Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress
Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali
Review
Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress
Ammara Latif1, Sun Ying1, Pu Cuixia1, Noman Ali2
()
Leaf rolling (LR) is one of the defensive mechanisms that plants have developed against adverse environmental conditions. LR is a typical drought response, promoting drought resistance in various gramineae species, including wheat, maize, and rice. Rice cultivation faces the formidable challenge of water deprivation because of its high water requirements, which leads to drought-related symptoms in rice. LR is an important morphological characteristic that plays a key role in controlling water loss during water insufficiency, thereby regulating leaf area and stature, which are crucial agronomic traits determining yield criteria. Bulliform, sclerenchyma, mesophyll, and vascular bundles are the cells that engage in LR and commonly exhibit adaxial or abaxial types of rolling in rice. The specific genes linked to rolling, either adaxially or abaxially, are discussed here. In addition to the factors influencing LR, here is a short review of the morphological, physiological and molecular responses of this adaptation under drought stress. Moreover, this review highlights how LR combats the consequences of drought stress. The eco-physiologicaland molecular mechanisms underlying this morphological adaptation in rice should be further explored, as they might be useful in dealing with various degrees of drought tolerance.
adaxial/abaxial rolling; drought stress; leaf rolling; molecular mechanism; rice; physiological response;transcript factor
More than half of the world’s population consumes rice (L.) asan essential food (Irshad et al, 2022). Cereal crops including rice, maize, wheat, sorghum, and millet are the primary food supply for the vast majority of people on earth. Rice is accountable for more than 20% of the calories among three cereal crops: rice, wheat and maize, which together contribute to 50% of the calories consumed by the entire population (Maclean et al, 2002; Yu and Tian, 2018;Ma et al, 2022). By 2050, when the world population is projected to be more than doubled, there won’t be enough rice to supply demand even with an average yearly rise in worldwide production (Fischer et al, 2009). Contrary to other cereal crops, rice grows naturally in submerged regions; as a result, water may play a crucial role in agricultural production of rice in dry and semi-arid environments (Venuprasad et al, 2012). More than 27 million hectares of rice are grown in upland fields (rainfed areas) rather than lowland fields (flooded areas), making them more susceptible to drought (Zu et al, 2017). Since lowland rice varieties need the second-highest amount of water of any other crops after cotton, as indicated in Table 1, they may also face drought if the rainfall is insufficient to maintain flooded paddy conditions (Bouman et al, 2007). Table 1 provides a comparative analysis of several important crops in specific regions during the past decade to underscore the agricultural water demands for cultivating rice. Notably, distinct units of measurement were employed by various researchers such as water level in the soil, litres of water required per kilogram of rice produced, and litres of water required per plant from emergence to maturity.
Rice production is significantly disrupted by drought at all morphological, physiological and molecular levels where morphological responses include decreased germination rate, plant biomass, plant stature, number of tillers, leaf size, shape, and rolling pattern as well as various root traits such as root depth and thickness. Leaf orientation and leaf rolling (LR) pattern are immediate responses to water deficit in rice and regarded as leading strategies to avoid water loss because less exposure to sunlight reduces water loss from the stomata, and bulliform cells can maintain water pressure in the cells for better water use efficiency in the leaves. The biomechanics of rice LR under drought stress have been hypothesized to be significantly influenced by turgor pressure in the bulliform cells (Zou et al, 2011; Cal et al, 2019; Matschi et al, 2020), though seedling height is also important in rice LR. According to Cal et al (2019), high rice genotypes have higher LR ratings than dwarf genotypes, independent of their sensitivity to dryness. In this review, we will embrace the morphological, physiological and molecular adaptations that support an essential morphological trait (LR) in rice, as well as why, when, where, and how LR occurs to combat the impacts of drought stress. Rice will thus have a collection of LR mechanisms on a single platform.

Table 1. Rice water requirements compared with main crops reported in the preceding decade.
Data are gathered from Praharaj et al (2016), Rahaman and Shehab, (2018), Upadhyaya (2019), Dharminder et al (2019) and Bharteey et al (2020), respectively. Data demonstrate that rice is a crop with high water demand. Drought-tolerant rice modifications such as leaf rolling are crucial to tackle drought stress. ‘‒’ indicates the data missing.
Why do leaves roll?
Every stage of rice growth requires a substantial amount of water, although water consumption varies according to growth stage, as shown in Fig. 1, which is why plants often exhibit LR in response to water deficit (Fang and Xiong, 2015). Other abiotic stressors such as temperature fluctuation, light and salt stress, heavy metals and ultraviolet exposure can also give rise to LR (Subashri et al, 2009; Kadioglu et al, 2012). One of the primary drought adaption mechanisms in rice is LR, which is a hydronastic movement that may control dehydration by lowering light perception and transpiration rate where water availability is limited (Kadioglu and Terzi, 2007). Although leaves are the primary photosynthetic tissues and water stress can diminish leaf expansion area, stomatal conductance, and storage assimilation, LR can be a helpful adaptation in these circumstances to counteract the effects of drought stress (Mangena, 2018).
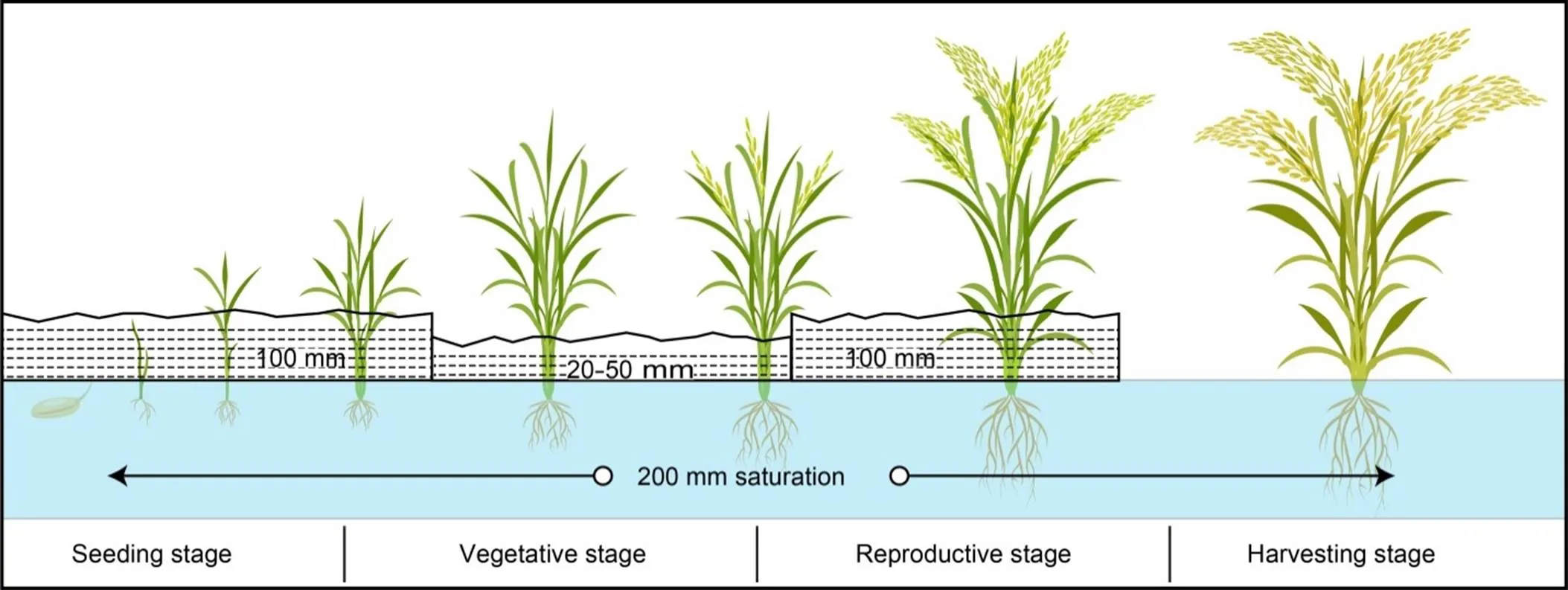
Fig. 1. A rice plant requires varying degrees of submergence at various growth stages.
Rice leaves typically show two LR patterns under drought stress: adaxial rolling (inward) and abaxial rolling (outward) (Tee, 2020). Optimum/partial LR is always more efficient for compensating drought impacts than completely rolled or fully flattened leaf structures (Xu et al, 2018). Partial LR not only promotes efficient photosynthetic activities, but also allows the energy load to decline, ensuring less water loss, which ultimately aids in maintaining plant turgor pressure under water deficits (Yuan et al, 2015; Islam et al, 2018). In addition, LR score can be clearly achieved in the afternoon, when the sunlight is at its peak (Baret et al, 2018).
Where does LR occur?
There are several cells that are involved in LR such as bulliform, parenchyma, collenchyma, sclerenchyma, mesophyll, and vascular bundles. However, the shrinkage of bulliform cells on either side of the leaf (adaxial/abaxial) due to lower turgor pressure and increased transpiration rate is a major cause behind LR in rice (Fang et al, 2012; Li et al, 2017). Bulliform cells’ number and size, along with their structure, are essential determinants in LR under water scarcity (Zou et al, 2014). Bulliform cells are positioned at the edges of vascular bundles adjacent to the midrib on the adaxial side of the leaf (Li et al, 2010). These cells are large, thin walled and highly vacuolated, and play a crucial role in the LR process by modulating their size and quantity (Fig. 2). The flat leaf phenotype is shown in Fig. 2-A, where fully expanded bulliform cells and thick sclerenchyma cells help retain the leaf structure flat by maintaining turgor pressure and providingmechanical strength. Likewise, LR phenotype under the drought stress is shown in Fig. 2-B, where contracted bulliform cells and defective sclerenchyma cellsallow the leaf to roll to reduce water loss during stress (Xiang et al, 2012; Zhang et al, 2015; Yang et al, 2016). Many genes regulating bulliform cell number and size have been revealed, including() (Chen et al, 2015) and() (Li et al, 2010), which are detailed in the molecular mechanism section. In addition to bulliform cells, defective sclerenchyma and cuticle development cells engage in LR via genes known as() and(), respectively, and will also be discussed in succeeding sections.
Physiology of LR
As a physiological response to water scarcity, plants normally aim for quick osmotic adjustment by accumulating osmolytes such as proteins, soluble carbohydrates, certain free amino acids, proline and glycine betaine (Mahmood et al, 2020). The accumulation of these osmolytes in the cytosol lowers the cell’s osmotic potential, which aids to withstand cellular dehydration (Valliyodan and Nguyen, 2006; Ahmad et al, 2007), and the most commonly found osmolytes that also act as osmoprotectants in rice are proline and glycine betaine (Rontein et al, 2002; Chen et al, 2023).
Water shortage in the soil is more commonly responsible for closing of stomata in rice to decrease the photorespiration rate, resulting in better water use efficiency (Li et al, 2009; Ullah et al, 2019). Furthermore, rice leaves display less chlorophyll content due to a decrease in photosynthetic rate. Nevertheless, leaf age really dictates the severity of chlorophyll loss (Young et al, 2004; Nikolaeva et al, 2010; Anjum et al, 2011). The thin and flat leaves of rice have vascular bundles running parallel to the midrib, which give them strength to stand upright during stress (Matsumoto et al, 2020). In addition, bulliform cells in rice are distinct epidermal cells on adaxial side of leaves, which are recognized from ordinary epidermal cells by their large size and unique shape and aid in maintaining the structure of leaf blade flat by absorbing water (Itoh et al, 2005; Li et al, 2010; Matschi et al, 2020).
Under dry conditions, loss of water in the bulliform cells causes LR on adaxial side, and abaxial rolling in some genotypes also caused by bulliform cells, but here the inflated bulliform cells increase in number, which is the major cause of abaxial side curling. According to Hibara et al (2009), a gene() is required for restricting differentiation of bulliform cells on the adaxial side, resulting in abaxial side curling, and its mutantvalidates its function in LR by showing adaxial rolling. Besides, the presence of strong and thick-walled sclerenchyma cells on both sides of vascular bundles contributes to LR by providing physical strength to the leaf blade under stress conditions (Sakurai et al, 2001; Crang et al, 2018). Fig. 3 depicts the cells and their potential genes involved in rice LR characteristics during drought stress.
Knockout/over-expression of genes involved in LR
Plants may experience cellular water deficits and alter gene expression (up- and down-regulation) when soil water content is low (Chaves et al, 2009), and various genes become transcriptionally activated under such conditions where their products help plants endure drought (Farooq et al, 2009). Stress conditions cantrigger gene expression on its own or in conjunction with subsequent stressors and/or damage responses. However, it is often assumed that several genes collaborate to source a complex phenomenon known as drought tolerance (Farooq et al, 2009). A considerable number of genes controlling leaf morphology and development have been identified in rice (Fujino et al, 2008; Rodriguez et al, 2014), and genes responsible for rice LR are listed in Fig. 4.
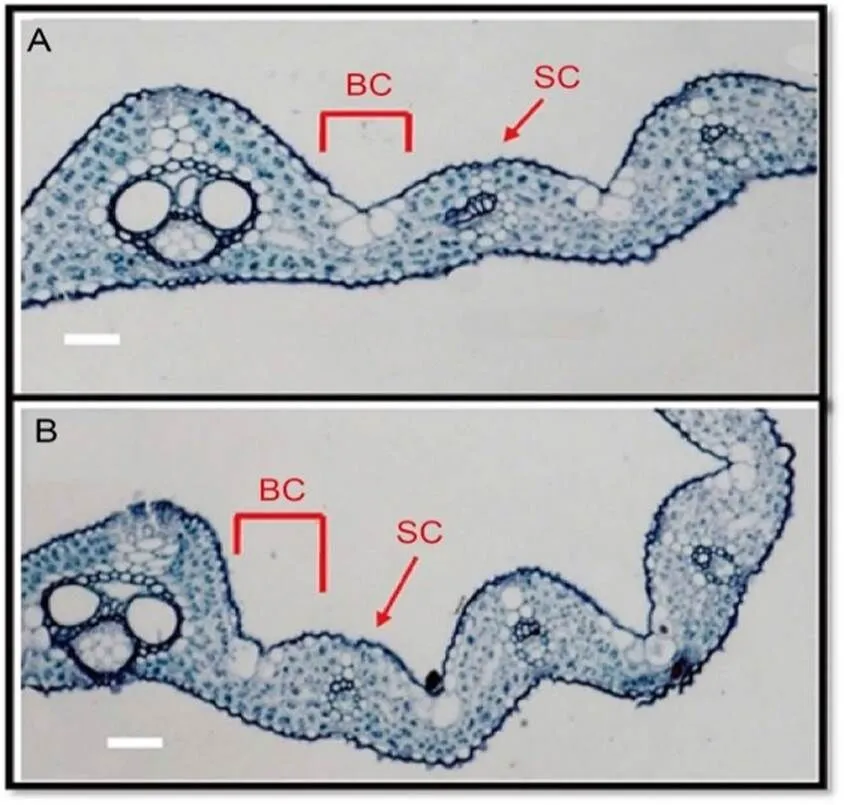
Fig. 2. Comparison of rice leaf anatomy under normal (A) and drought stress conditions (B)(Sun et al, 2020).
BC and SC represent bulliform cells and sclerenchyma cells, respectively. Scale bars, 20 μm.
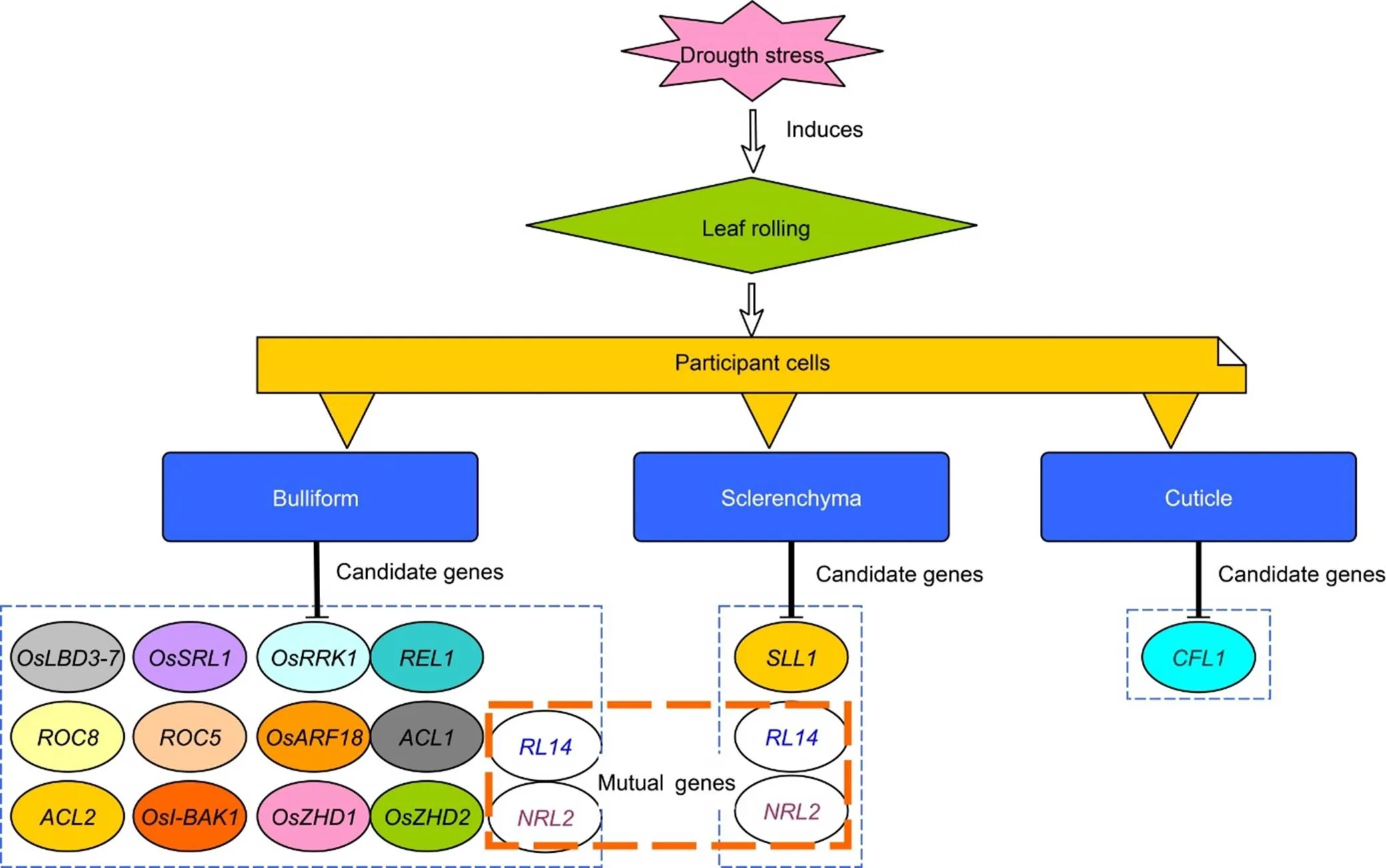
Fig. 3. Drought stress mediated leaf rolling involves modulated expression of different genes.
Fig. 4. Rice plants roll their leaves in response to drought stress, and genes that drive both adaxial and abaxial rolling are depicted individually.
Genes involved in adaxial LR
() gene in rice encodes a GPI (GLYCOSYL PHOSPHATIDYL INOSITOL) protein that is anchored and located in the plasma membrane. Theloss-of-function mutant promotes LR due to an increase in the number of bulliform cells on the adaxial surfaces of leaves. Further research revealed thatnegatively normalizes the expression of the vacuolarandgenes, which normally impede the formation of bulliform cells (Xiang et al, 2012).is an allelic variant of the() mutant, which exhibits LR abnormalities. The epidermis of bulliform cells in theloss-of-function mutant contains less lignin and cellulose, resulting in defects in cell wall formation, which diminishes the capacity to hold water and accelerates water loss, and then instigates leaf rolling (Li et al, 2017).
Similarly, over-expressed() causes adaxially curled thin leaves (Li et al, 2022), and histological analysis revealed thatreduces the size and quantity of bulliform cells. At the transcriptional level, genes involved in the negative regulation of bulliform cells are up-regulated, specifying that LR may occur owing to the less number of bulliform cells (Li et al, 2016). Another addition is(), which has an erect morphology and negatively regulates the number and size of bulliform cells. Hence,over-expression rice leaves validate adaxial rolling pattern (Ma et al, 2017).Moreover,positively regulates LR owing to changes in bulliform cell number and size (Chen et al, 2015). Over-expression()alsoresults in adaxially rolled leaves due to a decrease in number and size of bulliform cells, whereasknockdown results in abaxially rolled leaves due to an increase in the number and size of bulliform cells (Xu et al, 2021).
The() mutant in rice is responsible for undersized bulliform cells, which is the basic reason for adaxial curling phenotype. Themutant also has an effect on the size of leaf blade and spikelet (Matsumoto et al, 2018). The leaf blades of a loss-of-function mutant of thegene, which is linked to theKANADI family, are curled adaxially because abaxial side sclerenchyma cells fail to differentiate (Matsumoto et al, 2020). Rice() regulates adaxial leaf curling by influencing both the number and size of bulliform cells. Their transgenic plantsalso express pleiotropic defects, comprising dwarf plant stature and small seeds along with rolled leaves (Huang et al, 2016). Transgenic plants with lower expression levels of()or(), or over- expression of, demonstrate adaxial LR due to smaller bulliform cells. In addition,the protein interaction ofNRL2(NARROW AND ROLLED LEAF2)andRL14has a deleterious effect on lignin, cellulose and sclerenchyma differentiation (Zhao et al, 2016).
In general, adaxial LR in rice is typically brought on by variations in the number and size of bulliform cells as well as faults in sclerenchyma cells, but only a small number of genes linked to sclerenchyma cell defects have been identified so far.
Genes involved in abaxial LR
mediates abaxial LR by negatively regulating the number and area of bulliform cells, and transcription analysis disclosed thatpossibly controls LR through binding to the L1 motif box in the promoter region of PFL (pyruvate formate-lyase), because the PFL knockout phenotypes also exhibit abaxial LR characteristics (Zou et al, 2011). The T-DNA insertion in the promoter region ofandalso exhibit abaxial leaf rolling in the BY240 mutant due to a rise in epidermal cells on the adaxial side and a greater and higher number of bulliform cells on the abaxial side of the leaf (Li et al, 2010). The histological analysis of leaves from() over-expression plants expose a great number of bulliform cells and enlarged adaxial epidermal cells, hinting to the abaxial curling of leaves (Khew et al, 2015). Moreover, over-expression of()andshows abaxially rolled droopy leaves and the rolling phenotype is also due to greater number of bulliform cells (Xu et al, 2014). Similarly, a member of the YABBY gene family,also shows abaxial rolling when its expression is repressed due to the presence of additional number of bulliform cells (Zhang et al, 2020). In addition,negatively regulates the cuticle development by affecting the function ofthat mediates curly structure of leaf (Wu et al, 2011).
Vascular bundles are also crucial under drought stress, especially xylem, the main organ responsible for water transport throughout the plant. Theplays a critical role in vascular bundles to facilitate cold and salt tolerance(Saijo et al, 2000), but later studies revealed thatis controlled by, which directly stimulates the expression of drought related genes such as,,and, indicating thatmay function similarly under drought stress (Zhou et al, 2016). Rice leaves also roll outward when()is mutated, causing fewer mesophyll cells, more parenchyma cells and smaller vascular bundles in the leaf veins (Wang et al, 2016).
Overall, the bulliform cells are predominantly crucial for defining the LR phenomenon under drought stress; however, secondarily participating cells are parenchyma, mesophyll, sclerenchyma and vascular bundles.
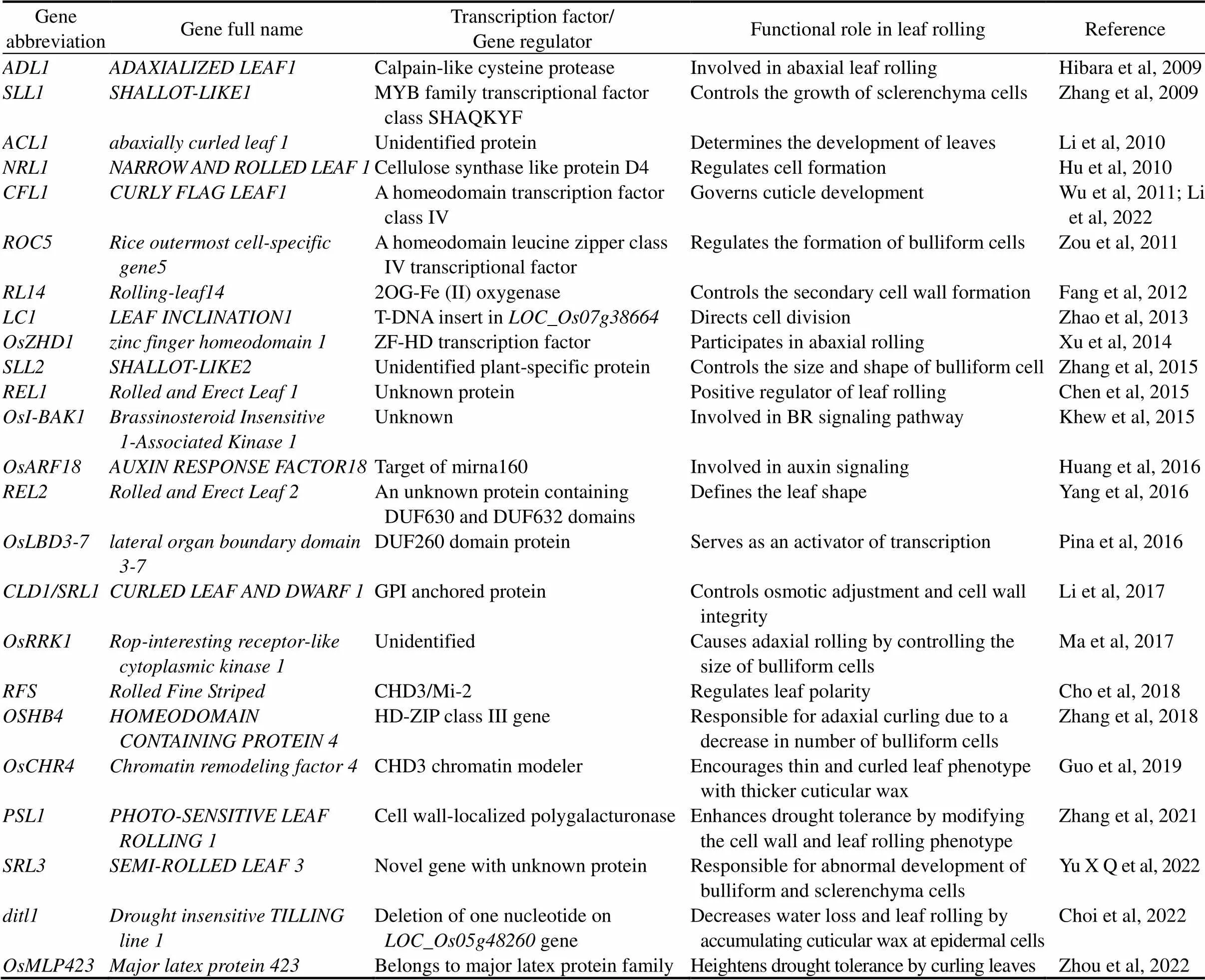
Table 2. List of genes/transcription factorsresponsible for adaxial and abaxial leaf rolling in rice.
Role of genes/transcription factors (TFs) in LR
Many rice drought responsive genes associated with their transcription factors such as MYB, MYC, NAC, WRKY, DREB/CBF (drought-responsive-element binding protein/C-repeat-binding factor) and ABF/ AREB are acknowledged (Osakabe et al, 2014). However, only a small number of regulating factors/ transcription factors associated with LR have been reported so far. The genes that have been linked to LR in previous studies are listed in Table 2 along with their regulating variables.
Crosstalk between drought and other abiotic stresses
The duration and severity of drought-related symptoms in plants are significantly influenced by environmental factors comprising drought intensity, frequency and duration (Zoghi et al, 2019). Plants get benefit from LR under short water supplies because it reduces water loss through stomata by lowering leaf expansion (Amal et al,2020). In comparison to loosely rolled leaves, optimally curled leaves produce higher yield (Liu et al, 2018), although other environmental factors such as low/high temperatures and solar radiations can also cause LR (Kadioglu and Terzi, 2007). Due to changes in leaf orientation and stomatal closure brought by reduction in bulliform cell size and number, LR is a critical adaptation for minimizing water loss under high temperatures (Sarkar et al, 2021). Bulliform cells play a vital role in LR in rice; however, certain cultivars also involve sclerenchyma and epidermal cells. Wheat and maize leaves also show a similar kind of LR pattern under water and heat stress due to changes in the number and size of bulliform cells, and this pattern is most pronounced from 11:00 am to 2:00 pm when the sunlight intensity is at its maximum (Kadioglu and Terzi, 2007; Matschi et al, 2020).() andare two genes responsible foradaxial/abaxial LR in maize (Juarez et al, 2004), and()is also a maize gene responsible for adaxial rolling (Canales et al, 2005). LR or withering, is not only a stress-induced response to dryness but also a defense mechanism to minimize water loss from plants (Li et al, 2017). For example,() is a photosensitive LR mutant in rice, which is also responsible for drought tolerance (Zhang et al, 2021). Moreover, numerous genes controlled by drought stress reveal a comparable response to cold stress, as exemplified by the contribution ofin stimulating both cold and drought-related genes, resulting in the distinctive rolling phenotype (Zhou et al, 2016). Similarly, an over-expressed NAC67 transcript factor identified from finger millet is also responsible for drought and salt tolerance in rice (Singh et al, 2021). Moreover,gene is responsible for LR in the presence of sunlight and moderates LR to boost photosynthetic efficiency, indicating that this gene may also be involved in drought tolerance. However, its impact on drought stress has not yet been identified (Yu N et al, 2022). These findings indicate that the genes responsible for LR due to water stress can also be involved in other stresses such as heat, cold, light and salt (Rahman et al, 2016).
What would happen in case of no LR?
Under dry conditions, plants roll their leaves and maintain an upright leaf angle to reduce transpiration rate by minimizing exposed leaf area and increasing water uptake to resist drought (Kadioglu and Terzi, 2007; Kahlown and Kemper, 2007). According to Cal et al (2019), LR is not a direct function of plant size, but there is a substantial correlation between LR in the stress treatment and leaf dimensions, including a positive correlation with leaf blade and sheath length and a negative correlation with specific leaf area and leaf blade width. Besides, xylem is found on the adaxial side of leaves, and the rolling phenotype is directly related to water movement through xylem (De Rybel et al, 2016). Therefore, it can be a leading strategy to avoid water loss under water deficit. However, if rolling doesn’t take place, more solar radiations would hit the leaf surface due to flattened leaf blade and more transpiration would take place through stomata to avoid heating up of plant (Lafitte et al, 2003). As a result, plants would quickly lose vigor and wilt, and if stress persists for a long period, it might cause burning or death of the plant by interfering photosynthetic activity. On the other hand, if a plant rolls its leaves, the expansion of the bulliform cells reduces water loss through stomata. Thus, it will help plants stand up straight and promote effective photosynthesis, boosting their ability to withstand dryness (Seleiman et al, 2021). In order to comprehend how the LR trait behaves in rice under drought stress, a comparison between the rolling and non-rolling phenotypes has been made as shown in Fig. 5.
Can LR be prevented?
The two necessary features for improving drought tolerance in rice are delayed leaf drying and LR, and high stress conditions lead to leaf curling at all growth stages of rice, such as the vegetative and reproductive stages (Barik et al, 2019). The ability to maintain turgor during drought stress by increasing water intake or adjusting osmotic pressure, shows delay in LR, however, partial LR protects against the negative effects of excessive solar radiations to prevent excessive water loss. According to Islam et al (2018), if the cells sustain their turgor pressure under drought stress, it would result in delayed LR. It is also reported that salicylic acid can prevent LR by altering the antioxidant system during water deficits, although this claim is only true for short periods of stress (Kadioglu et al, 2011). Assertively, osmotic adjustment can help rice tolerate drought by delaying LR, which keeps gas exchange going on and delays leaf death, but under severe water stress, it cannot be postponed for very long time (Hsiao et al, 1984).
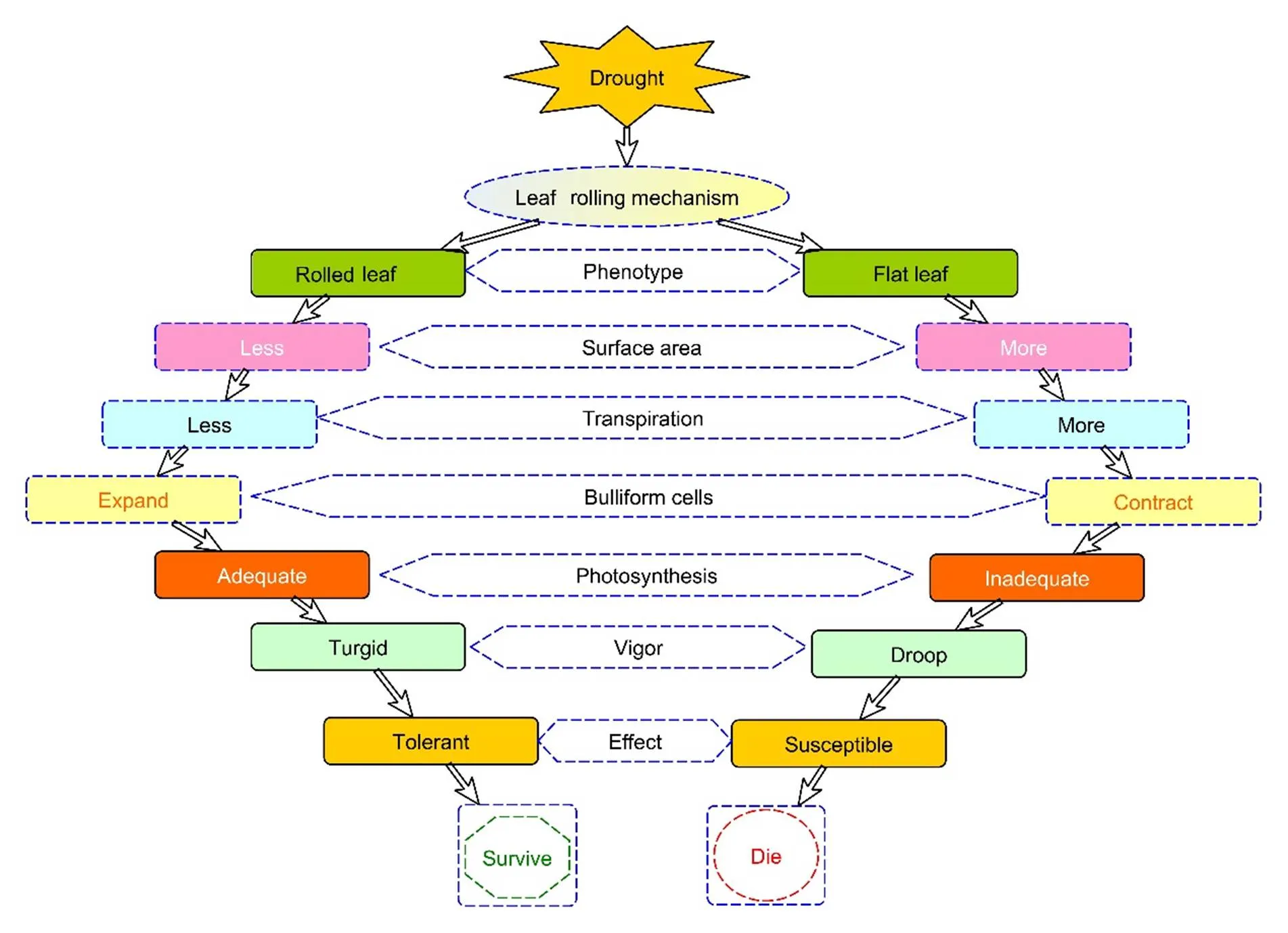
Fig. 5. Insightful demonstration of rice plant defense mechanism to combat impacts of drought stress under leaf rolling trait.
Is LR a necessary evil?
Photosynthesis is increased by moderate LR (Lang et al, 2004). According to Singh et al (2017), high levels of LR may be beneficial in mid-season drought situations to prevent water loss. However, enhanced/excessive LR may reduce photosynthetic effectivity and yield (Nar et al, 2009). Accordingly, delay in LR may be more helpful in rice than enhanced rolling. As the stress period increases, more rolled leaf phenotypes appear, but complete rolling is not considered beneficial because photosynthetic activity may cease in case of extreme rolling. For example,is a gene that has just come to light and has been linked to rice drought tolerance, and its over-expression transgenic lines result in delayed LR, demonstrating that LR is a crucial characteristic under the symptoms of drought stress (Zhou et al, 2022).
Conclusion
Drought resistance and tolerance is a multifarious characteristic including morphological, physiological and biochemical alterations (Hellal et al, 2018). According to mechanistic data, plant responses to water scarcity have been extensively investigated, and genes that aid in drought resistance have been inserted into agricultural plants to deal with drought in water- stressed areas, but plants with better drought resistance generally exhibit lower growth, underscoring the need of devising techniques to decouple drought resistance from growth (Xue et al, 2017). Therefore, rice plants have a variety of morphological, developmental and physiological methods to counteract drought stress and one major strategy for maintaining water content in leaves for photosynthesis is morphological adaptation like LR (Salehi-Lisar and Bakhshayeshan-Agdam, 2016). Depending upon the stress and environmental conditions, the expression of plant leaves ranges from tightly rolled to loosely rolled, and changes in LR pattern enhance the overall efficiency of photosynthetic machinery by improving assimilate source-to-sink translocation, which increases biomass. The mechanism behind both types of LR (adaxial and abaxial) shows that rolling expression is mostly due to an increase or decrease in the number of bulliform cells, but sclerenchyma, mesophyll, cuticle cells, and vascular bundles also participate. Thus, LR phenotype in rice is primarily caused by a shift in turgor pressure in bulliform cells. Hence, genes involvedin LR can be introduced into drought susceptible cultivars to enhance their tolerance against drought stress. This review will assist researchers in understanding the overview of the LR trait in rice during drought stress. Further studies are required to comprehend the genetic and molecular mechanisms behind this trait and determine its potential use in rice breeding.
Ahmad M S A, Javed F, Ashraf M. 2007. Iso-osmotic effect of NaCl and PEG on growth, cations and free proline accumulation in callus tissue of two indica rice (L.) genotypes., 53(1): 53–63.
Amal B A, Said M, Abdelaziz B, Mouhammed M, Nasser E N, Bouhmadi Keltoum E. 2020. Relationship between leaf rolling and some physiological parameters in durum wheat under water stress., 16(7): 1061–1068.
Anjum S A, Xie X Y, Wang L C, Saleem M F, Man C, Lei W. 2011. Morphological, physiological and biochemical responses of plants to drought stress., 6(9): 2026–2032.
Baret F, Madec S, Irfan K, Lopez J, Comar A, Hemmerlé M, Dutartre D, Praud S, Tixier M H. 2018. Leaf-rolling in maize crops: From leaf scoring to canopy-level measurements for phenotyping., 69(10): 2705–2716.
Barik S R, Pandit E, Pradhan S K, Mohanty S P, Mohapatra T. 2019. Genetic mapping of morpho-physiological traits involved during reproductive stage drought tolerance in rice., 14(12): e0214979.
Bharteey PK, Singh YV, Deka B, Dutta M. 2020. Assessment of water requirement for major crops of Mirzapur district in eastern Uttar Pradesh.,22(1): 100–106.
Bouman BAM, Lampayan R M, Tuong T P. 2007. Water Management in Irrigated Rice: Coping with Water Scarcity.Los Banos, the Philippines: International Rice Research Institute.
Cal A J, Sanciangco M, Rebolledo M C, Luquet D, Torres R O, McNally K L, Henry A. 2019. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought., 42(5): 1532–1544.
Canales C, Grigg S, Tsiantis M. 2005. The formation and patterning of leaves: Recent advances., 221(6): 752–756.
Chaves M M, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell., 103(4): 551–560.
Chen F, Aqeel M, Khalid N, Irshad M K, Farhat F, Nazir A, Ma J, Akhtar M S, Eldesoky G E, Aljuwayid A M, Noman A. 2023. Glutathione treatment suppresses the adverse effects of microplastics in rice., 322: 138079.
Chen Q L, Xie Q J, Gao J, Wang W Y, Sun B, Liu B H, Zhu H T, Peng H F, Zhao H B, Liu C H, Wang J, Zhang J L, Zhang G Q, Zhang Z M. 2015. Characterization ofin regulating leave morphology in rice., 66(19): 6047–6058.
Cho S H, Lee C H, Gi E, Yim Y, Koh H J, Kang K, Paek N C. 2018. The rice rolled fine striped (RFS) CHD3/Mi-2 chromatin remodeling factor epigenetically regulates genes involved in oxidative stress responses during leaf development., 9: 364.
Choi S Y, Lee Y J, Seo H U, Kim J H, Jang C S. 2022. Physio-biochemical and molecular characterization of a rice() mutant., 174(3): e13718.
Crang R, Lyons-Sobaski S, Wise R. 2018. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants. Springer Nature Swetzerland AG: Springer: 181–213.
De Rybel B, Mähönen A P, Helariutta Y, Weijers D. 2016. Plant vascular development: From early specification to differentiation., 17(1): 30–40.
Dharminder C, Singh R K, Kumar V, Devedee A K, Mruthyunjaya M, Reshu B. 2019. The clean water: The basic need of human and agriculture.,7(2): 1994–1998.
Fang J J, Guo T T, Xie Z W, Chun Y, Zhao J F, Peng L X, Zafar S A, Yuan S J, Xiao L T, Li X Y. 2021. The URL1-ROC5-TPL2 transcriptional repressor complex represses thegene to modulate leaf rolling in rice., 185(4): 1722–1744.
Fang L K, Zhao F M, Cong Y F, Sang X C, Du Q, Wang D Z, Li Y F, Ling Y H, Yang Z L, He G H. 2012. Rolling-leaf14 is a 2OG-Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves., 10(5): 524–532.
Fang Y J, Xiong L Z. 2015. General mechanisms of drought response and their application in drought resistance improvement in plants., 72(4): 673–689.
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S M A. 2009. Plant drought stress: Effects, mechanisms and management., 29: 185–212.
Fischer T R, Byerlee D, Edmeades G O. 2009. Can technology deliver on the yield challenge to 2050?: Conforti P. Looking Ahead in World Food and Agriculture: Perspectives to 2050. FAO: Agricultural Development Economics Division Economic and Social Development Department.
Fujino K, Matsuda Y, Ozawa K, Nishimura T, Koshiba T, Fraaije M W, Sekiguchi H. 2008.controls leaf shape mediated by auxin in rice., 279(5): 499–507.
Guo T T, Wang D F, Fang J J, Zhao J F, Yuan S J, Xiao L T, Li X Y. 2019. Mutations in the ricegene, encoding a CHD3 family chromatin remodeler, induce narrow and rolled leaves with increased cuticular wax., 20(10): 2567.
Hellal F A, El-Shabrawi H M, Abd El-Hady M, Khatab I A, El-Sayed S A, Abdelly C. 2018. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars., 16(1): 203–212.
Hibara K I, Obara M, Hayashida E, Abe M, Ishimaru T, Satoh H, Itoh J I, Nagato Y. 2009. Thegene functions in leaf and embryonic pattern formation in rice., 334(2): 345–354.
Hsiao T C, O’Toole J C, Yambao E B, Turner N C. 1984. Influence of osmotic adjustment on leaf rolling and tissue death in rice (L.)., 75(2): 338–341.
Hu J, Zhu L, Zeng D L, Gao Z Y, Guo L B, Fang Y X, Zhang G H, Dong G J, Yan M X, Liu J, Qian Q. 2010. Identification and characterization of, a novel gene regulating leaf morphology and plant architecture in rice., 73(3): 283–292.
Huang J, Li Z Y, Zhao D Z. 2016. Deregulation of the OsmiR160 target genecauses growth and developmental defects with an alteration of auxin signaling in rice., 6: 29938.
Irshad M K, Ibrahim M, Noman A, Shang J Y, Mahmood A, Mubashir M, Khoo K S, Ng H S, Show P L. 2022. Elucidating the impact of goethite-modified biochar on arsenic mobility, bioaccumulation in paddy rice (L.) along with soil enzyme activities., 160: 958–967.
Islam M M, Kayesh E, Zaman E, Urmi T A, Haque M M. 2018. Evaluation of rice (L.) genotypes for drought tolerance at germination and early seedling stage., 16(1): 44–54.
Itoh J I, Nonomura K I, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y. 2005. Rice plant development: From zygote to spikelet., 46(1): 23–47.
Juarez M T, Twigg R W, Timmermans M C. 2004. Specification of adaxial cell fate during maize leaf development., 131(18): 4533–4544.
Kadioglu A, Terzi R. 2007. A dehydration avoidance mechanism: Leaf rolling., 73(4): 290–302.
Kadioglu A, Saruhan N, Sağlam A, Terzi R, Acet T. 2011. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system., 64(1): 27–37.
Kadioglu A, Terzi R, Saruhan N, Saglam A. 2012. Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors., 182: 42–48.
Kahlown M A, Kemper W D. 2007. Factors affecting success and failure of trickle irrigation systems in Balochistan, Pakistan., 26(1): 71–79.
Khew C Y, Teo C J, Chan W S, Wong H L, Namasivayam P, Ho C L. 2015. Brassinosteroid insensitive 1-associated kinase 1 (OsI-BAK1) is associated with grain filling and leaf development in rice., 182: 23–32.
Lafitte H R, Blum A, Atlin G. 2003. Using secondary traits to help identify drought-tolerant genotypes.: Fischer K S, Lafitte R, Fukai S, Atlin G, Hardy B. Breeding Rice for Drought-Prone Environments. Los Banos, the Philippines: International Rice Research Institute: 37–48.
Lang Y Z, Zhang Z J, Gu X Y, Yang J C, Zhu Q S. 2004. Physiological and ecological effects of crimpy leaf character in rice (L.): II. Photosynthetic character, dry mass production and yield forming., 30(9): 883–887. (in Chinese with English abstract)
Li C, Zou X H, Zhang C Y, Shao Q H, Liu J, Liu B, Li H Y, Zhao T. 2016.overexpression induced adaxially rolled leaves in rice., 11(6): e0156413.
Li L, Shi Z Y, Li L, Shen G Z, Wang X Q, An L S, Zhang J L. 2010. Overexpression of() increasedbulliform cells and induced abaxial curling of leaf blades in rice., 3(5): 807–817.
Li M, Li X Z, Zhu L, Xue P B, Bao J L, Zhou B B, Jin J, Wang J. 2022. Genome-wide transcriptomic analysis reveals the gene regulatory network controlled byin regulating rice leaf rolling., 41(6): 2292–2304.
Li S X, Wang Z H, Malhi S S, Li S Q, Gao Y J, Tian X H. 2009. Chapter 7: Nutrient and water management effects on crop production, and nutrient and water use efficiency in dryland areas of China.,102: 223–265.
Li W Q, Zhang M J, Gan P F, Qiao L, Yang S Q, Miao H, Wang G F, Zhang M M, Liu W T, Li H F, Shi C H, Chen K M. 2017.modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice., 92(5): 904–923.
Liu F, Wang P D, Zhang X B, Li X F, Yan X H, Fu D H, Wu G. 2018. The genetic and molecular basis of crop height based on a rice model., 247(1): 1–26.
Ma J, Aqeel M, Khalid N, Nazir A, Alzuaibr F M, Al-Mushhin A A M, Hakami O, Iqbal M F, Chen F, Alamri S, Hashem M, Noman A. 2022. Effects of microplastics on growth and metabolism of rice (L.)., 307: 135749.
Ma Y H, Zhao Y, Shangguan X X, Shi S J, Zeng Y, Wu Y, Chen R Z, You A Q, Zhu L L, Du B, He G C. 2017. Overexpression ofchanges leaf morphology and defense to insect in rice., 8: 1783.
Maclean J L, Dawe D C, Hardy B, Hettel G P. 2002. Rice Almanac: Source Book for the Most Important Economic Activity on Earth. Wallingford: CABI.
Mahmood T, Abdullah M, Ahmar S, Yasir M, Iqbal M S, Yasir M, Ur Rehman S, Ahmed S, Rana R M, Ghafoor A, Nawaz Shah M K, Du X M, Mora-Poblete F. 2020. Incredible role of osmotic adjustment in grain yield sustainability under water scarcity conditions in wheat (L.)., 9(9): 1208.
Mangena P. 2018. Water stress: Morphological and anatomical changes in soybean (L.) plants.: Andjelkovic V. Plant, Abiotic Stress and Responses to Climate Change. IntechOpen: 9–31.
Matschi S, Vasquez M F, Bourgault R, Steinbach P, Chamness J, Kaczmar N, Gore M A, Molina I, Smith L G. 2020. Structure-function analysis of the maize bulliform cell cuticle and its potential role in dehydration and leaf rolling., 4(10): e00282.
Matsumoto H, Yasui Y, Kumamaru T, Hirano H Y. 2018. Characterization of amutant that exhibits a curled leaf phenotype., 92(6): 287–291.
Matsumoto H, Yasui Y, Ohmori Y, Tanaka W, Ishikawa T, Numa H, Shirasawa K, Taniguchi Y, Tanaka J, Suzuki Y, Hirano H Y. 2020.encoding the largest subunit of the Elongator complex has a unique role in leaf development and meristem function in rice., 104(2): 351–364.
Nar H, Saglam A, Terzi R, Várkonyi Z, Kadioglu A. 2009. Leaf rolling and photosystem II efficiency inexposed to drought stress., 47(3): 429–436.
Nikolaeva M K, Maevskaya S N, Shugaev A G, Bukhov N G. 2010. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity., 57(1): 87–95.
Osakabe Y, Osakabe K, Shinozaki K, Tran L S P. 2014. Response of plants to water stress., 5: 86.
Pina A L C B, Zandavalli R B, Oliveira R S, Martins F R, Soares A A. 2016. Dew absorption by the leaf trichomes ofin the Brazilian semiarid region., 43(9): 851–861.
Praharaj CS, Singh U, Singh SS, Singh NP, Shivay YS. 2016. Supplementary and life-saving irrigation for enhancing pulses production, productivity and water-use efficiency in India.,61: S249–S261.
Rahaman M M, Shehab M K. 2018. Is the propensity of increasing the rice production a sustainable approach? Experiences from India, Pakistan, and Bangladesh., 5(2): 12–28.
Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Muthurajan R. 2016. Over-expression of a NAC67 transcription factor from finger millet (L.) confers tolerance against salinity and drought stress in rice., 16(Suppl 1): 35.
Rodriguez R E, Debernardi J M, Palatnik J F. 2014. Morphogenesis of simple leaves: Regulation of leaf size and shape., 3(1): 41–57.
Rontein D, Dieuaide-Noubhani M, Dufourc E J, Raymond P, Rolin D. 2002. The metabolic architecture of plant cells: Stability of central metabolism and flexibility of anabolic pathways during the growth cycle of tomato cells., 277(46): 43948–43960.
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. 2000. Over- expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants., 23(3): 319–327.
Sakurai N, Katayama Y, Yamaya T. 2001. Overlapping expression of cytosolic glutamine synthetase and phenylalanine ammonia-lyase in immature leaf blades of rice., 113(3): 400–408.
Salehi-Lisar S Y, Bakhshayeshan-Agdam H. 2016. Drought stress in plants: Causes, consequences, and tolerance.: Hossain M A, Wani S H, Bhattacharjee S, Burritt D J, Tran L P. Drought Stress Tolerance in Plants: Vol. 1. Physiology and Biochemistry. Spring Nature Swetzerland AG: Springer Cham.
Sarkar S, Islam A K M A, Barma N C D, Ahmed J U. 2021. Tolerance mechanisms for breeding wheat against heat stress: A review., 138: 262–277.
Seleiman M F, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid H H, Battaglia M L. 2021. Drought stress impacts on plants and different approaches to alleviate its adverse effects., 10(2): 259.
Singh B, Reddy K R, Diaz Redoña E, Walker T. 2017. Screening of rice cultivars for morpho-physiological responses to early-season soil moisture stress., 24(6): 322–335.
Singh S, Koyama H, Bhati K K, Alok A. 2021. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement., 134: 475–495.
Subashri M, Robin S, Vinod K K, Rajeswari S, Mohanasundaram K, Raveendran T S. 2009. Trait identification and QTL validation for reproductive stage drought resistance in rice using selective genotyping of near flowering RILs., 166(2): 291–305.
Sun J, Cui X A, Teng S Z, Zhao K N, Wang Y W, Chen Z H, Sun X H, Wu J X, Ai P F, Quick W P, Lu T G, Zhang Z G. 2020. HD-ZIP IV generegulates the size of bulliform cells and lignin content in rice., 18(12): 2559–2572.
Tee E E. 2020. Journey and destination: KORRIGAN1 subcellular localization dynamically changes during plant growth and stress tolerance., 32(2): 291–292.
Ullah H, Santiago-Arenas R, Ferdous Z, Attia A, Datta A. 2019. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review.,156: 109–157.
Upadhyaya A. 2019. Multi-objective fuzzy linear programming for land allocation under different crops in bhagwanpur distributary., 6(4): 188–193.
Valliyodan B, Nguyen H T. 2006. Understanding regulatory networks and engineering for enhanced drought tolerance in plants., 9(2): 189–195.
Venuprasad R, Bool M E, Quiatchon L, Sta Cruz M T, Amante M, Atlin G N. 2012. A large-effect QTL for rice grain yield under upland drought stress on chromosome 1., 30(1): 535–547.
Wang L, Xu J, Nian J Q, Shen N W, Lai K K, Hu J, Zeng D L, Ge CW, Fang Y X, Zhu L, Qian Q, Zhang G H. 2016. Characterization and fine mapping of the rice generegulating leaf morphology and leaf vein development., 78(3): 345–356.
Wu R H, Li S B, He S, Wassmann F, Yu C H, Qin G J, Schreiber L, Qu L J, Gu H Y. 2011. CFL1, a WW domain protein, regulates cuticle development by modulating the function of HDG1, a class IV homeodomain transcription factor, in rice and., 23(9): 3392–3411.
Xiang J J, Zhang G H, Qian Q, Xue H W. 2012.encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells., 159(4): 1488–1500.
Xu P Z, Ali A, Han B L, Wu X J. 2018. Current advances in molecular basis and mechanisms regulating leaf morphology in rice., 9: 1528.
Xu Y, Wang Y H, Long Q Z, Huang J X, Wang Y L, Zhou K N, Zheng M, Sun J, Chen H, Chen S H, Jiang L, Wang C M, Wan J M. 2014. Overexpression of, a zinc finger homeodomain class homeobox transcription factor, induces abaxially curled and drooping leaf in rice., 239(4): 803–816.
Xu Y, Kong W Y, Wang F Q, Wang J, Tao Y J, Li W Q, Chen Z H, Fan F J, Jiang Y J, Zhu Q H, Yang J. 2021. Heterodimer formed by ROC8 and ROC5 modulates leaf rolling in rice., 19(12): 2662–2672.
Xue D W, Zhang X Q, Lu X L, Chen G, Chen Z H. 2017. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance., 8: 621.
Yang S Q, Li W Q, Miao H, Gan P F, Qiao L, Chang Y L, Shi C H, Chen K M. 2016., a gene encoding an unknown function protein which contains DUF630 and DUF632 domains controls leaf rolling in rice., 9(1): 37.
Young T E, Meeley R B, Gallie D R. 2004. ACC synthase expression regulates leaf performance and drought tolerance in maize., 40(5): 813–825.
Yu N, Liang Y P, Wang Q P, Peng X X, He Z H, Hou X W. 2022. Transcriptomic analysis ofoverexpression rice lines with rapid and dynamic leaf rolling morphology., 12(1): 6736.
Yu S, Tian L. 2018. Breeding major cereal grains through the lens of nutrition sensitivity., 11(1): 23–30.
Yu X Q, Xie W, Liu H, Liu W, Zeng D L, Qian Q, Ren D Y. 2022. Characterization and fine mapping of a semi-rolled leaf mutantin rice., 21(11): 3103–3113.
Yuan S, Li Y, Peng S B. 2015. Leaf lateral asymmetry in morphological and physiological traits of rice plant., 10(6): e0129832.
Zhang G H, Xu Q, Zhu X D, Qian Q, Xue H W. 2009. SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development., 21(3): 719–735.
Zhang G H, Hou X, Wang L, Xu J, Chen J, Fu X, Shen N W, Nian J Q, Jiang Z Z, Hu J, Zhu L, Rao Y C, Shi Y F, Ren D Y, Dong G J, Gao Z Y, Guo L B, Qian Q, Luan S. 2021.encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice., 229(2): 890–901.
Zhang J J, Wu S Y, Jiang L, Wang J L, Zhang X, Guo X P, Wu C Y, Wan J M. 2015. A detailed analysis of the leaf rolling mutantreveals complex nature in regulation of bulliform cell development in rice (L.)., 17(2): 437–448.
Zhang J S, Zhang H, Srivastava A K, Pan Y J, Bai J J, Fang J J, Shi H Z, Zhu J K. 2018. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development., 176(3): 2082–2094.
Zhang T P, Li C Y, Li D X, Liu Y, Yang X H. 2020. Roles of YABBY transcription factors in the modulation of morphogenesis, development, and phytohormone and stress responses in plants., 133(6): 751–763.
Zhao S Q, Xiang J J, Xue H W. 2013. Studies on the rice LEAF INCLINATION1 (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control., 6(1): 174–187.
Zhao S S, Zhao L, Liu F X, Wu Y Z, Zhu Z F, Sun C Q, Tan L B. 2016.regulates leaf shape, male fertility, and seed size in rice., 58(12): 983–996.
Zhou L G, Liu Z C, Liu Y H, Kong D Y, Li T F, Yu S W, Mei H W, Xu X Y, Liu H Y, Chen L, Luo L J. 2016. A novel geneimproves both drought avoidance and drought tolerance in rice., 6(1): 30264.
Zhou Z M, Fan J B, Zhang J, Yang Y M, Zhang Y F, Zan X F, Li X H, Wan J L, Gao X L, Chen R J, Huang Z J, Xu Z J, Li L H. 2022.is a positive regulator of tolerance to drought and salt stresses in rice., 11(13): 1653.
Zoghi Z, Hosseini S M, Kouchaksaraei M T, Kooch Y, Guidi L. 2019. The effect of biochar amendment on the growth, morphology and physiology ofseedlings under water-deficit stress., 138(6): 967–979.
Zou L P, Sun X H, Zhang Z G, Liu P, Wu J X, Tian C J, Qiu J L, Lu T G. 2011. Leaf rolling controlled by the homeodomain leucine zipper class IV genein rice., 156(3): 1589–1602.
Zou L P, Zhang Z G, Qi D F, Peng M, Lu T G. 2014. Cytological mechanisms of leaf rolling in rice., 54(1): 198–209.
Zu X F, Lu Y K, Wang Q Q, Chu P F, Miao W, Wang H Q, La H G. 2017. A new method for evaluating the drought tolerance of upland rice cultivars., 5(6): 488–498.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.04.002
27 December 2022;
23 April 2023
Ammara Latif (ammaralatif94@gmail.com)
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa

