K, Singh N K, Mohapatra T. 2013. Haplotype structure in grain weight geneand its association with grain characteristics in rice., 192(1): 55–61.
Du Z X, Huang Z, Li J B, Bao J Z, Tu H, Zeng C H, Wu Z, Fu H H, Xu J, Zhou D H, Zhu C L, Fu J R, He H H. 2021., a naturally varying QTL, regulates grain weight in rice., 134(9): 2767–2776.
Duan P G, Rao Y C, Zeng D L, Yang Y L, Xu R, Zhang B L, Dong G J, Qian Q, Li Y H. 2014., which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice., 77(4): 547–557.
Duan P G, Ni S, Wang J M, Zhang B L, Xu R, Wang Y X, Chen H Q, Zhu X D, Li Y H. 2015. Regulation ofby OsmiR396 controls grain size and yield in rice., 2: 15203.
Duan P G, Xu J S, Zeng D L, Zhang B L, Geng M F, Zhang G Z, Huang K, Huang L J, Xu R, Ge S, Qian Q, Li Y H. 2017. Natural variation in the promoter ofcontributes to grain size diversity in rice., 10(5): 685–694.
Duan P G, Li Y H. 2021. Size matters: G protein signaling is crucial for grain size control in rice., 14(10): 1618–1620.
Fan C C, Xing Y Z, Mao H L, Lu T T, Han B, Xu C G, Li X H, Zhang Q F. 2006., a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein., 112(6): 1164–1171.
Fan C C, Yu S B, Wang C R, Xing Y Z. 2009. A causal C-A mutation in the second exon ofhighly associated with rice grain length and validated as a functional marker., 118(3): 465–472.
Feng Z M, Wu C Y, Wang C M, Roh J, Zhang L, Chen J, Zhang S Z, Zhang H, Yang C Y, Hu J L, You X M, Liu X, Yang X M, Guo X P, Zhang X, Wu F Q, Terzaghi W, Kim S K, Jiang L, Wan J M. 2016.controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice., 67(14): 4241–4253.
Gao S P, Fang J, Xu F, Wang W, Sun X H, Chu J F, Cai B D, Feng Y Q, Chu C C. 2014.integrates cytokinin and auxin signaling to control rice crown root formation., 165(3): 1035–1046.
Gao X Y, Zhang J Q, Zhang X J, Zhou J, Jiang Z S, Huang P, Tang Z B, Bao Y M, Cheng J P, Tang H J, Zhang W H, Zhang H S, Huang J. 2019. Rice qGL3/OsPPKL1 functions with the GSK3/ SHAGGY-like kinase OsGSK3 to modulate brassinosteroid signaling., 31(5): 1077–1093.
Gao X Y, Zhang J Q, Cai G, Du H Y, Li J B, Wang R Q, Wang Y J, Yin J, Zhang W C, Zhang H C, Huang J. 2022a. qGL3/OsPPKL1 induces phosphorylation of 14-3-3 protein OsGF14b to inhibit OsBZR1 function in brassinosteroid signaling., 188: 624–636.
Gao X Y, Zhang J Q, Li J B, Wang Y J, Zhang R, Du H Y, Yin J, Cai G, Wang R Q, Zhang B Y, Zhao Z, Zhang H C, Huang J, 2022b. The phosphoproteomic and interactomic landscape of qGL3/OsPPKL1-mediated brassinosteroid signaling in rice., 109(5): 1048–1063.
Guo M L, Zhang W C, Mohammadi M A, He Z M, She Z Y, Yan M K, Shi C, Lin L W, Wang A Q, Liu J D, Tian D G, Zhao H M, Qin Y. 2022.controls grain size by influencing cell cycling and regulating homeostasis and signaling of brassinosteroid in rice., 13: 873993.
Guo T, Chen K, Dong N Q, Shi C L, Ye W W, Gao J P, Shan J X, Lin H X. 2018.negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice., 30(4): 871–888.
Guo T, Lu Z Q, Shan J X, Ye W W, Dong N Q, Lin H X. 2020.acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice., 32(9): 2763–2779.
Hao J Q, Wang D K, Wu Y B, Huang K, Duan P G, Li N, Xu R, Zeng D L, Dong G J, Zhang B L, Zhang L M, Inzé D, Qian Q, Li Y H. 2021. The GW2-WG1-OsbZIP47 pathway controls grain size and weight in rice., 14(8): 1266–1280.
Harberd N P. 2015. Shaping taste: The molecular discovery of rice genes improving grain size, shape and quality., 42(11): 597–599.
Harlan J. 1992. Crops and Man. WI, USA: Am Soc Agronomy CSSA: 284.
He Y, Li L Y, Shi W B, Tan J H, Luo X X, Zheng S Y, Chen W T, Li J, Zhuang C X, Jiang D G. 2022. Florigen repression complexes involving rice CENTRORADIALIS2 regulate grain size., 190(2): 1260–1274.
Hirano K, Yoshida H, Aya K, Kawamura M, Hayashi M, Hobo T, Sato-Izawa K, Kitano H, Ueguchi-Tanaka M, Matsuoka M. 2017. Small organ size 1 and small organ size 2/dwarf and low-tillering form a complex to integrate auxin and brassinosteroid signaling in rice., 10(4): 590–604.
Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. 2003. A rice brassinosteroid-deficient mutant,(), is caused by a loss of function of a new member of cytochrome P450., 15(12): 2900–2910.
Hou J L, Xi X Y, Li S M, Li Z H, Li G, Fu Q, Li Y F, Yan Y T, Yang Q S, Yu H, Xia Q J, Liu B L, Li B, Li Y, Zhang G Y, Zhao S Q, Ni X M. 2019. Molecular markers and RAD sequencing assistant selection for improvement of grain size in two-line sterile rice., 18(8): 2552–2558. (in Chinese with English abstract)
Hu J, Wang Y X, Fang Y X, Zeng L J, Xu J, Yu H P, Shi Z Y, Pan J J, Zhang D, Kang S J, Zhu L, Dong G J, Guo L B, Zeng D L, Zhang G H, Xie L H, Xiong G S, Li J Y, Qian Q. 2015. A rare allele ofenhances grain size and grain yield in rice., 8(10): 1455–1465.
Hu J H, Huang L Y, Chen G L, Liu H, Zhang Y S, Zhang R, Zhang S L, Liu J T, Hu Q Y, Hu F Y, Wang W, Ding Y. 2021. The elite alleles ofregulate grain size and increase grain yield in rice., 14(1): 90.
Hu Z J, He H H, Zhang S Y, Sun F, Xin X Y, Wang W X, Qian X, Yang J S, Luo X J. 2012. A Kelch motif-containing serine/ threonine protein phosphatase determines the large grain QTL trait in rice., 54(12): 979–990.
Hu Z J, Lu S J, Wang M J, He H H, Sun L, Wang H R, Liu X H, Jiang L, Sun J L, Xin X Y, Kong W, Chu C C, Xue H W, Yang J S, Luo X J, Liu J X. 2018. A novel QTLencodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice., 11(5): 736–749.
Huang K, Wang D K, Duan P G, Zhang B L, Xu R, Li N, Li Y H. 2017., which encodes an otubain-like protease with deubiquitination activity, influences grain size and shape in rice., 91(5): 849–860.
Huang X Z, Qian Q, Liu Z B, Sun H Y, He S Y, Luo D, Xia G M, Chu C C, Li J Y, Fu X D. 2009. Natural variation at thelocus enhances grain yield in rice., 41(4): 494–497.
Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B I, Onishi A, Miyagawa H, Katoh E. 2013. Loss of function of the IAA-glucose hydrolase geneenhances rice grain weight and increases yield., 45(6): 707–711.
Jiang Y H, Bao L, Jeong S Y, Kim S K, Xu C G, Li X H, Zhang Q F. 2012.is involved in the control of organ size by contributing to the regulation of signaling and homeostasis of brassinosteroids and cell cycling in rice., 70(3): 398–408.
Kerr I D, Bennett M J. 2007. New insight into the biochemical mechanisms regulating auxin transport in plants., 401(3): 613–622.
Khush G S. 1997. Origin, dispersal, cultivation and variation of rice., 35(1/2): 25–34.
Kim J H, Tsukaya H. 2015. Regulation of plant growth and development by the growth-regulating factor and grf-interacting factor Duo., 66(20): 6093–6107.
Koh S, Lee S C, Kim M K, Koh J H, Lee S, An G, Choe S, Kim S R. 2007. T-DNA tagged knockout mutation of rice, an orthologue ofBIN2, with enhanced tolerance to various abiotic stresses., 65: 453–466.
Lee C M, Park J, Kim B, Seo J, Lee G, Jang S, Koh H J. 2015. Influence of multi-gene allele combinations on grain size of rice and development of a regression equation model to predict grain parameters., 8(1): 33.
Lei J, Teng X, Wang Y F, Jiang X K, Zhao H H, Zheng X M, Ren Y L, Dong H, Wang Y L, Duan E C, Zhang Y Y, Zhang W W, Yang H, Chen X L, Chen R B, Zhang Y, Yu M Z, Xu S B, Bao X H, Zhang P C, Liu S J, Liu X, Tian Y L, Jiang L, Wang Y H, Wan J M. 2022. Plastidic pyruvate dehydrogenase complex E1 component subunit Alpha1 is involved in galactolipid biosynthesis required for amyloplast development in rice., 20: 437–453.
Leyser O. 2018. Auxin signaling., 176: 465–479.
Li D, Wang L, Wang M, Xu Y Y, Luo W, Liu Y J, Xu Z H, Li J, Chong K. 2009. Engineeringgene as a molecular tool to improve rice architecture for high yield., 7(8): 791–806.
Li J, Chu H W, Zhang Y H, Mou T M, Wu C Y, Zhang Q F, Xu J. 2012. The ricegene encodes a ubiquitin-associated (UBA) domain protein that regulates heading date and grain weight., 7(3): e34231.
Li N, Li Y H. 2016. Signaling pathways of seed size control in plants., 33: 23–32.
Li N, Xu R, Li Y H. 2019. Molecular networks of seed size control in plants., 70: 435–463.
Li Q F, Yu J W, Lu J, Fei H Y, Luo M, Cao B W, Huang L C, Zhang C Q, Liu Q Q. 2018. Seed-specific expression of, a rate-limiting gene involved in brassinosteroids biosynthesis, improves both grain yield and quality in rice., 66(15): 3759–3772.
Li R S, Li Z, Ye J, Yang Y Y, Ye J, Xu S L, Liu J R, Yuan X P, Wang Y P, Zhang M C, Yu H Y, Xu Q, Wang S, Yang Y L, Wang S, Wei X H, Feng Y. 2022. Identification of, a QTL coordinately controls grain size, grain number per panicle, and grain weight in rice., 13: 880919.
Li S C, Gao F Y, Xie K L, Zeng X H, Cao Y, Zeng J, He Z S, Ren Y, Li W B, Deng Q M, Wang S Q, Zheng A P, Zhu J, Liu H N, Wang L X, Li P. 2016. The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice., 14(11): 2134–2146.
Li X B, Tao Q D, Miao J, Yang Z F, Gu M H, Liang G H, Zhou Y. 2019. Evaluation of differential qPE9-1/DEP1 protein domains in rice grain length and weight variation., 12(1): 5.
Li Y B, Fan C C, Xing Y Z, Jiang Y H, Luo L J, Sun L, Shao D, Xu C J, Li X H, Xiao J H, He Y Q, Zhang Q F. 2011. Natural variation inplays an important role in regulating grain size and yield in rice., 43(12): 1266–1269.
Liu J F, Chen J, Zheng X M, Wu F Q, Lin Q B, Heng Y Q, Tian P, Cheng Z J, Yu X W, Zhou K N, Zhang X, Guo X P, Wang J L, Wang H Y, Wan J M. 2017.acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice., 3: 17043.
Liu L C, Tong H N, Xiao Y H, Che R H, Xu F, Hu B, Liang C Z, Chu J F, Li J Y, Chu C C. 2015. Activation ofsignificantly improves grain size by regulating auxin transport in rice., 112: 11102–11107.
Liu Q, Han R X, Wu K, Zhang J Q, Ye Y F, Wang S S, Chen J F, Pan Y J, Li Q, Xu X P, Zhou J W, Tao D Y, Wu Y J, Fu X D. 2018. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice., 9(1): 852.
Liu S Y, Hua L, Dong S J, Chen H Q, Zhu X D, Jiang J E, Zhang F, Li Y H, Fang X H, Chen F. 2015. OsMAPK6, a mitogen- activated protein kinase, influences rice grain size and biomass production., 84(4): 672–681.
Liu X Z, Zhang S W, Jiang Y L, Yan T W, Fang C W, Hou Q C, Wu S W, Xie K, An X L, Wan X Y. 2022. Use of CRISPR/ Cas9-Based gene editing to simultaneously mutate multiple homologous genes required for pollen development and male fertility in maize., 11(3): 439.
Ma G H, Yuan L P. 2015. Hybrid rice achievements, development and prospect in China., 2: 197–205.
Ma H G, Gao Y J, Wang Y G, Dai Y, Ma H X. 2022. Regulatory mechanisms of mitogen-activated protein kinase cascades in plants:More than sequential phosphorylation., 23(7): 3572.
Mao H L, Sun S Y, Yao J L, Wang C R, Yu S B, Xu C G, Li X H, Zhang Q F. 2010. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice., 107: 19579–19584.
Miao C B, Wang D, He R Q, Liu S K, Zhu J K. 2020. Mutations inandincrease grain size and modulate shoot architecture in rice., 18(2): 491–501.
Miao J, Yang Z F, Zhang D P, Wang Y Z, Xu M B, Zhou L H, Wang J, Wu S J, Yao Y L, Du X, Gu F F, Gong Z Y, Gu M H, Liang G H, Zhou Y. 2019. Mutation of, which encodes a type B heterotrimeric G protein γ subunit, increases grain size and yield production in rice., 17(3): 650–664.
Mitchell J W, Mandava N, Worley J F, Plimmer J R, Smith M V. 1970. Brassins: A new family of plant hormones from rape pollen., 225: 1065–1066.
Mori M, Nomura T, Ooka H, Ishizaka M, Yokota T, Sugimoto K, Okabe K, Kajiwara H, Satoh K, Yamamoto K, Hirochika H, Kikuchi S. 2002. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis., 130(3): 1152–1161.
Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M. 2006. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice., 141(3): 924–931.
Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, Hasegawa Y, Kitano H, Matsuoka M. 2006. The role ofand its homologous genes,and, in rice., 140: 580–590.
Nayak A K, Anilkumar C, Behera S, Sah R P, Lavanya G R, Kumar A, Behera L, Tp M A. 2022. Genetic dissection of grain size traits through genome-wide association study vased on genic markers in rice., 29(5): 462–472.
Ofoe R. 2021. Signal transduction by plant heterotrimeric G-protein., 23(1): 3–10.
Oh M H, Honey S H, Tax F E. 2020. The control of cell expansion, cell division, and vascular development by brassinosteroids: A historical perspective., 21(5): 1743.
Oki K, Fujisawa Y, Kato H, Iwasaki Y. 2005. Study of the constitutively active form of the alpha subunit of rice heterotrimeric G proteins., 46(2): 381–386.
Oki K, Kitagawa K, Fujisawa Y, Kato H, Iwasaki Y. 2009. Function of alpha subunit of heterotrimeric G protein in brassinosteroid response of rice plants., 4(2): 126–128.
Pan W B, Wu Y R, Xie Q. 2019. Regulation of ubiquitination is central to the phosphate starvation response., 24: 755–769.
Qi P, Lin Y S, Song X J, Shen J B, Huang W, Shan J X, Zhu M Z, Jiang L W, Gao J P, Lin H X. 2012. The novel quantitative trait locuscontrols rice grain size and yield by regulating Cyclin-T1;3., 22(12): 1666–1680.
Qiao J Y, Jiang H Z, Lin Y Q, Shang L G, Wang M, Li D M, Fu X D, Geisler M, Qi Y H, Gao Z Y, Qian Q. 2021. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice., 14(10): 1683–1698.
Reyes-Turcu F E, Ventii K H, Wilkinson K D. 2009. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes., 78: 363–397.
Rogers K, Chen X M. 2013. Biogenesis, turnover, and mode of action of plant microRNAs., 25(7): 2383–2399.
Sadras V O. 2007. Evolutionary aspects of the trade-off between seed size and number in crops., 100(2/3): 125–138.
Saichompoo U, Narumol P, Nakwilai P, Thongyos P, Nanta A, Tippunya P, Ruengphayak S, Itthisoponkul T, Bueraheng N, Cheabu S, Malumpong C. 2021. Breeding novel short grain rice for tropical region to combine important agronomical traits, biotic stress resistance and cooking quality in Koshihikari background., 28(5): 479–792.
Sakakibara H. 2006. Cytokinins: Activity, biosynthesis, and translocation., 57: 431–449.
Sakamoto T, Matsuoka M. 2008. Identifying and exploiting grain yield genes in rice., 11(2): 209–214.
Shang J Y, He X J. 2022. Chromatin-remodeling complexes: Conserved and plant-specific subunits in., 64(2): 499–515.
Shi C L, Ren Y L, Liu L L, Wang F, Zhang H, Tian P, Pan T, Wang Y F, Jing R N, Liu T Z, Wu F Q, Lin Q B, Lei C L, Zhang X, Zhu S S, Guo X P, Wang J L, Zhao Z C, Wang J, Zhai H Q, Cheng Z J, Wan J M. 2019. Ubiquitin specific protease 15 has an important role in regulating grain width and size in rice., 180(1): 381–391.
Shi C L, Dong N Q, Guo T, Ye W W, Shan J X, Lin H X. 2020. A quantitative trait locuscontrols rice grain size and yield through the gibberellin pathway., 103(3): 1174–1188.
Shomura A, Izawa T, Ebana K, Ebitani T, Kanegae H, Konishi S, Yano M. 2008. Deletion in a gene associated with grain size increased yields during rice domestication., 40(8): 1023–1028.
Si L Z, Chen J Y, Huang X H, Gong H, Luo J H, Hou Q Q, Zhou T Y, Lu T T, Zhu J J, Shangguan Y Y, Chen E W, Gong C X, Zhao Q, Jing Y F, Zhao Y, Li Y, Cui L L, Fan D L, Lu Y Q, Weng Q J, Wang Y C, Zhan Q L, Liu K Y, Wei X H, An K, An G, Han B. 2016.controls grain size in cultivated rice., 48(4): 447–456.
Smalle J, Vierstra R D. 2004. The ubiquitin 26S proteasome proteolytic pathway., 55: 555–590.
Song X J, Huang W, Shi M, Zhu M Z, Lin H X. 2007. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase., 39(5): 623–630.
Song X J, Kuroha T, Ayano M, Furuta T, Nagai K, Komeda N, Segami S, Miura K, Ogawa D, Kamura T, Suzuki T, Higashiyama T, Yamasaki M, Mori H, Inukai Y, Wu J Z, Kitano H, Sakakibara H, Jacobsen S E, Ashikari M. 2015. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice., 112(1): 76–81.
Spinner L, Gadeyne A, Belcram K, Goussot M, Moison M, Duroc Y, Eeckhout D, De Winne N, Schaefer E, Van De Slijke E, Persiau G, Witters E, Gevaert K, De Jaeger G, Bouchez D, van Damme D, Pastuglia M. 2013. A protein phosphatase 2A complex spatially controls plant cell division., 4: 1863.
Sun H Y, Qian Q, Wu K, Luo J J, Wang S S, Zhang C W, Ma Y F, Liu Q, Huang X Z, Yuan Q B, Han R X, Zhao M, Dong G J, Guo L B, Zhu X D, Gou Z H, Wang W, Wu Y J, Lin H X, Fu X D. 2014. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice., 46(6): 652–656.
Sun S Y, Wang L, Mao H L, Shao L, Li X H, Xiao J H, Ouyang Y D, Zhang Q F. 2018. A G-protein pathway determines grain size in rice., 9(1): 851.
Takano-Kai N, Jiang H, Kubo T, Sweeney M, Matsumoto T, Kanamori H, Padhukasahasram B, Bustamante C, Yoshimura A, Doi K, McCouch S. 2009. Evolutionary history of, a gene conferring grain length in rice., 182(4): 1323–1334.
Tan Y F, Xing Y Z, Li J X, Yu S B, Xu C G, Zhang Q F. 2000. Genetic bases of appearance quality of rice grains in Shanyou 63, an elite rice hybrid., 101(5): 823–829.
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y. 2005. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant,, with reduced seed length., 17(3): 776–790.
Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang C J, Dubouzet J G, Kikuchi S, Sekimoto H, Yokota T, Asami T, Kamakura T, Mori M. 2009., encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice., 151(2): 669–680.
Tang W B, Zhang G L, Deng H B. 2020. Technology exploration and practice of hybrid rice mechanized seed production., 34(2): 95–103. (in Chinese with English abstract)
Tang W B, Chen X J, Zhang G L, Deng H B, Hu Y Y, Tian Y. 2022. Current situation and technical breakthrough of mechanized hybrid rice seed production., 28(5): 20–27. (in Chinese with English abstract)
Tao Y J, Miao J, Wang J, Li W Q, Xu Y, Wang F Q, Jiang Y J, Chen Z H, Fan F J, Xu M B, Zhou Y, Liang G H, Yang J. 2020., involved in the cytokinin regulatory pathway, controls grain size in rice., 13(1): 76.
Tian X J, Li X F, Zhou W J, Ren Y K, Wang Z Y, Liu Z Q, Tang J Q, Tong H N, Fang J, Bu Q Y. 2017. Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture., 175(3): 1337–1349.
Tian X J, He M L, Mei E Y, Zhang B W, Tang J Q, Xu M, Liu J L, Li X F, Wang Z Y, Tang W Q, Guan Q J, Bu Q Y. 2021. WRKY53 integrates classic brassinosteroid signaling and the mitogen-activated protein kinase pathway to regulate rice architecture and seed size., 33(8): 2753–2775.
Tong H N, Chu C C. 2018. Functional specificities of brassinosteroid and potential utilization for crop improvement., 23(11): 1016–1028.
Tong H N, Liu L C, Jin Y, Du L, Yin Y H, Qian Q, Zhu L H, Chu C C. 2012. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice., 24(6): 2562–2577.
Uji Y, Kashihara K, Kiyama H, Mochizuki S, Akimitsu K, Gomi K. 2019. Jasmonic acid-induced VQ-motif-containing protein OsVQ13 influences the OsWRKY45 signaling pathway and grain size by associating with OsMPK6 in rice., 20(12): 2917.
Unnevehr L J, Duff B, Juliano B O. 1992. Consumer Demand for Rice Grain Quality. Manila, the Philippine: International Rice Research Institute.
Utsunomiya Y, Samejima C, Takayanagi Y, Izawa Y, Yoshida T, Sawada Y, Fujisawa Y, Kato H, Iwasaki Y. 2011. Suppression of the rice heterotrimeric G protein β-subunit gene,, causes dwarfism and browning of internodes and lamina joint regions., 67(5): 907–916.
Varshney V, Majee M. 2022. Emerging roles of the ubiquitin- proteasome pathway in enhancing crop yield by optimizing seed agronomic traits., 41(9): 1805–1826.
Verma A, Prakash G, Ranjan R, Tyagi A K, Agarwal P. 2021. Silencing of an ubiquitin ligase increases grain width and weight inrice., 11: 600378.
Wan X Y, Weng J F, Zhai H Q, Wang J K, Lei C L, Liu X L, Guo T, Jiang L, Su N, Wan J M. 2008. Quantitative trait loci (QTL) analysis for rice grain width and fine mapping of an identified QTL allelein a recombination hotspot region on chromosome 5., 179(4): 2239–2252.
Wang M, Qiao J Y, Yu C L, Chen H, Sun C D, Huang L Z, Li C Y, Geisler M, Qian Q, Jiang D A, Qi Y H. 2019. The auxin influx carrier, OsAUX3, regulates rice root development and responses to aluminium stress., 42(4): 1125–1138.
Wang S K, Wu K, Yuan Q B, Liu X Y, Liu Z B, Lin X Y, Zeng R Z, Zhu H T, Dong G J, Qian Q, Zhang G Q, Fu X D. 2012. Control of grain size, shape and quality byin rice., 44(8): 950–954.
Wang S K, Li S, Liu Q, Wu K, Zhang J Q, Wang S S, Wang Y, Chen X B, Zhang Y, Gao C X, Wang F, Huang H X, Fu X D. 2015. Theregulatory module determines grain shape and simultaneously improves rice yield and grain quality., 47(8): 949–954.
Wang S S, Wu K, Qian Q, Liu Q, Li Q, Pan Y J, Ye Y F, Liu X Y, Wang J, Zhang J Q, Li S, Wu Y J, Fu X D. 2017. Non-canonical regulation of SPL transcription factors by a human OTUB1-like deubiquitinase defines a new plant type rice associated with higher grain yield., 27(9): 1142–1156.
Wang S X, Zong Y, Lin Q P, Zhang H W, Chai Z Z, Zhang D D, Chen K L, Qiu J L, Gao C X. 2020. Precise, predictable multi- nucleotide deletions in rice and wheat using APOBEC-Cas9., 38(12): 1460–1465.
Wang Y D, Zhang T, Wang R C, Zhao Y D. 2018. Recent advances in auxin research in rice and their implications for crop improvement., 69(2): 255–263.
Wang Y X, Xiong G S, Hu J, Jiang L, Yu H, Xu J, Fang Y X, Zeng L J, Xu E B, Xu J, Ye W J, Meng X B, Liu R F, Chen H Q, Jing Y H, Wang Y H, Zhu X D, Li J Y, Qian Q. 2015. Copy number variation at thelocus contributes to grain size diversity in rice., 47(8): 944–948.
Weng J F, Gu S H, Wan X Y, Gao H, Guo T, Su N, Lei C L, Zhang X, Cheng Z J, Guo X P, Wang J L, Jiang L, Zhai H Q, Wan J M. 2008. Isolation and initial characterization of, a major QTL associated with rice grain width and weight., 18(12): 1199–1209.
Workman J, Kingston R. 1998. Althernation of nucleosome structure as a mechanism of transcriptional regulation., 67: 545–579.
Wu J X, Qiu S J, Wang M L, Xu C J, Deng X W, Tang X Y. 2021. Construction of a weight-based seed sorting system for the third-generation hybrid rice., 14(1): 66.
Wu M L, Xie F P, Tang L, Yu Y H, Wang H M, Deng P, Liu Y. 2010. Design of separating cylinder of pocket hole separating machine on large-scale seeding production for hybrid rice., 10(26): 1–4. (in English with Chinese abstract)
Xia D, Zhou H, Liu R J, Dan W H, Li P B, Wu B, Chen J X, Wang L Q, Gao G J, Zhang Q L, He Y Q. 2018., a novel QTL encoding a GSK3/SHAGGY-like kinase, epistatically interacts withto produce extra-long grains in rice., 11(5): 754–756.
Xiao Y H, Liu D P, Zhang G X, Tong H N, Chu C C. 2017. Brassinosteroids regulate OFP1, a DLT interacting protein, to modulate plant architecture and grain morphology in rice., 8: 1698.
Xiao Y H, Liu D P, Zhang G X, Gao S P, Liu L C, Xu F, Che R H, Wang Y Q, Tong H N, Chu C C. 2019., encoding a purine permease, regulates grain size via modulating cytokinin transport in rice., 61(5): 581–597.
Xiao Y H, Zhang G X, Liu D P, Niu M, Tong H N, Chu C C. 2020a. GSK2 stabilizes OFP3 to suppress brassinosteroid responses in rice., 102(6): 1187–1201.
Xiao Y H, Zhang J W, Yu G Y, Lu X D, Mei W T, Deng H B, Zhang G L, Chen G H, Chu C C, Tong H N, Tang W B. 2020b. Endoplasmic reticulum-localized PURINE PERMEASE1 regulates plant height and grain weight by modulating cytokinin distribution in rice., 11: 618560.
Xie K B, Wu C Q, Xiong L Z. 2006. Genomic organization, differential expression, and interaction ofpromoter-binding-like transcription factors and microRNA156 in rice., 142(1): 280–293.
Xu C J, Liu Y, Li Y B, Xu X D, Xu C G, Li X H, Xiao J H, Zhang Q F. 2015. Differential expression ofregulates grain size in rice., 66(9): 2611–2623.
Xu E B, Wang Y X, Ni S, Chen H Q, Zhu X D. 2015. Application of recessive small grain gene in mechanical sorting of hybrid rice seeds., 21(3): 8–11. (in Chinese)
Xu R, Duan P G, Yu H Y, Zhou Z K, Zhang B L, Wang R C, Li J, Zhang G Z, Zhuang S S, Lyu J, Li N, Chai T Y, Tian Z X, Yao S G, Li Y H. 2018. Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice., 11(6): 860–873.
Yan Y, Wei M X, Li Y, Tao H, Wu H Y, Chen Z F, Li C, Xu J H. 2021. MiR529a controls plant height, tiller number, panicle architecture and grain size by regulatingtarget genes in rice (L.)., 302: 110728.
Yang C, Shen W J, He Y, Tian Z H, Li J X. 2016. OVATE family protein 8 positively mediates brassinosteroid signaling through interacting with the GSK3-like kinase in rice., 12(6): e1006118.
Yang X F, Zhao X L, Dai Z Y, Ma F L, Miao X X, Shi Z Y. 2021. OsmiR396/growth regulating factor modulate rice grain size through direct regulation of embryo-specific miR408., 186(1): 519–533.
Yin W C, Xiao Y H, Niu M, Meng W J, Li L L, Zhang X X, Liu D P, Zhang G X, Qian Y W, Sun Z T, Huang R Y, Wang S P, Liu C M, Chu C C, Tong H N. 2020. ARGONAUTE2 enhances grain length and salt tolerance by activatingto modulate cytokinin distribution in rice., 32(7): 2292–2306.
Yu J, Miao J, Zhang Z, Xiong H, Zhu X, Sun X, Pan Y, Liang Y, Zhang Q, Abdul Rehman R M, Li J, Zhang H, Li Z. 2018. Alternative splicing ofcontrols grain length and yield inrice., 16(9):1667–1678.
Yuan H, Fan S J, Huang J, Zhan S J, Wang S F, Gao P, Chen W L, Tu B, Ma B T, Wang Y P, Qin P, Li S G. 2017.regulates grain size and number, and functions differently inandbackgrounds in rice., 10(1): 25.
Yuan H, Gao P, Hu X L, Yuan M, Xu Z Y, Jin M Y, Song W C, Zhan S J, Zhu X B, Tu B, Li T, Wang Y P, Ma B T, Qin P, Chen W L, Li S G. 2022a. Fine mapping and candidate gene analysis of, a novel quantitative trait locus coordinating grain size and grain number in rice., 135(1): 51–64.
Yuan H, Xu Z Y, Chen W L, Deng C Y, Liu Y, Yuan M, Gao P, Shi H, Tu B, Li T, Kang L Z, Ma B T, Wang Y P, Wang J, Chen X W, Li S G, Qin P. 2022b. OsBSK2, a putative brassinosteroid- signalling kinase, positively controls grain size in rice., 73(16): 5529–5542.
Zhang B W, Wang X L, Zhao Z Y, Wang R J, Huang X H, Zhu Y L, Yuan L, Wang Y C, Xu X D, Burlingame A L, Gao Y J, Sun Y, Tang W Q. 2016. OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation., 170(2): 1149–1161.
Zhang C, Xu Y Y, Guo S Y, Zhu J Y, Huan Q, Liu H H, Wang L, Luo G Z, Wang X J, Chong K. 2012. Dynamics of brassinosteroid response modulated by negative regulator LIC in rice., 8(4): e1002686.
Zhang D P, Zhang M Y, Liang J S. 2021. RGB1 regulates grain development and starch accumulation through its effect on-mediated auxin biosynthesis in rice endosperm cells., 12: 585174.
Zhang H G, Wang R X, Xu Z P, Liu Q Q, Yan C J, Liang G H, Zhou Y, Tang S Z, Gu M H. 2021. Breeding of Yangnongjing 1030, a new medium maturityrice variety with long grains., 49(4): 62–65. (in Chinese with English abstract)
Zhang J Q, Gao X Y, Cai G, Wang Y J, Li J B, Du H Y, Wang R Q, Zhang H S, Huang J. 2021. An adenylate kinase OsAK3 involves brassinosteroid signaling and grain length in rice (L.)., 14(1): 105.
Zhang L, Ma B, Bian Z, Li X Y, Zhang C Q, Liu J Y, Li Q, Liu Q Q, He Z H. 2020. Grain size selection using novel functional markers targeting 14 genes in rice., 13(1): 63.
Zhang X J, Wang J F, Huang J, Lan H X, Wang C L, Yin C F, Wu Y Y, Tang H J, Qian Q, Li J Y, Zhang H S. 2012. Rare allele ofassociated with grain length causes extra-large grain and a significant yield increase in rice., 109(52): 21534–21539.
Zhang Y D, Zhu Z, Zhao Q Y, Chen T, Yao S, Zhou L H, Zhao L, Zhao C F, Wang C L. 2016. Haplotypes ofand their roles in grain size regulation withalleles in rice., 15(1): gmr.15017587.
Zhang Z X, Zhao H, Huang F L, Long J F, Song G, Lin W X. 2019. The 14-3-3 protein GF14f negatively affects grain filling of inferior spikelets of rice (L.)., 99(2): 344–358.
Zhang Z Y, Li J J, Tang Z S, Sun X M, Zhang H L, Yu J P, Yao G X, Li G L, Guo H F, Li J L, Wu H M, Huang H G, Xu Y W, Yin Z G, Qi Y H, Huang R F, Yang W C, Li Z C. 2018. Gnp4/LAX2, a RAWUL protein, interferes with the OsIAA3-OsARF25 interaction to regulate grain length via the auxin signaling pathway in rice., 69(20): 4723–4737.
Zhao D S, Li Q F, Zhang C Q, Zhang C, Yang Q Q, Pan L X, Ren X Y, Lu J, Gu M H, Liu Q Q. 2018.acts as a transcriptional activator to regulate rice grain shape and appearance quality., 9(1): 1240.
Zhao J F, Wu C X, Yuan S J, Yin L, Sun W, Zhao Q L, Zhao B H, Li X Y. 2013. Kinase activity of OsBRI1 is essential for brassinosteroids to regulate rice growth and development., 199/200: 113–120.
Zhao Y D. 2010. Auxin biosynthesis and its role in plant development., 61: 49–64.
Zheng Y M, Zhu Y S, Mao X H, Jiang M R, Wei Y D, Lian L, Xu H B, Chen L P, Xie H A, Lu G D, Zhang J F. 2022. SDR7-6, a short-chain alcohol dehydrogenase/reductase family protein, regulates light-dependent cell death and defence responses in rice., 23(1): 78–91.
Zhou J Q, Zhang G L, Deng H B, Ming X Q, Lei B, Li F, Tang W B. 2022. Advantages of small grain male sterile lines in seed production for a new combination Zhuoliangyou 141 through the mixed-sowing manner., 48(2): 320–331. (in Chinese with English abstract)
Zhou Y, Zhu J Y, Li Z Y, Yi C D, Liu J, Zhang H G, Tang S Z, Gu M H, Liang G H. 2009. Deletion in a quantitative trait geneassociated with panicle erectness improves plant architecture during rice domestication., 183(1): 315–324.
Zhou Y, Miao J, Gu H Y, Peng X R, Leburu M, Yuan F H, Gu H W, Gao Y, Tao Y J, Zhu J Y, Gong Z Y, Yi C D, Gu M H, Yang Z F, Liang G H. 2015. Natural variations inregulate grain shape in rice., 201(4): 1591–1599.
Zhou Y, Tao Y J, Zhu J Y, Miao J, Liu J, Liu Y H, Yi C D, Yang Z F, Gong Z Y, Liang G H. 2017., a novel allele of, regulates grain number and grain size in a high-yield rice variety., 10(1): 34.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.03.014
5 July 2022;
8 March 2023
Tang Wenbang (tangwenbang@163.com)
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa

